Abstract
Background
The thienopyridine clopidogrel is one of the most commonly prescribed drugs worldwide. Both clopidogrel and the third-generation thienopyridine prasugrel are subject to efflux via P-glycoprotein (encoded by ABCB1, also known as MDR1). In vitro and clinical studies suggest that ABCB1 polymorphisms, particularly C3435T, may be associated with altered drug metabolism and efficacy.
Methods
We genotyped 2,932 patients with an acute coronary syndrome (ACS) in TRITON-TIMI 38 treated with clopidogrel or prasugrel and 321 healthy individuals in whom we measured the pharmacologic response to clopidogrel or prasugrel.
Findings
Among ACS patients treated with clopidogrel, ABCB1 C3435T genotype was significantly associated with risk for the primary endpoint of cardiovascular death, MI, or stroke (P=0.0064). TT homozygotes (804/2,932 [27%] of the population) had a 72% increased risk of the primary endpoint as compared with CT /CC individuals (52/414 [12.9%] vs. 80/1,057 [7.8%], HR 1.72, 95% CI 1.22–2.44, P=0.002). ABCB1 C3435T and CYP2C19 genotypes were significant, independent predictors of the primary endpoint, and the 47% (681/1454) of the population who were either CYP2C19 reduced-function allele carriers, ABCB1 3435 TT homozygotes, or both were at significantly increased risk of cardiovascular death, MI, or stroke (HR 1.97, 95% CI 1.38–2.82, P=0.0002). In healthy subjects, 3435 TT homozygotes had a reduction in platelet aggregation with clopidogrel that was 7.3 absolute percentage points lower (i.e., less platelet inhibition) vs. CT/CC individuals (P=0.0127). ABCB1 genotypes were not significantly associated with clinical or pharmacologic outcomes among ACS or healthy individuals treated with prasugrel.
Interpretation
Individuals with the ABCB1 3435 TT genotype have less platelet inhibition and are at significantly increased risk of recurrent ischemic events in the setting of clopidogrel treatment. Taking into account both ABCB1 and CYP2C19, nearly half of the population carries a genotype associated with an increased risk for major adverse cardiovascular events while on standard doses of clopidogrel.
INTRODUCTION
In patients presenting with acute coronary syndromes (ACS) and in those undergoing percutaneous coronary interventions (PCI) with stenting, dual antiplatelet therapy with aspirin and the thienopyridine clopidogrel is the guideline-approved standard of care.1, 2 As such, clopidogrel is one of the most commonly prescribed medications worldwide. However, there is substantial inter-patient variability in the pharmacodynamic response to clopidogrel,3 and those individuals with less platelet inhibition in the setting of treatment with clopidogrel are at increased risk of cardiovascular events.4 Prasugrel is a third-generation thienopyridine that has been found to achieve greater platelet inhibition with less inter-patient variability.5 In the TRITON-TIMI 38 trial, treatment with prasugrel as compared with clopidogrel resulted in a significantly lower rate of ischemic events and more bleeding.6
Both clopidogrel and prasugrel are pro-drugs that require intestinal absorption and biotransformation to active metabolites by cytochrome P450 (CYP) enzymes. In multiple studies, reduced-function genetic variants in the CYP2C19 gene (located on chromosome 10) have been associated with lower active drug metabolite levels, diminished platelet inhibition, and higher rates of adverse cardiovascular events in the setting of treatment with clopidogrel but not prasugrel.7–15 To that end, the United States Food and Drug Administration has now incorporated CYP2C19 genetic information into the updated clopidogrel label in the form of a black box warning, indicating that carriers of two reduced-function CYP2C19 alleles may have a reduced response to standard doses of clopidogrel.
In addition, there are key proteins involved in thienopyridine absorption, including the efflux pump P-glycoprotein (P-gp) encoded by the ABCB1 gene (also known as MDR1; located on chromosome 7). P-gp is an ATP-dependent efflux pump that transports various molecules across extra- and intra-cellular membranes. It is expressed, among other places, on intestinal epithelial cells where increased expression or function can alter bioavailability of drugs that are substrates. Data suggest that when treated with clopidogrel, individuals with genetic variants in ABCB1 (specifically those who are TT homozygotes for the C3435T variant), have lower levels of the active drug metabolite,16 and may have higher rates of adverse clinical outcomes.10 Further investigation of the impact of this polymorphism on outcomes in patients treated with clopidogrel, the effect in relation to CYP2C19 reduced-function variants, and the influence in those treated with the third generation thienopyridine prasugrel is needed.
Therefore, we genotyped the subset of subjects who provided samples for genetic analysis in the TRITON-TIMI 38 trial to evaluate the relationship between the C3435T ABCB1 polymorphism and adverse cardiovascular outcomes in the setting of treatment with clopidogrel or prasugrel. To assess for supporting pharmacologic data, ABCB1 genotyping was also performed in healthy individuals in whom platelet inhibition and drug levels were measured in response to clopidogrel or prasugrel. We also evaluated the contribution of the C3435T ABCB1 polymorphism in the context of CYP2C19 status to understand the independent contribution of variants in these two genes.
METHODS
Clinical Outcomes
The design and primary results of the TRITON-TIMI 38 trial have been described.6 Patients with ACS with planned PCI were randomly allocated to treatment with clopidogrel (300 mg loading dose followed by 75 mg daily) or prasugrel (60 mg loading dose followed by 10 mg daily) for up to 15 months. This pharmacogenetic analysis was conducted in a TRITON-TIMI 38 genetic substudy that included 2,932 patients who provided both a genetic sample and had ABCB1genotyped (N=1,471 for clopidogrel and N=1,461 for prasugrel). This study was approved by institutional review boards, and informed consent was obtained from all subjects.
The prespecified primary efficacy endpoint was a composite of cardiovascular death, myocardial infarction, or stroke.6 A secondary endpoint was definite or probable stent thrombosis as defined by the Academic Research Consortium (ARC).17 Safety endpoints included non-CABG related TIMI major or minor bleeding. All outcomes were adjudicated by a clinical events committee unaware of treatment assignment.
Pharmacodynamics and Pharmacokinetics in Healthy Subjects
Healthy subjects in 7 studies (N=321) involving treatment with clopidogrel and/or prasugrel were included in the pharmacodynamic and pharmacokinetic analyses (Supplemental Table 1 and Methods).8 These studies were approved by institutional review boards, and informed consent was obtained from all subjects.
The pharmacodynamic response was assessed using light transmission aggregometry in response to 20 μM ADP, and was expressed as absolute reduction in maximal platelet aggregation (ΔMPA) from baseline to four hours. Plasma concentrations of clopidogrel and prasugrel active drug metabolite were measured by liquid chromatography with mass spectrometry.18 The area under the plasma concentration-time curve was analyzed using the log-linear trapezoidal method from time of dose to the four hour measurable concentration (AUC0–4).
Genotyping Methodology
Genotyping for ABCB1 was completed using the Affymetrix Targeted Human DMET 1.0 Assay (Affymetrix, Santa Clara, CA, USA) and Illumina Infinium Beadchip Assay (Illumina, San Diego, CA, USA) to minimize missing data.19 Based on prior studies,10, 16 the main variant of interest was C3435T (rs1045642), and subjects were classified as homozygous for the C allele (CC), heterozygous (CT), or homozygous for the T allele (TT). As some in vitro studies have also assessed a haplotype consisting of C3435T and two other ABCB1 variants, G2677T/A (rs2032582) and C1236T (rs1128503), we also genotyped these polymorphisms (Supplemental Methods).20 Genotypes were in Hardy-Weinberg equilibrium (Supplemental Table 2).
Because genetic variation in CYP2C19 has been associated with the pharmacologic response and cardiovascular outcomes among subjects taking clopidogrel,8–10, 21–24 the combined influence of genetic variants in CYP2C19 and ABCB1 C3435T was evaluated. For the CYP2C19 gene, subjects were genotyped and dichotomized into two groups based on whether or not they possessed at least one significantly reduced-function allele (termed carriers) or if they possessed no reduced-function alleles (termed non-carriers).8
Statistical Analyses
Clinical Outcomes
Based on prior studies, the primary objective was to evaluate the association between ABCB1 C3435T genotypes and rates of the primary efficacy endpoint (cardiovascular death, myocardial infarction, or stroke) in patients in the TRITON-TIMI 38 study. To be consistent with the main trial analyses, the Gehan-Wilcoxon test was used for the primary efficacy endpoint and log-rank for other endpoints. Event rates were expressed as Kaplan-Meier estimates at 15 months.25 Hazard ratios and 95% confidence intervals were calculated based on Cox proportional hazards regression models with clinical syndrome (non-ST-elevation vs. ST-elevation ACS) as a stratification factor. Two-sided P values were calculated to test for differences in cardiovascular event rates between patients stratified by genotype. If a significant association for the primary efficacy evaluation was found among subjects treated with clopidogrel, then additional efficacy endpoints were also tested, including the hazards for the components of the composite primary endpoint, the primary endpoint at 30 days, and stent thrombosis. In terms of safety endpoints, non-CABG related TIMI major or minor bleeding was assessed through 15 months. Parallel analyses were performed among those patients allocated to treatment with prasugrel.
To understand further the contribution of ABCB1 variants, the associations between additional ABCB1 genotypes (G2677T/A, C1236T, and the haplotype that included C1236T, G2677T/A, and C3435T, see the Supplemental Methods) and cardiovascular outcomes were tested in each treatment arm, using the same methodology. We then evaluated C3435T in the context of CYP2C19, for which tests stratified by and Cox proportional hazards regression models adjusted for CYP2C19 status were performed. A meta-analysis that included results from FAST-MI10 was performed by combining HRs for each study using a fixed-effects model with weighting based on inverse variance.
Pharmacodynamics and Pharmacokinetics
The associations between genetic variation and pharmacodynamic and pharmacokinetic parameters were tested using likelihood ratio tests based on linear regression or mixed-effects models. The primary outcomes were platelet inhibition (ΔMPA) and exposure to active drug metabolite [log(AUC0–4)]. The models contained subject as a random effect when repeated measures were present, genotype as the predictor of main interest, and other fixed effects including study, dose, and ethnicity, and for pharmacodynamics, baseline MPA. Other demographic variables, including body weight, age, gender, and smoking, were included as determined to be appropriate for each drug as has been done previously.8 Additional models were also created adjusting for CYP2C19.
Role of the Funding Source
The TRITON-TIMI 38 genetic study was designed and performed in collaboration between the TIMI Study Group and the sponsors, Daiichi Sankyo Co., Ltd and Eli Lilly and Company. The academic authors directed and had access to all the analyses and the full clinical database, wrote all drafts of the manuscript, decided to publish the results, and vouch for the accuracy and completeness of the data.
RESULTS
Patient Population in the Clinical Outcomes Study
For the 2,932 patients analyzed in the TRITON-TIMI 38 trial, the average age was 60.2±10.9 years, 28.3% (831/2,932) were female, 70.4% (2,064/2,932) presented with non-ST-elevation ACS, and 29.6% (868/2,932) presented with ST-elevation myocardial infarction. In total, 27% of the genetic study population were TT homozygotes (n=804), 50% CT heterozygotes (n=1,459), and 23% CC homozygotes (n=669). Baseline characteristics in the TRITON-TIMI 38 trial by C3435T genotype are provided in Supplemental Table 3.
ABCB1 C3435T and Clinical Outcomes in Clopidogrel Treated Subjects
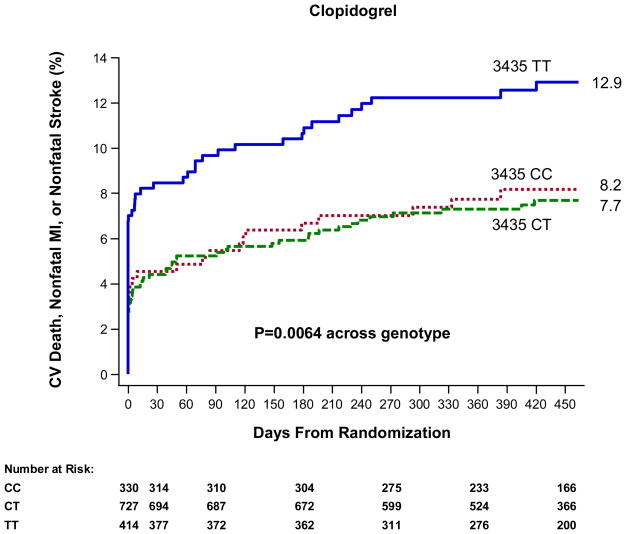
Among patients in TRITON-TIMI 38 genetic study allocated to treatment with clopidogrel (N=1,471), the C3435T genotype was significantly associated with the risk of the primary endpoint of cardiovascular death, myocardial infarction, or stroke (P=0.0064, Figure 1). TT homozygotes were at significantly increased risk as compared with CC individuals (HR 1.69, 95% CI 1.05–2.72); CT heterozygotes were at similar risk as CC individuals (HR 0.94, 95% CI 0.58–1.51). Thus, TT homozygotes for C3435T had a 72% increased risk of the primary endpoint as compared with CT/CC individuals (52/414 [12.9%] vs. 80/1,057 [7.8%], HR 1.72, 95% CI 1.22–2.44, P=0.0020) when evaluated through 15 months. Among 3435 TT vs. CT/CC patients for each of the components of the primary endpoint, the HR for cardiovascular death was 1.63 (5/414 [1.3%] vs. 8/1,057 [0.9%], 95% CI 0.53–4.98, P=0.388), the HR for non-fatal myocardial infarction was 1.82 (48/414 [12.0%] vs. 70/1,057 [6.8%], 95% CI 1.26–2.62, P=0.0013), and the HR for non-fatal stroke was 1.66 (2/414 [0.6%] vs. 3/1,057 [0.3%], 95% CI 0.28–9.93, P=0.575). Moreover, the increased risk was evident by 30 days, by which time 3435 TT homozygotes versus CT/CC individuals experienced an approximately two-fold increase in risk for the primary endpoint (35/414 [8.5%] vs. 47/1,057 [4.5%], HR 1.96, 95% CI 1.26–3.03, P=0.0022).
Figure 1. ABCB1 C3435T and Cardiovascular Outcomes in Patients Treated with Clopidogrel.
The cumulative risk of cardiovascular death, myocardial infarction, or stroke is presented across genotype with a p-value across genotype.
Rates of stent thrombosis did not differ significantly when comparing 3435 TT vs. CT/CC individuals (5/396 [1.3%] vs. 12/1,004 [1.3%], HR 1.07, 95% CI 0.38–3.04, P=0.900). Rates of non-CABG related TIMI major or minor bleeding did not differ statistically by ABCB1 C3435T genotype (TT vs. CT/CC: 15/414 [3.6%] vs. 26/1,052 [2.5%], HR 1.49, 95% CI 0.79–2.82, P=0.214).
Other ABCB1 Variants and Clinical Outcomes in Clopidogrel Treated Subjects
To understand further the contribution of ABCB1 variants, two other ABCB1 polymorphisms were explored: G2677T/A and C1236T. Neither variant was significantly associated with the risk of cardiovascular death, myocardial infarction, or stroke, although there was a non-significant trend for 2677 TT homozygotes to be at increased risk (Supplemental Figure 1, 2, and Results). Haplotypes were constructed using the 3 loci (C1236T, G2677T/A, and C3435T) and did not demonstrate associations beyond what was seen for C3435T alone (Supplemental Results).
ABCB1 C3435T, CYP2C19, and Clinical Outcomes in Clopidogrel Treated Subjects
In a model containing both the ABCB1 C3435T genotype and CYP2C19 carrier status, both variants were significant, independent predictors of cardiovascular death, myocardial infarction, or stroke (ABCB1 3435 TT vs. CT/CC: HR 2.01, 95% CI 1.30–3.11, P=0.0017; CYP2C19 reduced-function allele carrier vs. non-carrier: HR 1.77, 95% CI 1.11–2.80, P=0.0155). When dividing the subjects into four groups based on ABCB1 C3435T genotype and CYP2C19 status (Figure 3), the 53% (773/1,454) of the population who did not carry at-risk genotypes in either gene had a low rate of cardiovascular death, myocardial infarction, or stroke at 15 months (KM event rate 6.3%,48/773). In contrast, the event rates were significantly higher among the 47% (681/1,454) of the population who were either carriers of a CYP2C19 reduced-function allele only (KM event rate 11.5%, 29/268), ABCB1 3435 TT homozygotes only (KM event rate 12.6%, 35/288), or both (KM event rate 13.6%, 17/125) (pooled HR 1.97, 95% CI 1.38–2.82, P=0.0002).
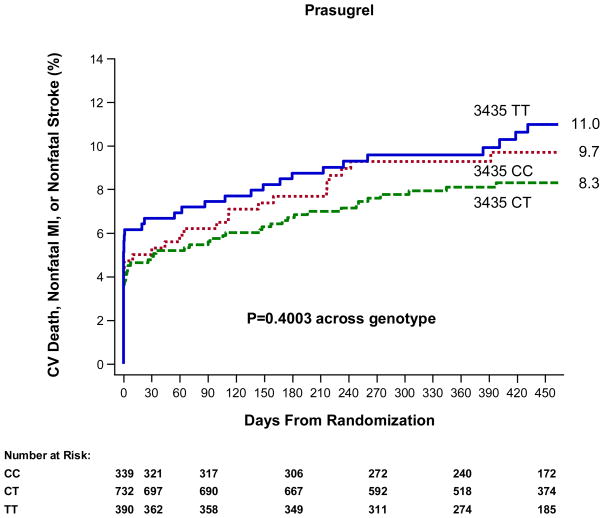
Figure 3. ABCB1 C3435T and Cardiovascular Outcomes in Patients Treated with Prasugrel.
The cumulative risk of cardiovascular death, myocardial infarction, or stroke is presented across genotype with a p-value across genotype.
When examining the early timepoint of 30 days, individuals who did not carry either at-risk variant were at low risk (KM event rate 4.0%, 31/773), individuals who were either ABCB1 3435 TT homozygotes or carriers of a CYP2C19 reduced-function allele were at intermediate risk (KM event rate 7.0%, 20/288 and 6.0%, 16/268, respectively, pooled HR 1.64, 95% CI 1.01–2.65, P=0.0441 vs. carriers of neither) and individuals who were both CYP2C19 reduced-function allele carriers and ABCB1 3435 TT homozygotes were at high risk (KM event rate 12.0%, 15/125, HR 3.16, 95% CI 1.71–5.85, P=0.0003 vs. carriers of neither).
ABCB1 Variants and Clinical Outcomes in Prasugrel Treated Subjects
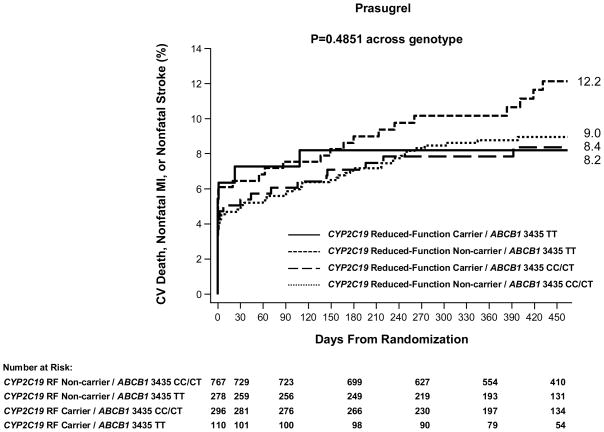
There was not a significant association between the ABCB1 C3435T genotype and risk of cardiovascular death, myocardial infarction, or stroke among patients in TRITON-TIMI 38 genetic study allocated to treatment with prasugrel (N=1,461, Figure 3 and Supplemental Results). Specifically, TT homozygotes did not have a significantly increased risk of the primary efficacy endpoint of CV death, MI, or stroke as compared with CT/CC carriers (41/390 [11.0%] vs. 91/1,071 [8.7%], HR 1.25, 95% CI 0.86–1.81, P=0.235) when evaluated through 15 months. Rates of non-CABG related TIMI major or minor bleeding did not differ statistically by ABCB1 C3435T genotype (Supplemental Results). In terms of other ABCB1 variants, the G2677T/A and C1236T genotypes overall were not significantly associated with the risk of cardiovascular death, myocardial infarction, or stroke among prasugrel treated subjects, although there were non-significant trends for 2677 TT homozygotes vs. CT/CC individuals to be at increased risk (29/253 [11.8%] vs. 94/1,104 [8.8%], HR 1.38, 95% CI 0.91–2.09, P=0.1290, Supplemental Figure 3, 4 and Results). When dividing the subjects based on ABCB1 C3435T genotypes and CYP2C19 status, rates of cardiovascular death, myocardial infarction, or stroke at 15 months were statistically similar among the four groups (P=0.4851, Figure 4).
Figure 4. ABCB1 C3435T, CYP2C19, and Cardiovascular Outcomes in Patients Treated with Prasugrel.
The cumulative risk of cardiovascular death, myocardial infarction, or stroke is presented across the four genotype categories. The P-value across genotype category is presented.
Pharmacodynamic and Pharmacokinetic Findings in Healthy Subjects Treated with Clopidogrel and Prasugrel
Among healthy subjects treated with clopidogrel, ABCB1 3435 TT homozygotes had a diminished pharmacodynamic effect, with a reduction of platelet aggregation in response to a clopidogrel loading dose that was 7.3 absolute percentage points lower (i.e., less platelet inhibition) than that seen in CT/CC individuals (P=0.0127). After adjusting for CYP2C19 genotype, the response was 6.6 absolute percentage points lower (P=0.022). The pharmacodynamic effects of clopidogrel on TT carriers were observed only after a loading dose (for both 300 mg and 600 mg); no significant association was observed during maintenance dosing. There was no significant relationship between C3435T genotype and exposure to clopidogrel active metabolite levels. In prasugrel treated individuals, there were no significant relationships between C3435T genotype and platelet response (1.3 percentage points higher; P=0.4345) or exposure to prasugrel’s active metabolite. There was no relationship between genotype status for either G2677T/A or C1236T and pharmacodynamic outcomes among clopidogrel or prasugrel treated subjects.
DISCUSSION
The pharmacologic and clinical response to clopidogrel varies widely among patients and genetic variants in the CYP2C19 gene have been shown to influence the response. P-gp is another important protein involved in the transport of drugs, and pharmacogenetic interactions with a variety of classes of medications have been suggested.26 In the present analysis, we evaluated the C3435T variant in ABCB1 and found that TT homozygotes (27% of the study population) as compared with CT/CC individuals experienced significantly lesser degrees of platelet inhibition with clopidogrel loading dosing when testing a healthy population and a significantly increased risk of adverse cardiovascular events among ACS subjects treated with clopidogrel. When considering ABCB1 C3435T status in the context of CYP2C19 among subjects treated with clopidogrel, variants in the two genes offered significant, independent information about the risk of cardiovascular death, myocardial infarction, or stroke. Conversely, there were no statistically significant associations between the ABCB1 variants tested and the response to prasugrel.
ABCB1 encodes the P-gp efflux transporter. Clopidogrel is a P-gp substrate and inhibition of P-gp has been shown to influence the bioavailability of clopidogrel.16 The C3435T variant in ABCB1 is among the most studied polymorphisms in the pharmacogenetic literature, and the synonymous C3435T variant has been associated with altered disposition of several drugs.26 Although a genome-wide association study only identified CYP2C19 as being associated with the pharmacodynamic response to clopidogrel, the same study indicated that platelet response to clopidogrel was highly heritable and not entirely explained by CYP2C19, suggesting that additional genetic variants may be relevant. To that end, in a study of patients treated with clopidogrel following elective PCI, 3435 TT homozygotes as compared with CT/CC individuals had significantly lower active clopidogrel metabolite levels, suggesting enhanced intestinal efflux possibly mediated by higher P-gp expression associated with the 3435 TT genotype.16 Although there is variability in the data regarding P-gp expression and activity, mRNA expression levels in duodenal enterocytes have been found to be two to three-fold higher with the ABCB1 3435 TT genotype as compared with either the CC or CT genotype.27–29 In the present analysis, among the healthy subjects, TT homozygotes had an absolute reduction in maximal platelet aggregation after a loading dose of clopidogrel that was 7.3 percentage points lower (i.e., less platelet inhibition) versus CC or CT individuals. Although we did not find a significant relationship between the C3435T genotype and pharmacokinetic data, others have demonstrated this relationship, and the differences in results may be related to subjects, methodologies, and single center vs. multicenter study design.
In terms of clinical outcomes, we found that ABCB1 3435 TT homozygotes experienced a 72% increased risk of adverse cardiovascular events as compared with CT/CC individuals in the setting of treatment with clopidogrel in TRITON-TIMI 38. Likewise, in a prior study, among patients receiving clopidogrel therapy after an acute myocardial infarction, those who were 3435 TT homozygotes had an approximately 70% increase in cardiovascular events during follow-up.10 In that prior study, however, 3435 CT heterozygotes were also at increased risk of adverse cardiovascular events, albeit less so than the TT homozygotes; the differences in the findings may be based on the patient populations. Combining the results of the prior study and ours yielded an apparent graded allele-dose response with a HR for adverse cardiovascular events of 1.29 (95% 0.99–1.69) for 3435 CT vs. CC individuals and a HR of 1.70 (95% CI 1.28–2.26) for 3435 TT vs. CC individuals. In the present analysis, G2677T/A and C1236T did not add additional statistically significant information. Nonetheless, additional basic genetic pharmacology studies may be helpful in further defining the actual functional ABCB1 variants and the most appropriate genetic model with respect to response to clopidogrel.
In the present study, evaluating the contribution of ABCB1 variants in the context of CYP2C19 showed that variants in the two genes offered complementary information about cardiovascular risk. When dividing the subjects into four groups based on ABCB1 C3435T and CYP2C19 status, rates of cardiovascular death, myocardial infarction, or stroke through 15 months were nearly twice as high among the study population who were either carriers of a CYP2C19 reduced-function allele, 3435 TT homozygotes, or both, compared with individuals who did not carry either. Moreover, taking into account both ABCB1 and CYP2C19, nearly half of the population carries a genotype associated with an increased risk for major adverse cardiovascular events while on standard doses of clopidogrel.
With prasugrel, there was no statistically significant association between ABCB1 C3435T polymorphisms and cardiovascular outcomes in TRITON-TIMI 38. Likewise, among the healthy subjects, no relationships between the C3435T variant and pharmacokinetic and pharmacodynamic outcomes were seen with prasugrel. The rapid metabolism of prasugrel may mitigate the genetic influence of ABCB1 C3435T polymorphisms, even though the drug is subject to the P-gp system. There was a trend towards 2677 TT homozygotes having higher rates of adverse cardiovascular events compared with the rest of the population. No association was seen with the pharmacologic data with these two variants. Future studies will assist in further examining these exploratory observations.
There are several limitations to this analysis. First, there was a small number of non-Caucasian individuals in these studies and future investigations in other populations would be useful. Second, due to the sample handling and the need for repeat measurements for the pharmacokinetic and pharmacodynamic assessments, these evaluations were conducted in healthy individuals, not in the acute clinical trial study population. Third, in our clinical outcomes study patients treated with clopidogrel received a 300 mg loading dose and 75 mg daily maintenance dose and patients treated with prasugrel received a 60 mg loading dose and 10 mg daily maintenance dose; we cannot comment on the impact of ABCB1 genetic variants in patients receiving different doses of these medications. Fourth, the number of bleeding and stent thrombosis events was small, and the present analysis had limited power to detect an association between the tested ABCB1 variants and these outcomes. Additional studies that include more bleeding and stent thrombosis events will be particularly important to further understand the relationships between ABCB1 genetic variants and outcomes. Finally, there may be other genetic variants that influence the relationship between treatment with clopidogrel and cardiovascular outcomes.
In conclusion, we found that ABCB1 3435 TT homozygotes were significantly more likely to experience adverse cardiovascular outcomes in the setting of treatment with clopidogrel following an ACS and PCI. Thus, the association between ABCB1 polymorphisms and ischemic risk among patients treated with clopidogrel has been observed now in multiple pharmacologic and clinical outcomes studies. The present analysis also demonstrates that the pharmacogenetic effects of ABCB1 C3435T are independent and complementary to CYP2C19. As clinicians, professional societies, and patients are integrating information about the genetic factors that influence the response to thienopyridines, the role of both ABCB1 and CYP2C19 should be considered.
Supplementary Material
Figure 2. ABCB1 C3435T, CYP2C19, and Cardiovascular Outcomes in Patients Treated with Clopidogrel.
The cumulative risk of cardiovascular death, myocardial infarction, or stroke is presented across the four genotype categories. The P-value across genotype category is presented.
Acknowledgments
Funding Source: Supported by research grants from Daiichi Sankyo and Eli Lilly.
In addition, Dr. Mega reports consulting fees from Sanofi-Aventis, Bristol Myers Squibb, and Astra-Zeneca. Dr. Mega is also supported in part by grant K99/R00 HL098461-01 from the National Institutes of Health. Dr. Wiviott reports consulting fees from Sanofi-Aventis, Bristol Myers Squibb, Astra-Zeneca, ARENA, Medco, and Portola, and lecture fees for CME from Schering Plough, Daiichi-Sankyo, Eli Lilly, Novartis and Astra-Zeneca. Dr. Simon reports research grant support from Servier, Pfizer, Daiichi Sankyo, Eli Lilly, Sanofi-Aventis, AstraZeneca, and Caisse d’Assurance Maladie and consulting fees from Eli Lilly, Daiichi Sankyo, Sanofi-Aventis, and Astra-Zeneca. Dr. Antman has no additional relationships to disclose. Dr. Braunwald reports consulting fees Daiichi Sankyo and lecture fees from Schering Plough, Merck. Dr. Sabatine reports consulting fees from AstraZeneca, Bristol Myers Squibb, Sanofi-Aventis, Daiichi Sankyo, and Eli Lilly and lecture fees from Bristol Myers Squibb, Sanofi-Aventis, and Eli Lilly. Dr. Walker reports being an employee of Daiichi Sankyo and holding equity ownership or stock options therein. Dr. Close reports being a former employee of Eli Lilly and holding equity ownership or stock options therein. Dr. Shen reports being an employee of Eli Lilly and holding equity ownership or stock options therein.
Footnotes
Contributions: Each author declares that they participated in the following activities:
JLM and MSS conceived and designed the research. SDW and EMA acquired the data. JLM, SLC, and MSS analyzed and interpreted the data. LS performed statistical analyses. JLM and MSS drafted the initial manuscript. JRW and EB participated in funding and supervision. JLM, SLC, SDW, JRW, TS, EMA, EB, and MSS made critical revisions to the manuscript for important intellectual content.
Conflicts of Interest: The TIMI Study Group receives research grant support from Daiichi Sankyo, Eli Lilly, Sanofi-Aventis, Bristol Myers Squibb, AstraZeneca, Schering Plough/Merck, Johnson & Johnson, and Bayer Healthcare.
References
- 1.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(22):2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107(23):2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 4.Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, Gick M, Caputo A, Buttner HJ, Neumann FJ. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol. 2006;48(9):1742–1750. doi: 10.1016/j.jacc.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116(25):2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 6.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 7.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51(20):1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 9.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373(9660):309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 10.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 11.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103(6):806–811. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 12.Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, Morath T, Schomig A, Kastrati A, von Beckerath N. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 13.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JL, Mower CP, Horne BD, Muhlestein JB, Park JL, Bair TL, Carlquist JF. Carriage of the CYP2C19*2 allele increases one-year risk of myocardial infarction among recipients of drug-eluting stents treated with clopidogrel. J Am Coll Cardiol. 2009;53(10 Supplement A):A27. [Google Scholar]
- 15.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 16.Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, Kastrati A, Schomig A, Schomig E. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80(5):486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, Van de Werf F, Downey WE, Scirica BM, Murphy SA, Antman EM. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet. 2008;371(9621):1353–1363. doi: 10.1016/S0140-6736(08)60422-5. [DOI] [PubMed] [Google Scholar]
- 18.Farid NA, McIntosh M, Garofolo F, Wong E, Shwajch A, Kennedy M, Young M, Sarkar P, Kawabata K, Takahashi M, Pang H. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(2):169–179. doi: 10.1002/rcm.2813. [DOI] [PubMed] [Google Scholar]
- 19.Dumaual C, Miao X, Daly TM, Bruckner C, Njau R, Fu DJ, Close-Kirkwood S, Bauer N, Watanabe N, Hardenbol P, Hockett RD. Comprehensive assessment of metabolic enzyme and transporter genes using the Affymetrix Targeted Genotyping System. Pharmacogenomics. 2007;8(3):293–305. doi: 10.2217/14622416.8.3.293. [DOI] [PubMed] [Google Scholar]
- 20.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 21.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108(7):2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 22.Fontana P, Hulot JS, De Moerloose P, Gaussem P. Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J Thromb Haemost. 2007;5(10):2153–2155. doi: 10.1111/j.1538-7836.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 23.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5(12):2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 24.Gladding P, Webster M, Zeng I, Farrell H, Stewart J, Ruygrok P, Orminston J, El-Jack S, Armstrong G, Kay P, Scott D, Gunes A, Dahl M. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (plavix response in coronary intervention) trial. J Am Coll Cardiol: Cardiovascular Intervention. 2008;1(6):620–627. doi: 10.1016/j.jcin.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, Winters KJ, Warmke JW, McCabe CH, Braunwald E. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) Am Heart J. 2006;152(4):627–635. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhou SF, Di YM, Chan E, Du YM, Chow VD, Xue CC, Lai X, Wang JC, Li CG, Tian M, Duan W. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab. 2008;9(8):738–784. doi: 10.2174/138920008786049302. [DOI] [PubMed] [Google Scholar]
- 27.Moriya Y, Nakamura T, Horinouchi M, Sakaeda T, Tamura T, Aoyama N, Shirakawa T, Gotoh A, Fujimoto S, Matsuo M, Kasuga M, Okumura K. Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol Pharm Bull. 2002;25(10):1356–1359. doi: 10.1248/bpb.25.1356. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, Matsuo M, Kasuga M, Okumura K. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther. 2002;71(4):297–303. doi: 10.1067/mcp.2002.122055. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.