Abstract
Objectives. We estimated the relationship between mining and tuberculosis (TB) among countries in sub-Saharan Africa.
Methods. We used multivariate regression to estimate the contribution of mining activity to TB incidence, prevalence, and mortality, as well as rates of TB among people living with HIV, with control for economic, health system, and population confounders.
Results. Mining production was associated with higher population TB incidence rates (adjusted b = 0.093; 95% confidence interval [CI] = 0.067, 0.120; with an increase of mining production of 1 SD corresponding to about 33% higher TB incidence or 760 000 more incident cases), after adjustment for economic and population controls. Similar results were observed for TB prevalence and mortality, as well as with alternative measures of mining activity. Independent of HIV, there were significant associations between mining production and TB incidence in countries with high HIV prevalence (≥ 4% antenatal HIV prevalence; HIV-adjusted B = 0.066; 95% CI = 0.050, 0.082) and between log gold mining production and TB incidence in all studied countries (HIV-adjusted B = 0.053; 95% CI = 0.032, 0.073).
Conclusions. Mining is a significant determinant of countrywide variation in TB among sub-Saharan African nations. Comprehensive TB control strategies should explicitly address the role of mining activity and environments in the epidemic.
Tuberculosis (TB) has risen markedly in sub-Saharan African nations over the past 2 decades, with reported annual incidence doubling from 173.6 to 351.7 per 100 000 population between 1990 and 2007.1,2 Even in the wealthiest country on the continent, the Republic of South Africa, TB incidence more than tripled, from 305 per 100 000 in 1993 to 948 per 100 000 in 2007, and TB mortality rates quadrupled over this period.2 Although TB surveillance is poor in this region, the most recent estimates of TB data from the World Health Organization (WHO), which reflect the current TB burden among these countries, indicate that TB incidence declined in only 10 of 46 African countries between 2000 and 2007.2
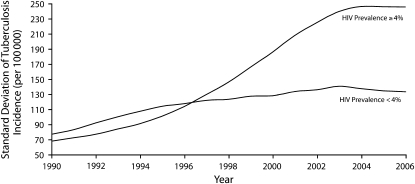
Although the main explanation for rising TB rates has been the growth of HIV,1,3 the persistence of TB in countries with low HIV prevalence suggests that TB transmission is related to other factors as well, such as late diagnosis and incomplete treatment, migration, and low socioeconomic status (itself associated with poor detection and treatment outcomes).1 Furthermore, as shown in Figure 1, the dispersion of TB incidence rates among countries has been widening in both low- and high-HIV-prevalence nations in Africa, which requires explanations that go beyond the known mediating factors.
FIGURE 1.
Trends in tuberculosis incidence among 46 African countries, stratified by antenatal HIV prevalence: 1990–2006.
Note. African countries for which comparative data are presented are as follows: those whose average antenatal HIV prevalence rates between 1990 and 2006 were 4% or higher (Botswana, Burkina Faso, Burundi, Central African Republic, Chad, Democratic Republic of Congo, Republic of Congo, Cote d'Ivoire, Djibouti, Eritrea, Ethiopia, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mozambique, Namibia, Niger, Rwanda, Sao Tome and Principe, Seychelles, Somalia, South Africa, Swaziland, Uganda, Zimbabwe), and those whose average antenatal HIV prevalence rates between 1990 and 2006 were below 4% (Angola, Benin, Cameroon, Cape Verde, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Mauritius, Nigeria, Senegal, Sierra Leone, Tanzania, Togo, Zambia).
Source. Data are from the World Health Organization Global Tuberculosis Database, 2009 edition.2 HIV prevalence data are based on Joint United Nations Programme on HIV/AIDS Estimation and Projections Package3 logistic estimates of antenatal HIV prevalence rates.
One group at exceptionally high risk of TB is mineral miners.4–7 Many mineworkers are migrants, which may expose them to multiple TB risk factors, including HIV, health care disruptions (despite the excellent tertiary health care available to the miners compared with the rest of the population), and low socioeconomic status.8 Their migration may also facilitate the transmission of TB to the general community.9–12 Miners in sub-Saharan Africa have greater incidence of TB than do any other working population in the world (reported at 3000–7000 per 100 000 miners per year in some areas),7 and constitute one of the largest pools of employed men in sub-Saharan Africa.13 The TB incidence among miners is estimated to be as much as ten times higher than in the populations from which they originate.7
Working conditions inside mines create a high-risk environment for TB transmission, resulting in part from silica dust exposure (which increases the risk of pulmonary TB, particularly in gold mines),5,14,15 as well as confined and poorly ventilated environments conducive to transmission.16 Outside the mines, circular migration continues to be the norm.11,17,18 Sex work remains common around all-male hostels at the mines, increasing the risk of HIV transmission,19 which in turn increases the risk of active TB.20 At 1 mining town near Johannesburg, 52% of migrant women were sex workers and more than two thirds of these workers were HIV positive.21 Recent estimates indicate that miners are 3 to 4 times more likely to be infected with HIV than are nonminers; partners of migrant miners have also been found to have significantly greater prevalence of HIV than prevalence among the general populations.12
Migration can also disrupt TB detection and care. Miners often have multiple treatment episodes with inappropriate therapy and high default rates, which can lead to the acquisition of drug-resistant TB.22–24 In Lesotho, most TB patients and 25% of drug-resistant TB patients have worked as miners in South Africa.9,25 Although miners at large sites in some countries have access to extensive tertiary-care health facilities during their periods of employment,26 primary-care facilities are often sparse near mines and in the rural areas to which miners return after seasonal work, and many care facilities are inaccessible to contract workers, who sometimes constitute the majority of the mining population. In some countries, mining companies report regular TB screening,27 but independent verification is lacking and there is some evidence that TB is undetected. One study of miners that was based on autopsies revealed that a significant fraction had active disease undetected during their lives.28
At least a half million migrant workers have been employed in the African mining industry at any one time during the last 2 decades.11 In large mining countries like South Africa, over 40% of these workers have been foreign,18 which can lead to transmission of TB from miners to others in their home populations. Thus, although there are a number of mechanisms by which mining could be related to TB, to date no study has assessed whether TB incidence rates are affected by mining by calculating the population-attributable TB risk of mining.
We tested the hypothesis that a higher rate of mining activity at the country level is associated with greater TB incidence in the population. We built on anecdotal reports of TB and multidrug-resistant TB in miners who returned home to rural areas9,25 and asked whether the evidence linking mining to increased HIV and associated TB in individuals can result in population-level effects on overall TB rates. We calculated the population risk of TB attributable to mining to evaluate whether and to what extent differences in mining production can account for the differences in TB burdens among sub-Saharan African countries, after we controlled for other important mediating variables such as HIV and income.
METHODS
We used ecological data on mining and TB for 44 of 46 countries in the WHO's African region (comparable data on mining were unavailable for Algeria and the Comoros). TB incidence, prevalence, and mortality data were drawn from the WHO Global Tuberculosis Database 2009 Edition.2 We took HIV/AIDS prevalence data from the Joint United Nations Programme on HIV/AIDS Estimation and Projections Package29 using antenatal HIV prevalence data from the 2008 United States Agency for International Development HIV/AIDS Surveillance Database.30 Demographic and economic data used to adjust for potential confounders are from the World Bank World Development Indicators 2008 Edition.31
We evaluated the population's exposure to the mining sector in several ways. First, as our principal measures, we used the latest available cross-national comparative data on overall mining production and mining production per capita from the (most recent) British and US Geological Surveys 2009 edition,32,33 which covered extraction of 62 minerals from 2001 to 2005. Second, we took estimates of the mining sector's size, based on number of mines in each country, from the Mbendi Global Mining Database 2009 edition,34 in which mines were most recently registered in 2005. Third, we tested the effects of specific minerals, such as gold, that have the strongest risks of silicosis. Thus, we included measures of mining sector activity, size, and type in the analysis.
We considered the 3 possible ways HIV could affect the association between mining and TB: as a confounder, mediating variable, or effect modifier. First, although in theory HIV could be a confounder of the relationship between mining and TB by being independently associated with both variables, because mining is determined fundamentally by the presence of mineral deposits, it is implausible that HIV could generate an increase in mining production; in contrast, there is extensive evidence that mining increases the risk of HIV infection.11,12,35 A second possibility is that HIV mediates the relationship between mining and TB. If this were the case, then HIV should be held constant as an independent variable in the regression models. To test this possibility, we sequentially included HIV prevalence rates as a control variable in the statistical model. Third, high rates of HIV could increase the mining–TB association. To evaluate potential effect modification, we stratified the countries into high–HIV-prevalence and low–HIV-prevalence groups, based on the WHO Stop TB Partnership's widely used cutpoint of 4% HIV prevalence rate in antenatal clinics. To assess whether HIV acts as a mediating variable, we used heterogeneity of effect testing to identify whether the mining–TB associations differed significantly in high- and low-HIV regions.
To hold constant other potential confounders, such as a country's wealth (a population-level control for socioeconomic status), export dependence, urbanization rates, and population size, we used multivariate regression analysis. We normalized positively skewed variables using a natural log transformation. We thus evaluated the population-level relationship between mining and TB using the basic regression model of log TB incidence rates, estimated using pooled ordinary least squares, a standard approach in cross-national analysis,36 as follows:
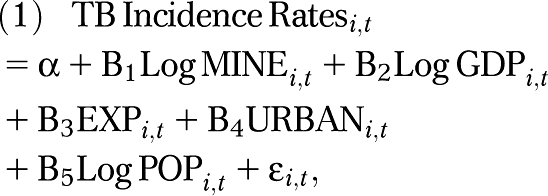 |
where i is country, t is year, MINE is 1 of the several measures of mining sector production, size, or type, GDP is the natural logarithm of the country's gross domestic product per capita in constant US dollars, EXP is exports as a fraction of GDP, URBAN is the percentage of the population living in urban settings, and POP is the logarithm of the population size. Unstandardized parameter estimates (b) and 95% confidence intervals (CIs) are presented from regression models estimated with STATA version 10.1 (StataCorp LP, College Station, TX).
RESULTS
Table 1 shows the results of our basic regression model. Mining production was significantly associated with higher TB incidence rates among the studied countries (unadjusted B = 0.068; 95% CI = 0.050, 0.086), so that each 10% increase in mining production was associated with about 0.7% higher TB incidence. After correction for differences in log gross domestic product per capita, urbanization rates, log population size, and exports as a fraction of GDP, the estimated relationship grew, with each 10% increase in mining now associated with a 0.9% higher rate of TB incidence (adjusted B = 0.093; 95% CI = 0.067, 0.120). To put this effect in perspective, an increase in mining production of 1 standard deviation from the sub-Saharan African mean corresponds to about 33% greater TB incidence. Given that there were about 2.3 million incident cases in the countries studied in 2005, the estimated higher population risk corresponds to 760 000 more incident TB cases. The size of the mining sector, measured as the number of mines operating in 2005, was also significantly associated with higher TB incidence (B = 0.197; 95% CI = 0.080, 0.314; Table 1). Similar significant associations of mining production were observed with TB prevalence and mortality rates (see Appendix 1, available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 1.
Associations Between Mining and Tuberculosis Incidence Rates: Sub-Saharan Africa, 2001–2005
| Mining Production, 2001–2005 |
No. of Mines, 2005 |
|||||
| Covariate | Unadjusted Model | All Countries | Mining Countries | Unadjusted Model | All Countries | Mining Countries |
| Mining production, B (SE) | 0.068*** (0.009) | 0.093*** (0.013) | 0.123*** (0.019) | … | … | … |
| No. of mines, B (SE) | … | … | … | 0.197** (0.058) | 0.446*** (0.115) | 0.406** (0.133) |
| GDP per capita, B (SE) | … | −0.074 (0.065) | −0.050 (0.068) | … | −0.180 (0.147) | −0.179 (0.165) |
| Exports as % of GDP, B (SE) | … | 0.007* (0.003) | 0.005 (0.003) | … | 0.003 (0.007) | 0.007 (0.008) |
| Urbanization rates, B (SE) | … | −0.017*** (0.004) | −0.019*** (0.004) | … | −0.017 (0.009) | −0.019* (0.009) |
| Population size, B (SE) | … | 0.011 (0.049) | −0.049 (0.050) | … | −0.226 (0.149) | −0.272 (0.160) |
| No. of countriesa | 44 | 36 | 33 | 44 | 34 | 31 |
| R2 | 0.20 | 0.34 | 0.27 | 0.22 | 0.44 | 0.32 |
Note. GDP = gross domestic product. Tuberculosis incidence, mining production, number of mines, GDP per capita, and population size are in log form to adjust for positive skew. Ellipses indicate not applicable.
Source. Tuberculosis incidence data are from the World Health Organization Global Tuberculosis Database 2009 edition.2 Mining data are from the British Geological Survey Map 2009 edition.32
Number of country-years in models using mining production is 220 country-years for unadjusted, 175 country-years for adjusted, and 158 country-years for mining countries.
*P < .05; **P < .01; ***P < .001.
Before proceeding, we performed a series of robustness checks to our sample and specification. First, we replicated our analysis using only countries with mines, removing from the data set countries such as Somalia and Djibouti that lacked mines (which were coded as zero in the logged variables). The estimated dose–response relationships between greater mining production and TB incidence rates were unchanged (Table 1). Second, we assessed mining production per capita, replicating all of the results reported and finding a stronger relationship between mining production and TB incidence (adjusted models, B = 0.104; 95% CI = 0.065, 0.144). Third, when we removed outliers (based on standardized residuals greater than 2 or less than −2), the mining–TB association was slightly strengthened. We also replicated our analysis after removing high labor-exporting countries, such as Swaziland and Lesotho (where many miners are exposed to risks from mines in neighboring South Africa), from the sample. Fourth, we adjusted for other potential confounders, such as Directly Observed Treatment, Short-Course (DOTS) surveillance and treatment infrastructure, which is the percentage of clinics in a country that use DOTS to treat TB as tracked by the WHO1,2; we also corrected for economic factors, such as foreign direct investment, inflation, and government spending, and found that our results were robust (see Appendix 1, available as an online supplement).
Testing HIV as a Mediating Variable
If the relationship between mining and TB is mediated by HIV, as hypothesized, we would expect that holding antenatal HIV prevalence rates constant would attenuate the observed relationship between mining activity and TB at the population level. As shown in Table 2, adjusting for HIV prevalence reduced the estimated association between mining production and TB incidence rates by roughly three quarters to nonsignificance at α = .05 (B = 0.018; 95% CI = −0.006, 0.043). The magnitude of the association of HIV prevalence rates with TB incidence rates, B = 0.047—interpreted as a semielasticity as each 1% higher HIV prevalence rate is associated with approximately 4% to 5% higher population TB incidence—was similar to the effect sizes observed at population level among countries of central and eastern Europe and the former Soviet Union.37
TABLE 2.
HIV-Adjusted Association of Mining With Tuberculosis Incidence Rates: Sub-Saharan Africa, 2001–2005
| All Countries |
HIV Prevalence < 4% |
HIV Prevalence ≥ 4% |
||||
| Covariates | Mining Production, 2001–2005 | No. of Mines, 2005 | Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model |
| Mining production, B (SE) | 0.018 (0.012) | … | 0.050a* (0.022) | 0.036 (0.020) | 0.126a*** (0.010) | 0.066*** (0.008) |
| No. of mines, B (SE) | … | 0.172 (0.126) | … | … | … | … |
| GDP per capita, B (SE) | −0.122* (0.051) | −0.175 (0.127) | −0.828*** (0.111) | −0.755*** (0.101) | 0.104* (0.047) | 0.117*** (0.027) |
| Exports as % of GDP, B (SE) | 0.003 (0.002) | 0.003 (0.006) | 0.010 (0.005) | 0.006 (0.005) | 0.005* (0.002) | 0.002 (0.001) |
| Urbanization rates, B (SE) | −0.001 (0.003) | −0.004 (0.008) | 0.028** (0.010) | 0.035*** (0.009) | −0.023*** (0.003) | −0.016*** (0.002) |
| Population size, B (SE) | 0.054 (0.039) | −0.062 (0.133) | −0.042 (0.095) | −0.099 (0.086) | 0.004 (0.034) | −0.057** (0.021) |
| Antenatal HIV prevalence, B (SE) | 0.047*** (0.005) | 0.041** (0.012) | … | 0.222** (0.065) | … | 0.013*** (0.003) |
| No. of countries | 34 | 32 | 11 | 10 | 28 | 26 |
| R2 | 0.53 | 0.52 | 0.71 | 0.78 | 0.62 | 0.72 |
Note. GDP = gross domestic product. Tuberculosis incidence, mining production, number of mines, GDP per capita, and population size are in log form to adjust for positive skew.
Source. Tuberculosis incidence data are from the World Health Organization Global Tuberculosis Database 2009 edition.2 Mining production data are from the British Geological Survey map 2009 edition.32
Test for heterogeneity of effect across models using seemingly unrelated estimation: χ21 = 11.27; P < .001.
*P < .05; **P < .01; ***P < .001.
Testing HIV as an Effect Modifier
We also tested the potential role of HIV as an effect modifier in the mining–TB relationship by stratifying the sample into high– and low–HIV-prevalence countries on the basis of the WHO Stop TB 4% antenatal prevalence threshold. Table 2 shows that in countries where antenatal HIV prevalence was less than 4%, the coefficient describing the association of log mining production with log TB was 0.050 (95% CI = 0.004, 0.095). In countries with antenatal HIV prevalence over 4%, this coefficient was 0.126 (95% CI = 0.106, 0.147). Tests for effect homogeneity indicated that these differences in the coefficients between the 2 groups were statistically significant (χ21 = 11.27; P < .001), providing evidence supporting the possibility that HIV mediates the mining–TB association.
Gold Mining Compared With Other Mining
We also compared gold mining, which is believed to expose miners to the highest silica dust exposure of any mineral,14,15,35 with other forms of mining that involve less silica exposure. Using a dummy variable for whether a country mined for gold or not, we observed that countries that mined for gold had, on average, 32.4% higher TB incidence rates (B = 0.324; 95% CI = 0.129, 0.519) than did non–gold-mining countries. After adjusting for the effects of income per capita, urbanization rates, population size, exports, and antenatal HIV prevalence rates, we found that mining for gold was associated with significantly higher log TB incidence rates (B = 0.485; 95% CI = 0.222, 0.747) than was not mining for gold. This finding was robust even after we corrected for differences in HIV prevalence. Although this effect size, roughly corresponding to 48.5% higher TB incidence rates, may seem large, it accounts for about one quarter of the variance in TB incidence rates among African countries. For example, in 2003, TB incidence rates were 394.1 per 100 000 in countries with an antenatal HIV prevalence of at least 4%, more than double the TB incidence rates of 185.1 per 100 000 recorded in countries with HIV antenatal prevalence of less than 4%.
When we further evaluated this relationship using the log of gold mining production, we also found a persistent significant association between gold mining and TB incidence (B = 0.053; 95% CI = 0.032, 0.073) even after holding HIV prevalence rates constant. This suggests that gold mining is associated with a specific increase in risk of TB independent of HIV, which may plausibly be a result of the increased risk of silicosis. One possible confounder is the military and ethnic conflicts associated with gold (although this has tended to be more associated with diamonds). As a robustness check, we included a measure of exposure to war (based on the sum of years exposed to military or ethnic conflict prior to 2000), and found the results unchanged.
Explaining Variations in HIV–Tuberculosis Coinfection Rates
We further evaluated whether mining was related to HIV–TB coinfection rates (the rate of incident TB infections among persons living with HIV, per 100 000 population). We again split countries into high and low prevalence of antenatal HIV. As shown in Table 3, mining was not significantly associated with HIV–TB coincidence in low–HIV-prevalence countries (B = −0.009; 95% CI = −0.058, 0.041), but mining was significantly associated with increased HIV–TB coincidence (B = 0.061; 95% CI = 0.021, 0.100) in high–HIV-prevalence countries.
TABLE 3.
Association of Mining With HIV–Tuberculosis Coinfection Rates, by HIV Prevalence: Sub-Saharan Africa, 2001–2005
| Covariates | HIV Prevalence < 4% (n = 10), B (95% CI) | HIV Prevalence ≥ 4% (n = 26), B (95% CI) |
| Mining production | −0.009 (0.024) | 0.061** (0.020) |
| GDP per capita | −1.286*** (0.122) | 0.546*** (0.067) |
| Exports as % of GDP | 0.014* (0.007) | −0.008* (0.003) |
| Urbanization rates | 0.025 (0.014) | −0.029*** (0.005) |
| Population size | 0.208 (0.113) | −0.069 (0.051) |
| Antenatal HIV prevalence | 0.513*** (0.079) | 0.031*** (0.008) |
| R2 | 0.91 | 0.64 |
Note. GDP = gross domestic product. Coinfection rates are rates of tuberculosis among people living with HIV, per 100 000 population. HIV–tuberculosis coinfection rates, mining production, GDP per capita, and population size are in log form to adjust for positive skew.
Source. Tuberculosis incidence data are from the World Health Organization Global Tuberculosis Database 2009 edition. Mining production data are from the British Geological Survey Map 2009 edition.
*P < .05; **P < .01; ***P < .001.
Other Robustness Checks
We performed a series of additional analyses to assess the mining–TB relationship and check its robustness to reduce the likelihood that the association was detected spuriously. These robustness checks included (1) evaluating the relationships between TB and specific minerals, such as gold and diamond production; (2) using a quasi-natural experiment to compare trends in HIV prevalence and TB incidence, prevalence, and mortality rates in west African countries where mining production dropped between 2001 and 2005 with those where mining production increased; (3) assessing variations in male–female standardized TB mortality rates (because men would be disproportionately affected directly by effects of mining on TB); and (4) adding controls for DOTS infrastructure and treatment. All results (which are shown in Appendices 1 through 4, available as supplements to the online version of this article at http://www.ajph.org) were consistent with our basic findings of a significant and robust association between mining and TB. Furthermore, the results were consistent with considerable microlevel evidence of the effects of mining on TB, as well as qualitative evidence from quasi-natural experiments (such as when mining production decreased because of external shocks). For example, we observed that neighboring countries with similar levels of GDP (“matching” countries) experienced differences in mining activity over the last few years and experienced corresponding changes in their TB incidence, prevalence, and mortality rates in parallel to their mining production levels (Appendix 2, available as an online supplement). The findings are also consistent with the observation that men of working age in post-apartheid South Africa have considerably higher age-specific mortality rates than do women (Appendix 3, available as an online supplement).
DISCUSSION
We found consistent evidence that differences in the population's exposure to mining sector production, numbers of mines, and type of mining can significantly explain cross-national variations in TB incidence, prevalence, and mortality rates as well as of HIV–TB coinfection rates in sub-Saharan Africa. Most of the observed association of greater population mining with higher population TB incidence appears to be mediated by HIV; however, not just migration and mining environments but also occupational hazards associated with mining appear to account for the observed patterns. Exposure to mining was significantly associated with higher population TB incidence, and we found that this association was independent of HIV prevalence among high–HIV-burden countries. Furthermore, the association between gold mining in particular and TB incidence was independent of HIV, possibly because of the independent risk of silicosis in gold mines as well as the confined environment in gold mines that is thought to be conducive to TB transmission.
As with any statistical analysis, this study has several important limitations. First, we could not directly estimate individuals’ exposure to mining, or individually catalog their rates of TB disease and secondary transmissions to others. We instead indirectly estimated the impact of mining by using multiple variables, from production rates to the number of mines in a country, to measure the exposure to mining conditions among miners. Second, as with any ecological study, there is a potential for ecological fallacies; that is, population-level associations do not correspond to individual-level mechanisms. However, the observed associations are consistent with a substantial body of empirical individual-level research, and were robust to numerous alternative data sources and types. Third, there is a risk that the observed associations could be spurious, resulting from confounding or outlier effects. The former can never be excluded completely, but our findings remained robust following numerous statistical checks of our models (Appendices 1–4, available as online supplements). Fourth, the available data on domestic mining production underestimate the population's overall exposure to mining-related TB risks in countries that have small domestic mining sectors but many mineworkers working abroad, like Swaziland or Lesotho. This bias would run counter to our hypothesis, rendering our methodology conservative in detecting a significant association between exposure to mining and higher rates of TB. Future studies should also assess the potential heterogeneity in the TB risks associated with mining on the basis of the size and type of mines, and should also examine the associations of mining with TB among HIV-negative populations.
Our estimates of the relationship between mining and TB outcomes are likely to be understated for at least 3 reasons. First, our analysis does not fully account for the role of foreign labor. Countries that were high exporters of labor, such as Swaziland, Lesotho, and Zambia, were above the regression line; that is, their TB incidence was much higher than would be expected on the basis of the number of mines or the level of mining production in the country, making it more difficult to detect a relationship between mining and TB. When we removed outliers, including countries that are high exporters of labor, we found that the mining–TB relationship was increased. Second, those countries that did not develop extensive migration-based systems of labor for the mines, such as Ghana (where communities developed alongside the mines rather than in all-male barracks), tended to fall below the regression line, meaning that TB incidence was lower than would be expected given the amount of mining production (or activity). When these countries were removed from the analysis, the strength of the mining–TB relationship also increased, consistent with our hypothesis that mining affects TB through increased HIV risk (most likely through reduced sexual partnership stability in the context of migration).10,19 Third, TB disease estimates are imprecise, hence we used multiple estimates of TB burden (incidence, prevalence, and mortality); however, such measurement error would be nondifferential, making it harder to identify an effect should one exist (with similar measurement issues for mining production estimates further attenuating the associations).
The magnitude of our findings is consistent with the notion that mining not only increases the hazards of TB among directly exposed individual miners but significantly increases the risks of TB spread to the general community in sub-Saharan African countries. Despite the importance to the economy of extractive industries in several countries, miners constitute less than 1% of the entire population, even in South Africa. Although the incidence of TB among miners is typically estimated to be 5 to 6 times higher than is in the general population, they still account directly for a small fraction of the population-attributable risk of TB from mining. Hence, mines appear to be generating a disproportionate number of secondary infections in the wider population, similar to the role played by prisons in the former Soviet Union.37
Our findings suggest that mining profoundly affects not only the health of miners but also the dynamics of TB incidence in sub-Saharan African nations. The implication for policy is not to close mines but to reduce levels of risk to those observed in Western mines, usually owned by the same companies operating in sub-Saharan Africa. This will involve more extensive collaboration among governments, health and labor ministries, and mining companies. Health care programs for miners should emphasize continuity of care as miners travel across borders (e.g., through patient-held charts listing previous medication regimens, clinical findings, and drug sensitivities), facilitating earlier diagnosis (e.g., enforcing standards of screening at minefields), and improving working conditions to reduce the risk of incident infection (e.g., reducing poor working conditions, cramped hostel living quarters, or exposure to silica dust).8 As shown by the population risk of mining, improved public health and health care conditions for miners may be necessary not only for the miners themselves, but for controlling TB more generally among sub-Saharan African populations.
Human Participant Protection
No protocol approval was needed for this study.
References
- 1.Global Tuberculosis Control—Epidemiology, Strategy, Financing. Geneva, Switzerland: World Health Organization; 2009 [Google Scholar]
- 2.World Health Organization Global tuberculosis database. 2009. Available at: http://www.who.int/tb/country/global_tb_database/en/index.html?language. Accessed March 29, 2010
- 3.Global Tuberculosis Control—Surveillance, Planning, Financing. Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 4.Packard R. White Plague, Black Labor: Tuberculosis and the Political Economy of Health and Disease in South Africa. Berkeley: University of California Press; 1987 [Google Scholar]
- 5.Rees D, Murray J. Silica, silicosis and tuberculosis. Int J Tuberc Lung Dis. 2007;11(5):474–484 [PubMed] [Google Scholar]
- 6.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191(2):150–158 [DOI] [PubMed] [Google Scholar]
- 7.Tuberculosis Strategic Plan for South Africa, 2007–2011. Pretoria, South Africa: Ministry of Health; 2007 [Google Scholar]
- 8.Basu S, Stuckler D, Gonsalves G, Lurie M. The production of consumption: addressing the impact of mineral mining on tuberculosis in southern Africa. Global Health. 2009;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Mining Sector, Tuberculosis and Migrant Labour in Southern Africa. Johannesburg: Aids and Rights Alliance South Africa (ARASA); 2008 [Google Scholar]
- 10.Lurie M, Williams B, Zuma K, et al. Who infects whom? HIV concordance and discordance among migrant and non-migrant couples in South Africa. AIDS. 2003;17(15):2245–2252 [DOI] [PubMed] [Google Scholar]
- 11.Crush J, Williams B, Gouws E, Lurie M. Migration and HIV/AIDS in South Africa. Dev South Afr. 2005;22(3):293–317 [Google Scholar]
- 12.Lurie M, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis. 2003;30(2):149–156 [DOI] [PubMed] [Google Scholar]
- 13.2008 World Factbook. Washington, DC: US Central Intelligence Agency; 2008 [Google Scholar]
- 14.Hnizdo E, Murray J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup Environ Med. 1998;55(7):496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchyard G, Kleinschmidt I, Corbett EL, Murray J, Smit J, De Cock KM. Factors associated with an increased case-fatality rate in HIV-infected and non-infected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(8):705–712 [PubMed] [Google Scholar]
- 16.Leon R, Davies A, Salomon M, Davies J. Commission of Enquiry Into Safety and Health in the Mining Industry. Pretoria, South Africa: Government Printers; 1995 [Google Scholar]
- 17.Crush JD, Dodson B. Another lost decade: the failures of South Africa's post-apartheid migration policy. Tijdschr Econ Soc Geogr. 2007;98(4):436–454 [Google Scholar]
- 18.Crush J, Jeeves A, Yudelman D. South Africa's Labor Empire: A History of Black Migrancy to the Gold Mines. Cape Town, South Africa: Westview and David Philip; 1991 [Google Scholar]
- 19.Campbell C. Migrancy, masculine identities and AIDS: the psychosocial context of HIV transmission on the South African gold mines. Soc Sci Med. 1997;45(2):273–281 [DOI] [PubMed] [Google Scholar]
- 20.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340(5):367–373 [DOI] [PubMed] [Google Scholar]
- 21.Auvert B. HSV-2 is a major risk factor for HIV infection among young women in Carletonville (South Africa). Paper presented at: XIII International AIDS Conference; July 9–14, 2000; Durban, South Africa. Available at: http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102242597.html. Accessed March 29, 2010
- 22.Sonnenberg P, Ross MH, Shearer SC, Murray J. The effect of dosage cards on compliance with directly observed tuberculosis therapy in hospitals. Int J Tuberc Lung Dis. 1998;2(2):168–171 [PubMed] [Google Scholar]
- 23.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–1693 [DOI] [PubMed] [Google Scholar]
- 24.Mqoqi N, Churchyard GA, Kleinschmidt I, Williams B. Attendance versus compliance with tuberculosis treatment in an occupational setting—a pilot study. S Afr Med J. 1997;87(11):1517–1521 [PubMed] [Google Scholar]
- 25.Murray J, Sonnenberg P, Shearer S, Godfrey-Fausset P. Drug-resistant pulmonary tuberculosis in a cohort of southern African goldminers with high prevalence of HIV infection. S Afr Med J. 2000;90(4):381–386 [PubMed] [Google Scholar]
- 26.Fourie I. Healthcare in the mining industry. : Williams B, Campbell C, HIV/AIDS Management in South Africa: Priorities for the Mining Industry. Johannesburg, South Africa: Epidemiology Research Unit; 1999:45–48 [Google Scholar]
- 27.AngloGold Ashanti Report to Society 2003. Johannesburg, South Africa: PricewaterhouseCoopers and AngloGold; 2003. Available at: http://www.anglogold.co.za/subwebs/InformationForInvestors/ReportToSociety03/index.htm. Accessed March 29, 2010 [Google Scholar]
- 28.Lowe R, Murray J. Tuberculosis at Autopsy in Black Mine Workers. Johannesburg, South Africa: National Center for Occupation Health; 1994 [Google Scholar]
- 29.Ghys P, Brown T, Grassly NC, et al. The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sex Transm Infect. 2004;80:i5–i9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HIV/AIDS Surveillance Database. Washington, DC: US Census Bureau; 2010 [Google Scholar]
- 31.World Development Indicators 2008 Edition. Washington DC: World Bank; 2008 [Google Scholar]
- 32.British Geological Survey. 2009. Available at: http://www.bgs.ac.uk/downloads/start.cfm?id=1574. Accessed March 29, 2010
- 33.US Geological Survey. 2009. Available at: http://www.bgs.ac.uk/mineralsuk/statistics/worldStatistics.html. Accessed March 29, 2010
- 34.Mbendi Global Mining Database 2009. Available at: http://www.mbendi.com/indy/ming/af/p0005.htm#10. Accessed March 29, 2010
- 35.Williams B, Campbell C, Mqoqi N, Kleinschmidt I. Occupational health, occupational illness: tuberculosis, silicosis and HIV on the South African Mines. : Banks D, Parker JE, Occupational Lung Disease: An International Perspective. London, England: Chapman & Hall; 1998:95–103 [Google Scholar]
- 36.Jones A. Health econometrics. : Cuyler A, Newhouse JP, Handbook of Health Economics. New York, NY: Elsevier Science; 2000:265–344 [Google Scholar]
- 37.Stuckler D, Basu S, McKee M, King L. Mass incarceration can explain population increases in TB and multi-drug resistant TB in European and central Asian countries. Proc Natl Acad Sci USA. 2008;105(36):13280–13285 [DOI] [PMC free article] [PubMed] [Google Scholar]