Abstract
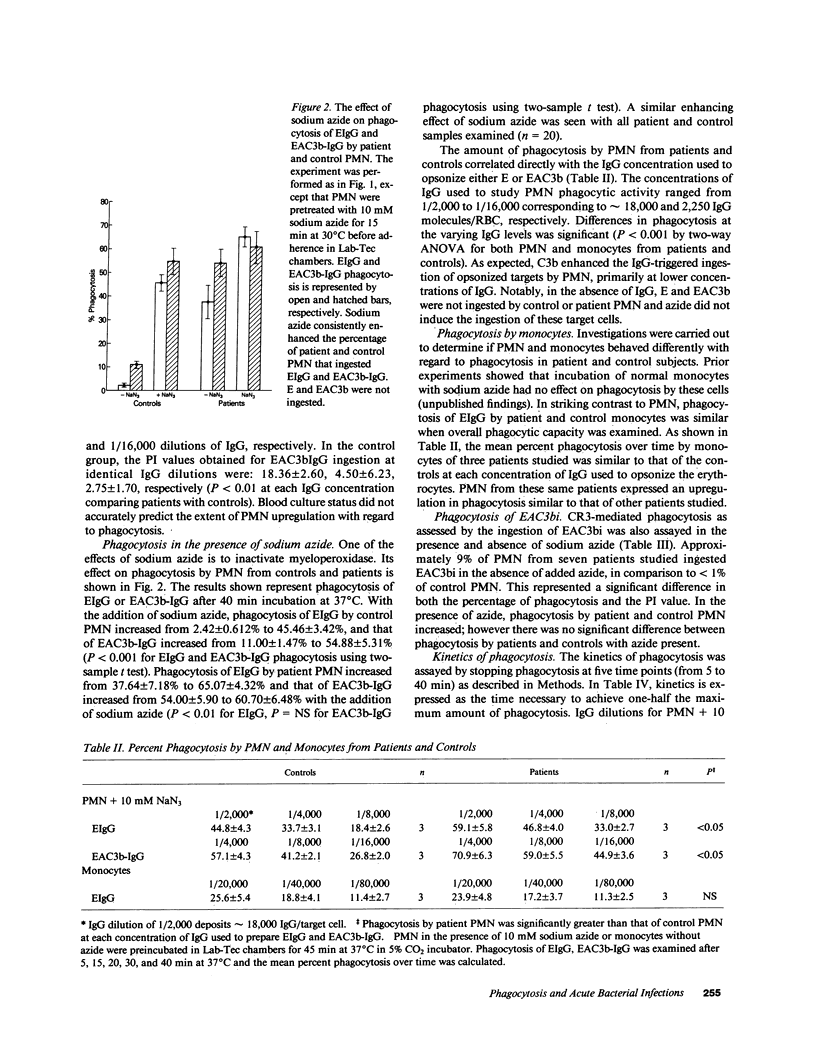
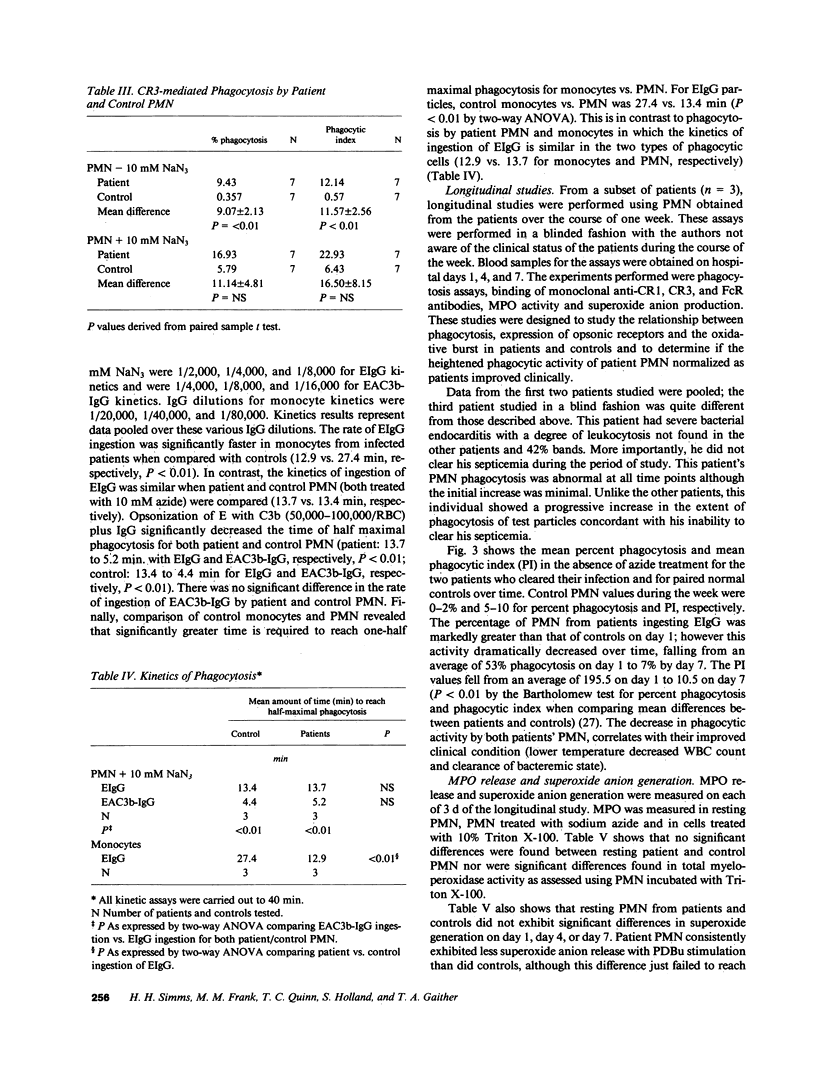
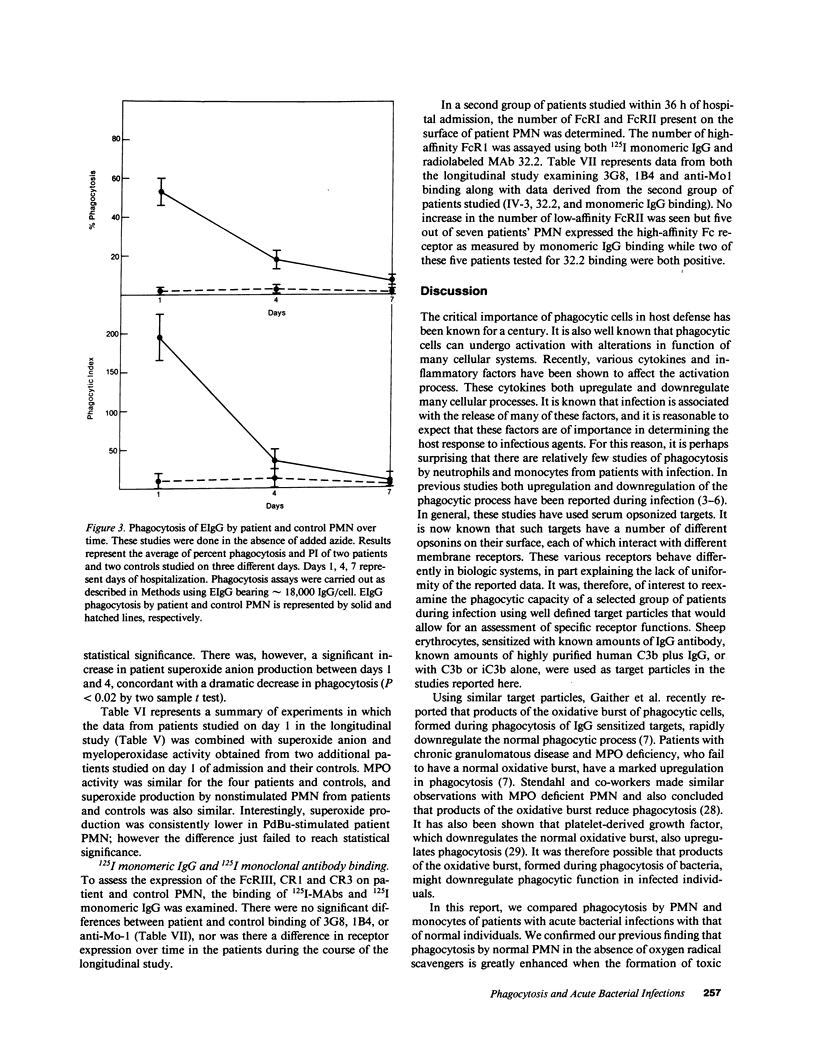
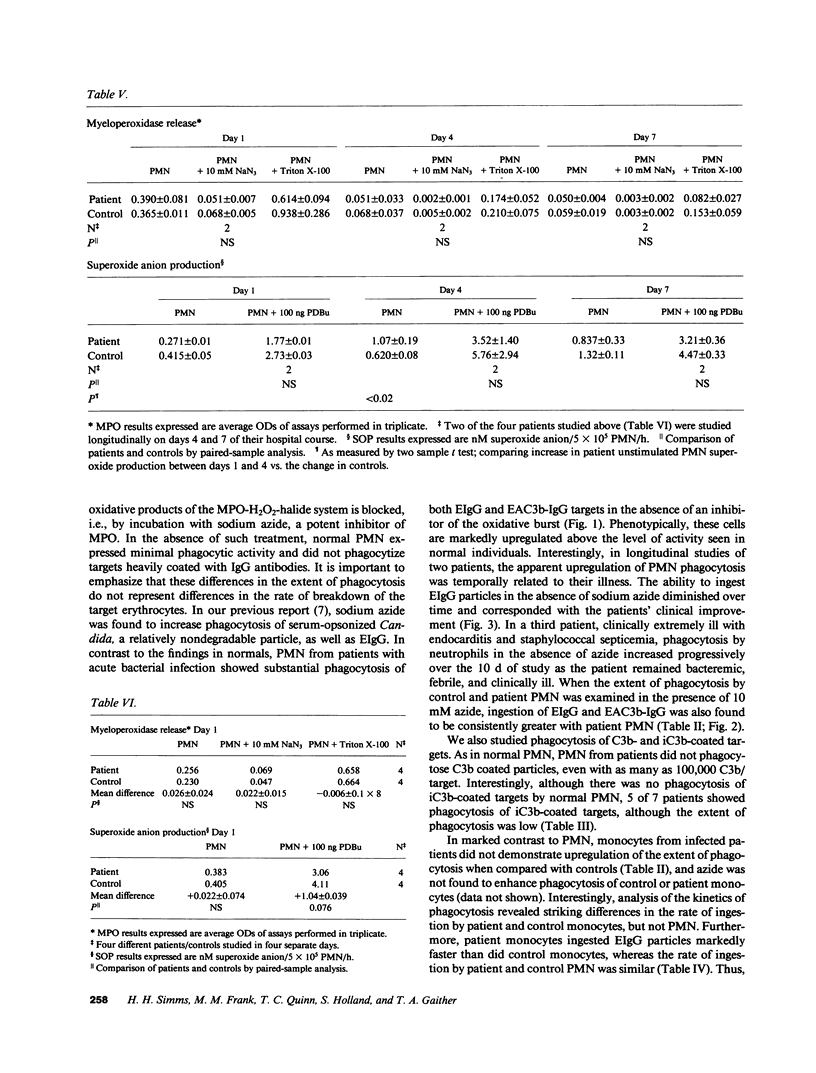
Polymorphonuclear leukocytes (PMN) and monocytes from 20 patients with acute bacterial infections were examined for phagocytic function. PMN of patients expressed markedly enhanced phagocytosis as measured by the ingestion of erythrocyte (E)IgG and IgG/C3b-coated E. Phagocytosis of E coated with C3b alone was not seen, while low levels of ingestion of iC3b-E by patients' PMNs was noted. Monocytes from patients and controls expressed similar phagocytic activity in a fixed endpoint assay; however, the kinetics of phagocytosis by patients' monocytes was strikingly faster. Superoxide anion (O2.) and myeloperoxidase activities were similar to controls in PMN of four patients studied on day 1 of admission. PMN from two of three patients studied longitudinally showed an initial elevation in EIgG phagocytosis, which fell to normal levels by day 4, concomitantly with increased O2. generation and clinical improvement. Phagocytosis remained elevated in the third patient who did not clear his septicemia. Surface membrane FcRII, FcRIII, CR1, and CR3 were similar on patient and control PMN. In contrast, FcRI was increased on PMN of five of seven patients by monomeric IgG binding, and on two of two patients by monoclonal anti-FcRI binding. Thus, PMN and monocytes of patients with acute bacterial infections are either upregulated with regard to phagocytic function or are less susceptible to downregulation than are normal cells. This presumably would have a beneficial effect on host defenses during infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Koch C., Vejlsgaard R., Wilken-Jensen K. Fatal granulomatous disease. Acta Paediatr Scand. 1968 Mar;57(2):110–114. doi: 10.1111/j.1651-2227.1968.tb04661.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Allred C. D., Solberg C. O., Hill H. R. Chemiluminescence by polymorphonuclear leukocytes from patients with active bacterial infection. J Infect Dis. 1980 Jan;141(1):14–26. doi: 10.1093/infdis/141.1.14. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Bradley P. P., Christensen R. D., Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982 Sep;60(3):618–622. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Gaither T. The effect of temperature on the reactivity of guinea-pig complement with gamma G and gamma M haemolytic antibodies. Immunology. 1970 Dec;19(6):967–974. [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Hall R. P., Lawley T. J., Crabtree G. R., Frank M. M. Monocyte receptors for the Fc portion of IgG studies with monomeric human IgG1: normal in vitro expression of Fc gamma receptors in HLA-B8/Drw3 subjects with defective Fc gamma-mediated in vivo clearance. J Immunol. 1982 Sep;129(3):1041–1049. [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Gadek J. E., Katusha K., Santaella M., Frank M. M. Cleavage of membrane-bound C3b and C3bi by viable human neutrophils (PMN). Mol Immunol. 1983 Jun;20(6):623–635. doi: 10.1016/0161-5890(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Medley S. R., Gallin J. I., Frank M. M. Studies of phagocytosis in chronic granulomatous disease. Inflammation. 1987 Jun;11(2):211–227. doi: 10.1007/BF00916022. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Vargas I., Inada S., Frank M. M. The complement fragment C3d facilitates phagocytosis by monocytes. Immunology. 1987 Nov;62(3):405–411. [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Krukowski Z. H., Smith G. A unifying concept of polymorphonuclear neutrophil function in acute bacterial infection. World J Surg. 1983 May;7(3):424–429. doi: 10.1007/BF01658095. [DOI] [PubMed] [Google Scholar]
- Looney R. J., Ryan D. H., Takahashi K., Fleit H. B., Cohen H. J., Abraham G. N., Anderson C. L. Identification of a second class of IgG Fc receptors on human neutrophils. A 40 kilodalton molecule also found on eosinophils. J Exp Med. 1986 Apr 1;163(4):826–836. doi: 10.1084/jem.163.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Guyre P. M., Anderson C. L., Fanger M. W. Heteroantibody-mediated cytotoxicity: antibody to the high affinity Fc receptor for IgG mediates cytotoxicity by human monocytes that is enhanced by interferon-gamma and is not blocked by human IgG. J Immunol. 1986 Dec 1;137(11):3378–3382. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Neutrophil granulocyte function in bacterial infections. Lancet. 1972 Oct 7;2(7780):727–730. doi: 10.1016/s0140-6736(72)92022-3. [DOI] [PubMed] [Google Scholar]
- Solomkin J. S., Cotta L. A., Brodt J. K., Hurst J. M. Regulation of neutrophil superoxide production in sepsis. Arch Surg. 1985 Jan;120(1):93–98. doi: 10.1001/archsurg.1985.01390250081013. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Coble B. I., Dahlgren C., Hed J., Molin L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J Clin Invest. 1984 Feb;73(2):366–373. doi: 10.1172/JCI111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Henson P. M. Rapid micromeasurement of neutrophil exocytosis. Inflammation. 1978 Jun;3(2):129–135. doi: 10.1007/BF00910734. [DOI] [PubMed] [Google Scholar]
- Wilson E., Laster S. M., Gooding L. R., Lambeth J. D. Platelet-derived growth factor stimulates phagocytosis and blocks agonist-induced activation of the neutrophil oxidative burst: a possible cellular mechanism to protect against oxygen radical damage. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2213–2217. doi: 10.1073/pnas.84.8.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Seligmann B., Gallin J. I. Exudation primes human and guinea pig neutrophils for subsequent responsiveness to the chemotactic peptide N-formylmethionylleucylphenylalanine and increases complement component C3bi receptor expression. J Clin Invest. 1986 Mar;77(3):925–933. doi: 10.1172/JCI112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Peterson P. K., Quie P. G., Verhoef J. Interactions of phagocytic and bacterial cells in patients with bacteremia caused by gram-negative rods. J Infect Dis. 1980 Apr;141(4):441–449. doi: 10.1093/infdis/141.4.441. [DOI] [PubMed] [Google Scholar]



