Abstract
Objectives. We conducted a randomized clinical trial to test an integrated behavioral intervention designed to enhance using HIV treatment as prevention by improving medication adherence, reducing risks for other sexually transmitted infections, and minimizing risk compensation beliefs.
Methods. Individuals living with HIV/AIDS (n = 436) participated in a randomized clinical trial testing an intensive behavioral intervention aimed at reducing HIV transmission risks compared with an attention control condition. We used unannounced pill counts to monitor antiretroviral therapy adherence and computerized interviews to measure risk behaviors.
Results. The integrated transmission risk reduction intervention demonstrated increased antiretroviral therapy adherence and less unprotected intercourse with nonseroconcordant partners at 3- and 6-month follow-ups as well as fewer new sexually transmitted infections diagnosed over the 9-month follow-up period (adjusted odds ratio = 3.0; P < .05; 95% confidence interval = 1.01, 9.04). The integrated intervention also reduced behavioral risk compensation beliefs.
Conclusions. A theory-based integrated behavioral intervention can improve HIV treatment adherence and reduce HIV transmission risks. HIV treatment as prevention should be bundled with behavioral interventions to maximize effectiveness.
Antiretroviral therapy (ART) improves the health and increases the longevity of people infected with HIV.1 Growing evidence indicates that ART can also reduce HIV infectiousness, raising the possibility of using HIV treatment as prevention.2–3 Mathematical models suggest that HIV testing with immediate treatment may have a substantial preventative effect in high HIV prevalence populations.4–6 The potential preventative benefits of treating HIV infection are shifting prevention policies. Most notably, the Swiss Federal AIDS Commission has stated that repeated undetectable HIV RNA (viral load) tests can render individuals noninfectious.7–9 Although biologically and epidemiologically plausible,10,11 using HIV treatment as prevention will fail when medication adherence is poor and when there are co-occurring sexually transmitted infections (STIs).
Most ART regimens demonstrate suppressive effects in the genital tract that are similar to those in blood plasma,12 and the genital tract suffers similar detrimental ramifications of ART nonadherence.13 The most forgiving ART regimens require at least 85% adherence to suppress HIV replication, avoid treatment-resistant variants of the virus, and reduce infectiousness.14–16 Evidence also shows that individuals who experience difficulty adhering to ART engage in higher-risk sexual behaviors.17 Even under optimal adherence, persons with undetectable peripheral blood viral loads will be highly infectious in their genital secretions when they have co-occurring STIs.18
Co-occurring STIs are prevalent among people living with HIV/AIDS19,20 and cause HIV shedding in genital fluids.21 Individuals who are coinfected with HIV and other STIs are therefore far more infectious than their blood plasma viral load indicates. The poor concordance between blood plasma and semen HIV RNA is at least in part the result of inflammatory processes caused by co-occurring STIs.22 The interplay between treatment, viral load, and sexual transmission is further complicated by risk compensation; individuals who believe they are less infectious take fewer precautions against infecting partners.23–26
To succeed, the use of HIV treatment as prevention, or so-called test and treat strategies, will require a comprehensive approach that encompasses adherence support, sexual risk reduction, and the amelioration of risk compensation.27 HIV transmission risk reduction interventions for people infected with HIV have thus far focused exclusively on reducing unprotected sex with non-HIV–positive partners.28 Similarly, ART adherence interventions have not directly addressed HIV transmission risks. One example of an intervention that addressed treatment adherence and transmission risk reduction was the Healthy Living Project.29–30 In a multisite trial, the Healthy Living Project targeted mental health, treatment adherence, and risk behaviors in separate intervention modules delivered several weeks apart. The investigators of the Healthy Living Project examined the 3 modules independently in separate analyses. The Healthy Living Project medication module demonstrated significant increased treatment adherence,28 and the prevention module resulted in significant reductions in HIV transmission risk behaviors.29 Unfortunately, the Healthy Living Project did not test the synergistic effects of the adherence and risk reduction modules. We are not aware of any unified behavioral intervention that has attempted to simultaneously reduce HIV infectiousness by improving treatment adherence and reducing HIV exposures. We sought to fill this gap by testing an integrated adherence and risk-reduction intervention designed for use in conjunction with HIV treatment as prevention.
The aim of this clinical trial was to test the effects of a theory-based integrated behavioral intervention for reducing HIV transmission risks in individuals living with HIV/AIDS. Our primary hypothesis was that an integrated intervention approach would improve treatment adherence and reduce risk behaviors relative to a matched-contact control intervention. The secondary hypothesis was that the integrated intervention would reduce risk compensation beliefs concerning undetectable HIV RNA.
METHODS
Participants were 310 men and 126 women recruited from AIDS service providers in Atlanta, Georgia. The study commenced March 2005, enrollment ended October 2008, and follow-ups were completed November 2009. Atlanta has among the fastest growing HIV epidemics in the United States,31 with more than 23 000 reported cases of AIDS and an HIV/AIDS rate of 23 per 100 000 population, exceeding the average 15 per 100 000 population in other major US cities. For recruitment, we notified AIDS service providers and infectious disease clinics about the study opportunity. Recruitment brochures were placed in providers' lobbies and waiting areas. Interested persons telephoned the research site to schedule an intake appointment.
The study entry criteria were (1) aged 18 years or older and (2) name-matching proof of positive HIV status and photo identification. We did not screen participants for dependant variables (ART adherence and risk behavior) to ensure ecological validity of the intervention groups, because they would occur in community and clinical services.
Overview of Intervention Conditions
We implemented the 2 conditions in this trial using the same operational procedures. We conducted all intervention group sessions at the same community-based AIDS service provider. We formatted both arms to deliver a 45-minute one-on-one orientation and goal-setting session with 1 of the group facilitators before five 120-minute group sessions and a 60-minute postgroup one-on-one counseling session. The same interventionists delivered both conditions in male–female facilitator pairs. All facilitators received 2 weeks of training, delivered their first group sessions with an experienced facilitator, and received weekly supervision. We conducted group sessions with 8–10 participants of mixed gender and sexual orientations.
Integrated Risk Reduction and Adherence Intervention
We grounded the experimental condition in conflict theory of decision making.32 Conflict theory posits that weighing risks and benefits of behavioral options leads to more personally effective decisions. We therefore used a single model of decision-making skills that afforded a fully integrated and unified intervention for 2 targeted outcomes, both of which hinge on effective decision making: improved medication adherence and reduced sexual transmission risk behavior.
We conducted the first individual counseling session to set personal treatment and prevention goals for the upcoming group. The first group session built cohesion and trust, and in it we discussed how risk reduction and treatment goals are related. The session included a team-building game designed to educate participants about the basics of HIV transmission, treatment resistance, and viral load. We covered myths and facts about infectiousness in detail. The second group session focused on understanding HIV treatment, including deciding when to start medications. We applied decisional balance exercises to treatment decisions and sexual relationships in contexts of detectable and undetectable HIV RNA. Group session 3 focused entirely on sexual decision making under various nuanced conditions of moods, substance use, relationships, HIV status disclosure, treatment status, and viral load.
Group session 4 aimed to build treatment and safer sex decision skills in relation to substance use. A core activity in this session had participants wear vision-disorienting goggles to simulate intoxication while filling a pillbox with mints and then apply a condom to a penis model. We then trained participants in medication management and safer sex strategies, including using male and female condoms. Group session 5 emphasized treatment adherence to improve health and reduce infectiousness. We also offered skill-building activities for recognizing symptoms of STIs. The final individual counseling session occurred within 1 week of the last group session and delivered a personalized plan for treatment decisions, adherence, and safer sex.
Attention Control Condition
The control arm was a contact-matched noncontaminating support group for individuals living with HIV/AIDS. Participants received 1 individual orientation session. The first group session focused on building group cohesion and discussed how to access quality health information. The remaining 4 group sessions covered detecting early warning signs of cancer, breast and testicular self-examination, nutrition decision making, healthy food selection, planning exercise, and relaxation. The final individual counseling session set personalized health improvement goals.
Outcome Measures
We assessed demographics, health, and behavior at baseline and 3-, 6-, and 9-month follow-ups using audio computer-assisted self-interviews.33 We asked participants their age, ethnicity, years of education, income, HIV symptoms, employment, and disability status. Participants also indicated whether they had drunk alcohol or used other drugs in the previous 3 months. We monitored HIV treatment adherence using unannounced pill counts.
ART adherence and viral load. We monitored HIV treatment adherence with monthly, unannounced telephone-based pill counts. Unannounced pill counts are reliable and valid in assessing HIV treatment adherence when conducted in participants' homes and on the telephone.34,35 We gave participants a free cell phone that restricted service to project contacts and emergency use (e.g., 911). After office-based training in the pill-counting procedure, participants were called at unscheduled times by a telephone assessor. We took pill counts over 21- to 35-day intervals and conducted them for each antiretroviral medication the participants were taking. We also collected pharmacy information from pill bottles to verify the number of pills dispensed between calls. We calculated adherence as the ratio of pills counted to pills prescribed, taking into account the number of pills dispensed. Two consecutive pill counts were necessary for computing adherence. Adherence data represents the percentage of pills taken as prescribed following the initial office-based assessment averaged across antiretroviral medications. Telephone assessors were blind to assigned treatment condition. The first 3 pill counts occurred before the baseline assessment, allowing us to calculate at least 1 preintervention adherence value. As part of the monthly telephone assessment, participants also reported their CD4 cell count and HIV viral load.
Sexual risk behaviors and STIs. Participants responded to questions assessing their number of male and female sexual partners and frequency of unprotected and protected sexual behaviors (anal and vaginal intercourse) with seroconcordant (same HIV status) and nonseroconcordant (HIV negative and unknown HIV status) partners in the previous 3 months.36,37 Outcome analyses focused on testing the primary hypothesis regarding HIV transmission risks, specifically reducing unprotected sexual behaviors with non-HIV-positive partners. Participants also reported whether they had been diagnosed with a non-HIV sexually transmitted infection (gonorrhea, chlamydia, syphilis, and nongonococcal urethritis) during each 3-month retrospective period.
Adherence and prevention strategies and risk compensation beliefs. To assess adherence strategies, we asked participants to indicate whether they had used 15 common strategies for improving medication adherence by responding on 5-point scales in which 0 = “never” and 4 = “always” (α = 0.70).38,39 To determine their use of prevention strategies, we asked participants to report how often they had used 9 common behavioral strategies for sexual risk reduction in the previous 3 months. We summed these items to create an index of safer sex strategies.40 We adapted risk compensation beliefs from previous research41 and included 7 items (e.g., “People with HIV who take HIV medications are less likely to infect their sexual partners during unsafe sex” and “It is safe to have sex without a condom when my viral load is undetectable”) on 6-point scales in which 1 = “strongly disagree” and 6 = “strongly agree.” The mean risk compensation score was internally consistent (α = 0.84).
Sample Size, Randomization, and Blinding
We used a moderate effect size (d = 0.35) to calculate statistical power for both projected outcomes.28,42 We assumed 80% retention and estimated a sample of 110 for each primary outcome to achieve a 90% chance of detecting differences between groups.
After 3 unannounced pill counts and the baseline audio computer-assisted self-interview assessment, we randomly assigned participants to conditions. The project director performed the allocation, manually drawing equal numbers of condition-coded markers without replacement. We did not breach randomization throughout the trial.
Recruitment, screening, office-based assessment, and telephone assessment staff remained blinded to condition throughout the study, and interventionists never conducted assessments.
Statistical Analyses
In our outcome analyses, we used an intent-to-treat approach, in which we included all available follow-up data from participants in the analyses regardless of their exposure to the intervention sessions. Preliminary analyses involved procedures suggested by Jurs and Glass43 to test baseline equivalence between conditions and effects of attrition on dependent measures.
In primary and secondary outcome analyses, we used generalized estimating equations with unstructured working correlation matrixes. We used Poisson distribution for count data and normal distribution for scaled data. All outcome analyses controlled for baseline scores, gender, and date of study enrollment. We entered condition, time of assessment, and condition × time interactions as model effects. We used planned contrasts with a least significant difference adjustment to test for simple effects. We tested sexual risk reduction outcomes, including safer sex strategies, for all sexually active participants and continuous adherence values for all participants receiving ART with < 90% baseline adherence. We analyzed adherence strategies for all participants taking HIV treatment and analyzed risk compensation beliefs for the entire sample. We employed logistic regression analysis to analyze the dichotomous STI outcome for all sexually active participants, controlling for potentially confounding HIV physical symptoms. We used SPSS version 18 (SPPS, Inc, Chicago, IL) for all primary outcome analyses.
We tested the simultaneous effect of the intervention on multiple outcomes using structural equation modeling with AMOS version 18 (AMOS Development Corp, Crawfordville, FL). Specifically, we tested the intervention condition as a predictor of 6-month follow-up nonseroconcordant unprotected intercourse, medication adherence, and subsequent infectiousness beliefs. We entered STI diagnoses over the 9-month follow-ups as the dependant variable and unprotected intercourse and infectiousness beliefs as predictor variables. In addition, we included adherence as a predictor of viral load. We report model coefficients for paths and goodness of fit indexes.
RESULTS
The majority (91%) of participants were African American, 55% of men reported current male sexual partners, and the mean age was 44.1 years (SD = 6.8). Seventy-one percent of participants were receiving ART that included nonnucleoside reverse transcriptase inhibitors (n = 54; 24%), protease inhibitors (n = 31; 14%), or protease inhibitors boosted by a pharmacokinetic enhancer (n = 132; 58%). Nine (4%) were using other regimens. No differences were found between conditions on any baseline measures (Table 1).
TABLE 1.
Demographic Characteristics and Baseline Behaviors for Integrated and Comparison Interventions: Integrated HIV Intervention Trial, Atlanta, GA, March 2005–November 2009
| Characteristics | Integrated Intervention (n = 217), No. (%) or Mean (SD) | Comparison Intervention (n = 219), No. (%) or Mean (SD) | χ2 or t |
| Gender | |||
| Men | 161 (74) | 149 (68) | |
| Women | 56 (29) | 70 (32) | 2.00 |
| Transgender (men) | 13 (6) | 17 (8) | 0.50 |
| Race | |||
| African American | 199 (92) | 198 (90) | |
| White | 12 (6) | 14 (6) | |
| Latino | 4 (2) | 3 (1) | |
| Other | 2 (1) | 4 (2) | 2.20 |
| Unemployed | 77 (35) | 78 (35) | 1.60 |
| Disabled | 113 (52) | 118 (54) | 1.60 |
| Income < $10 000 | 152 (71) | 155 (71) | 0.20 |
| Education ≤ 12 y | 129 (59) | 144 (66) | 1.80 |
| Substance use in past 3 mo | |||
| Alcohol | 114 (48) | 96 (56) | 3.30 |
| Marijuana | 54 (25) | 43 (20) | 1.70 |
| Other drugs | 49 (22) | 47 (21) | 0.10 |
| Sexually active | 141 (65) | 145 (66) | 0.01 |
| HIV nonseroconcordant | |||
| Sexual partner | 64 (30) | 66 (30) | 0.10 |
| STI in the past 3 mo | 6 (3) | 13 (6) | 2.60 |
| Taking ART | 152 (70) | 157 (72) | 3.20 |
| ≤ 90% adherent to ART | 62 (44) | 51 (33) | 3.70 |
| Undetectable viral load | 94 (44) | 104 (48) | 0.80 |
| Age | 44.2 (7.0) | 44.7 (6.9) | 0.8 |
| Years since testing HIV+ | 13.1 (6.7) | 12.4 (5.9) | 1.1 |
| Risk compensation beliefs | 0.9 (1.0) | 1.0 (1.1) | 0.7 |
| Sex behaviors for entire sample | |||
| No. of sexual partners | 1.3 (2.9) | 1.3 (3.3) | 1.4 |
| No. of nonpositive sexual partners | 0.6 (1.8) | 0.7 (3.1) | 0.2 |
| Unprotected intercourse with nonpositive partners | 0.5 (2.9) | 0.8 (5.6) | 0.8 |
| Condom-protected intercourse with nonpositive partners | 1.6 (7.9) | 0.9 (2.9) | 1.2 |
| Sex behaviors for sexually active only | |||
| No. of sexual partners | 2.0 (3.4) | 2.1 (3.9) | 0.9 |
| No. of nonpositive sexual partners | 1.0 (2.1) | 1.0 (3.7) | 0.8 |
| Unprotected intercourse with nonpositive partners | 0.8 (3.6) | 1.3 (6.8) | 0.4 |
| Condom-protected intercourse with nonpositive partners | 2.5 (9.7) | 1.4 (3.5) | 1.3 |
| Unannounced pill count adherence | |||
| ART adherence entire sample | 85.7 (27.9) | 90.7 (22.5) | 1.7 |
| ART adherence for ≤ 90% adherent | 63.5 (25.5) | 68.8 (23.6) | 1.1 |
Note. ART = antiretroviral therapy; STI = sexually transmitted infection. All comparisons were nonsignificant.
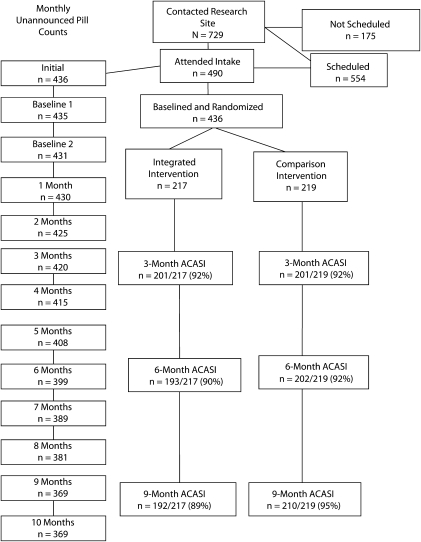
As shown in Figure 1, the trial retained more than 90% of participants randomized to conditions for audio computer-assisted self-interviews and 84% for monthly telephone assessments. Six participants were known to have died during the trial, 5 withdrew, and 3 moved out of state. Attrition was proportional for the 2 conditions at the 3- and 6-month follow-ups. However, significantly more participants in the integrated intervention were lost at the 9-month follow-up (χ2(1490) = 8.3; P < .01); 89% of experimental and 95% of control participants were retained more than 9 months. Overall retention was higher than the 80% initially expected, with > 90% of participants retained. In the planned attrition analyses suggested by Jurs and Glass,43 we did not find differences between retention and attrition by condition for participant characteristics or outcome variables.
FIGURE 1.
Flow of participants through the randomized trial: Integrated HIV Intervention Trial, Atlanta, GA, March 2005–November 2009.
Note. ACASI = audio computer-assisted self-interview.
Intervention attendance was also proportional across conditions. Participants in both conditions attended the same average of 3.5 (SD = 2.1) group sessions and 1.6 (SD = 0.1) individual sessions; 196 of 217 (90%) participants in the integrated intervention attended at least 1 group session as did 196 of 219 (89%) in the comparison group. Similarly, 135 of 217 (62%) integrated participants and 138 of 219 (63%) comparison participants attended the final individual goal-setting session.
Treatment Adherence Outcomes
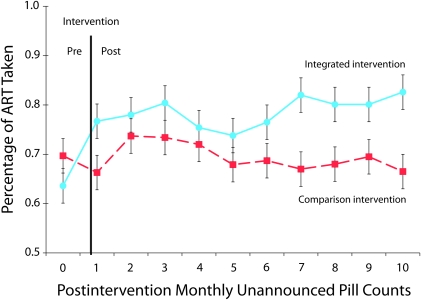
Figure 2 shows adjusted estimated means (SEs) for unannounced pill count adherence by condition over the 12-month observation period. Results indicated a significant intervention condition effect (Wald χ2 = 4.1; P < .05); integrated intervention participants demonstrated significantly greater adherence over the follow-up period than did the comparison group. The time effect (Wald χ2 = 6.4) and the condition × time interaction (Wald χ2 = 9.6) were not significant.
FIGURE 2.
Estimated means (SE) for ART adherence outcomes over 12 months of observation: Integrated HIV Intervention Trial, Atlanta, GA, March 2005–November 2009.
Note. ART = antiretroviral therapy.
Analyses of monthly reports of viral load did not indicate significant differences between conditions. Between 45% and 55% of participants in both conditions reported undetectable HIV RNA throughout the study.
Sexual Transmission Risk Reduction Outcomes
Results indicated significant differences between conditions for HIV exposure risks to uninfected sexual partners. There was a significant intervention × time interaction for unprotected intercourse; the integrated intervention engaged in significantly less nonseroconcordant unprotected intercourse at the 3- and 6-month follow-ups (Table 2). The integrated intervention group reported 121 nonseroconcordant unprotected intercourse acts between the baseline and the 6-month follow-up compared with 356 nonseroconcordant unprotected sex acts reported by the comparison group. The difference between conditions was no longer significant at the 9-month follow-up. Inspection of disaggregated anal and vaginal intercourse indicated a nonsignificant trend for a condition effect on nonseroconcordant unprotected anal intercourse and a significant condition effect for nonseroconcordant unprotected vaginal intercourse; integrated intervention participants reported lower rates of both behaviors. We found no significant differences between groups in number of sexual partners and condom-protected intercourse.
TABLE 2.
Nonseroconcordant Sexual Behavior Outcomes, Skills, and Strategies and Risk Compensation Beliefs for Integrated and Comparison Interventions: Integrated HIV Intervention Trial, Atlanta, GA, March 2005–November 2009
| Measures | Integrated Intervention, Mean (SD) | Comparison Intervention, Mean (SD) | Condition, χ2 | Time, χ2 | Condition × Time, χ2 |
| No. of nonpositive partners | |||||
| 3 mo | 0.5 (1.4) | 0.6 (1.3) | 0.7 | 4.5 | 0.1 |
| 6 mo | 0.6 (1.1) | 0.9 (5.1) | |||
| 9 mo | 0.04 (0.6) | 0.4 (0.6) | |||
| Unprotected intercourse | |||||
| 3 mo | 0.9 (5.3) | 2.3 (15.0) | 0.8 | 10.3*** | 8.1*** |
| 6 mo | 0.2 (1.0) | 1.0 (3.8) | |||
| 9 mo | 1.1 (4.5) | 0.9 (4.3) | |||
| Unprotected anal intercourse | |||||
| 3 mo | 0.2 (1.4) | 0.4 (2.4) | 2.7* | 6.5*** | 3.2 |
| 6 mo | 0.1 (0.7) | 0.6 (2.9) | |||
| 9 mo | 0.4 (1.7) | 0.6 (3.7) | |||
| Unprotected vaginal intercourse | |||||
| 3 mo | 0.1 (1.1) | 0.3 (2.0) | 4.6** | 13.4*** | 3.6 |
| 6 mo | 0.1 (0.3) | 0.1 (0.2) | |||
| 9 mo | 0.1 (0.5) | 0.1 (0.2) | |||
| Condom-protected intercourse | |||||
| 3 mo | 0.8 (4.9) | 2.0 (14.0) | 0.5 | 0.1 | 3.9 |
| 6 mo | 0.2 (0.9) | 0.8 (3.5) | |||
| 9 mo | 1.0 (4.2) | 0.8 (4.0) | |||
| Safer sex strategies | |||||
| 3 mo | 47.4 (104.2) | 31.8 (59.3) | 4.9** | 7.2 | 4.4 |
| 6 mo | 29.4 (38.7) | 31.3 (37.5) | |||
| 9 mo | 43.5 (89.3) | 25.9 (44.9) | |||
| Adherence strategies | |||||
| 3 mo | 16.4 (13.4) | 15.2 (10.3) | 4.0** | 7.9** | 1.0 |
| 6 mo | 16.2 (11.7) | 16.2 (13.6) | |||
| 9 mo | 15.5 (13.4) | 13.8 (10.8) | |||
| Risk compensation beliefs | |||||
| 3 mo | 0.9 (1.0) | 1.1 (1.1) | 4.8** | 1.6 | 1.7 |
| 6 mo | 1.0 (1.0) | 1.1 (1.2) | |||
| 9 mo | 0.9 (1.0) | 1.1 (1.1) |
Note. All analyses control for baseline scores, gender, and date of study enrollment
*P < .10; **P < .05; ***P < .01.
Analyses for acquiring a new bacterial STI aggregated over the 9-month postintervention showed a significant difference between conditions; fewer participants in the integrated intervention (5 of 143 sexually active; 3.5%) reported new STIs than did those in the comparison intervention (13 of 150 sexually active; 8.6%; adjusted odds ratio = 3.0; P < .05; 95% confidence interval [CI] = 1.01, 9.04).
Behavioral Strategies and Beliefs
The integrated intervention participants reported greater use of safer sex risk reduction and medication adherence strategies (Table 3). A significant time effect also showed that adherence strategies increased across groups. The integrated intervention demonstrated less endorsement of risk compensation beliefs than did the comparison condition. The condition × time interactions were not significant.
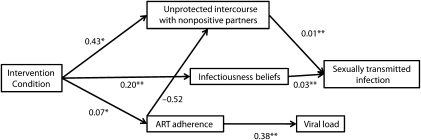
Structural Model of Integrated Intervention Outcomes
Structural equation modeling simultaneously tested intervention outcomes and their association to intervention endpoints (Figure 3; all baseline scores controlled not shown). Results demonstrated a good fit of the data to the model (χ2 = 38.4; P > .05; root mean square error of approximation = 0.031; 95% CI = 0.000, 0.051). The intervention condition demonstrated significant effects on nonseroconcordant unprotected intercourse and ART adherence. The intervention also significantly affected subsequent risk compensation beliefs. As expected, adherence and risk reduction behavioral outcomes predicted their respective endpoints.
FIGURE 3.
Structural equation model for intervention effects on primary outcomes and association of primary outcomes to health-related endpoints: Integrated HIV Intervention Trial, Atlanta, GA, March 2005–November 2009.
Note. ART = antiretroviral therapy. *P < .05; **P < .01
DISCUSSION
The current trial demonstrated significant improvement in ART adherence and reductions in HIV transmission risks resulting from an integrated behavioral intervention designed to improve adherence and to prevent HIV transmission. The magnitude of effects parallel those reported by an intervention that aimed to reduce risks and increase adherence in separate modules.29–30 To our knowledge, this trial is the first test of an intervention designed to simultaneously and synergistically reduce HIV transmission risks by improving adherence, reducing unprotected intercourse, and minimizing risk compensation. The pattern of results for the primary and secondary outcomes suggests that an integrated behavioral intervention has the potential to bolster the use of HIV treatments to avert new HIV infections.
Although adherence improvements were relatively stable over the 9 months of follow-up, the sexual risk reduction outcomes were shorter lived, dissipating before 9 months after the intervention. The durability of behavioral outcomes may prove more optimistic when the intervention is delivered in clinical care given the opportunities for ongoing support. Another limitation of this trial was its reliance on self-reported measures for sexual risk and biological outcomes. Although the consistency of findings supports the study's internal validity, further testing of this intervention should be undertaken with biological endpoints. We emphasized ecological validity in designing the intervention, focusing on simple and low-cost strategies for reducing risk and improving adherence.44 Although we strived to maintain implementation simplicity, the intervention included 2 individual and 5 group sessions. Operations research is needed to examine whether the intervention can be shortened and whether it can be infused within existing clinical services, such as support groups, medication adherence groups, and case management.
Risk compensation beliefs conceptually link adherence and HIV transmission risk behaviors. We have previously proposed that some individuals who believe that they are not infectious after learning they have undetectable HIV RNA will adjust their sexual practices by using condoms less and engaging in higher rates of unprotected sex, therefore exposing themselves to co-occurring STIs and greater infectivity. In the integrated intervention tested in this trial, we used a uniform decision-making model to simultaneously affect adherence, risk behaviors, and risk compensation beliefs. These findings confirm the conceptual basis for the intervention and suggest that other integrated intervention models may be effective in addressing multiple related health behaviors.
HIV treatment used for prevention offers great hope for extending the benefits of ART to avert new HIV infections. Behavioral interventions can be bundled with HIV treatments used for prevention and offered as a unified prevention package. Failure to directly address factors such as ART adherence, risk behaviors, co-occurring STIs, and risk compensation will undermine the promise of using HIV treatment for prevention. It is incumbent on “test and treat” programs, as well as other approaches that use HIV treatment for prevention, to provide adequate resources for integrating behavioral interventions to sustain adherence, risk reduction, and STI control.
Acknowledgments
The National Institute of Mental Health (grant R01-MH71164) supported this study.
Cynthia Grossman was the program officer.
The authors wish to thank Jeff Parsons, Dan Dunable, Juanita Williams, Richard Anderson, George Burgess, and the AIDS Survival Project of Atlanta for their contributions.
S. C. Kalichman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Human Participant Protection
The University of Connecticut institutional review board approved all study protocols.
References
- 1.Lima VD, Harrigan R, Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301(22):2380–2382 [DOI] [PubMed] [Google Scholar]
- 3.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57 [DOI] [PubMed] [Google Scholar]
- 5.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287(5453):650–654 [DOI] [PubMed] [Google Scholar]
- 6.Wilson DP, Law MG, Grulich AE. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372(9635):314–320 [DOI] [PubMed] [Google Scholar]
- 7.Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV transmission under highly active antiretroviral therapy. Lancet. 2008;372(9652):1806–1807; author reply 1807 [DOI] [PubMed] [Google Scholar]
- 8.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV infection results in suppression of the seminal shedding of HIV. AIDS. 2000;14(2):117–121 [DOI] [PubMed] [Google Scholar]
- 9.Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV-positive individuals without additional sexually transmitted diseases (STD) and on effective anti-retroviral therapy are sexually non-infectious. Schweiz Arzteztg. 2008;89(5):165–169 [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929 [DOI] [PubMed] [Google Scholar]
- 11.Wawer MJ, Gray RH, Sewankarnbo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409 [DOI] [PubMed] [Google Scholar]
- 12.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43(8):1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernazza PL, Gilliam BL, Dyer J, Fiscus SA, Eron JJ, Cohen F. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997;11(8):987–993 [PubMed] [Google Scholar]
- 14.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE. 2008;3(7):e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangsberg DR, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4(2):65–72 [DOI] [PubMed] [Google Scholar]
- 16.Parienti JJ, Ragland K, Lucht F, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis. 2010;50(8):1192–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond C, Richardson JL, Milan J, et al. Use of and adherence to antiretroviral therapy is associated with decreased sexual risk behavior in HIV clinic patients. J Acquir Immune Defic Syndr. 2005;39(2):211–218 [PubMed] [Google Scholar]
- 18.Ping LH, Cohen MS, Hoffman I, et al. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74(19):8946–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer TH, Metsch LR, Zenilman JM. Use of a public sexually transmitted disease clinic by known HIV-positive adults: decreased self-reported risk behavior and increased disease incidence. J Acquir Immune Defic Syndr. 2002;29(3):289–294 [DOI] [PubMed] [Google Scholar]
- 20.Kalichman SC, Rompa D, Cage M. Sexually transmitted infections among HIV seropositive men and women. Sex Transm Infect. 2000;76(5):350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilcher CD, Chuan Tien H, Eron JJ, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792 [DOI] [PubMed] [Google Scholar]
- 22.Kalichman SC, DiBerto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35(1):55–60 [DOI] [PubMed] [Google Scholar]
- 23.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual behavior: a meta-analytic review. JAMA. 2004;292(2):224–236 [DOI] [PubMed] [Google Scholar]
- 24.Kalichman SC, Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect. 2003;79(1):59–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalichman SC, Rompa D, Austin J, Luke W, DiFonzo K. Viral load, perceived infectivity, and unprotected intercourse. J Acquir Immune Defic Syndr. 2001;28(3):303–305 [DOI] [PubMed] [Google Scholar]
- 26.Eaton LA, Kalichman S. Risk compensation in HIV prevention: implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Curr HIV/AIDS Rep. 2007;4(4):165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalichman SC. Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: implications for positive prevention interventions. Psychosom Med. 2008;70(5):593–597 [DOI] [PubMed] [Google Scholar]
- 28.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20(2):143–157 [DOI] [PubMed] [Google Scholar]
- 29.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the Healthy Living Project Randomized Controlled Study. J Acquir Immune Defic Syndr. 2007;46(5):574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Healthy Living Project Team Effects of a behavioral intervention to reduce risk of transmission among people living with HIV/AIDS: the Healthy Living Project Randomized Controlled Study. J Acquir Immune Defic Syndr. 2007;44(2):213–221 [DOI] [PubMed] [Google Scholar]
- 31.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med. 2010;362(11):967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janis IL, Mann L. Decision-Making: A Psychological Analysis of Conflict, Choice, and Commitment. New York: Free Press; 1977 [Google Scholar]
- 33.Gribble JN, Miller HG, Cooley PC, Catania JA, Pollack L, Turner CF. The impact of T-ACASI interviewing on reported drug use among men who have sex with men. Subst Use Misuse. 2000;35(6–8):869–890 [DOI] [PubMed] [Google Scholar]
- 34.Bangsberg DR, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic medication monitors and unannounced pill counts. AIDS Behav. 2001;5(3):275–281 [Google Scholar]
- 35.Kalichman SC, Amaral CM, Cherry C, et al. Monitoring antiretroviral adherence by unannounced pill counts conducted by telephone: reliability and criterion-related validity. HIV Clin Trials. 2008;9(5):298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Napper L, Fisher DG, Reynolds GL, Johnson ME. HIV risk behavior self-report reliability at different recall periods. AIDS Behav. 2010;35(4):350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder KE, Carey MP, Vanable P. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytic options. Ann Behav Med. 2003;26(2):76–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19(2):124–133 [PubMed] [Google Scholar]
- 39.Kalichman SC, Cain D, Cherry C, Kalichman M, Pope H. Pillboxes and antiretroviral adherence: prevalence of use, perceived benefits, and implications for electronic medication monitoring devices. AIDS Patient Care STDS. 2005;19(12):833–839 [DOI] [PubMed] [Google Scholar]
- 40.Kalichman SC, Cain D, Weinhardt L, et al. Experimental components analysis of brief theory-based HIV-AIDS risk reduction counseling for sexually transmitted infection patients. Health Psychol. 2005;24(2):198–208 [DOI] [PubMed] [Google Scholar]
- 41.Kalichman SC, Eaton L, Cain D, et al. Changes in HIV treatment beliefs and sexual risk behaviors among gay and bisexual men, 1997–2002. Health Psychol. 2007;26(5):650–656 [DOI] [PubMed] [Google Scholar]
- 42.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–297 [DOI] [PubMed] [Google Scholar]
- 43.Jurs SG, Glass GV. The effect of experimental mortality on the internal and external validity of the randomized comparative experiment. J Exp Educ. 1971;40(1):62–66 [Google Scholar]
- 44.Mills EJ, Cooper C. Simple, effective interventions are key to improving adherence in marginalized populations. Clin Infect Dis. 2007;45(7):916–917 [DOI] [PubMed] [Google Scholar]