Abstract
Introduction
Our objective was to compare the impact of extra-capsular (ECAN) versus intracapsular allograft nephrectomy (ICAN) on allosensitization and surgical outcomes.
Methods
Between 1990 and 2004, 96 allograft nephrectomies were performed at our institution. Of these, 29 procedures were performed within 1 month of the transplant and were therefore omitted from analysis. Overall, the results of 44 ECAN and 23 ICAN were reviewed.
Results
The mean operative times were 110.9 versus 130.4 min for ICAN versus ECAN (p = 0.02) and the estimated blood loss was 226 mL for ICAN versus 483 mL for ECAN (p = 0.004). Intraoperative and postoperative complications were low using either technique and differences were not statistically significant. Overall, the preoperative to postoperative change in the percentage of panel reactive antibody was +2.1% for ICAN versus +1.2% for ECAN (NS) at 3 to 12 months postoperatively, respectively (NS). The percentage of patients relisted was 33.3% versus 54.3% (NS), and the percentage of patients re-transplanted once relisted was also very similar: 63.2% for ECAN versus 66.7% for ICAN (NS), after a mean follow-up of 4.5 and 8.4 years, respectively.
Conclusions
ICAN can be performed with shorter operative times and less blood loss versus the extracapsular approach. As well, this operative approach does not appear to affect allosensitization and the ability to re-transplant patients.
Abstract
Introduction
Notre objectif était de comparer les résultats d’une néphrectomie extracapsulaire et d’une néphrectomie intracapsulaire d’un greffon allogénique et leur effet sur l’allosensibilisation.
Méthodologie
Entre 1990 et 2004, 96 néphrectomies de greffon allogénique ont été réalisées à notre établissement. De ce nombre, 29 interventions ont été effectuées dans le mois suivant la transplantation et ont donc été omises dans les analyses. Au total, on a examiné les résultats de 44 néphrectomies extracapsulaires et 23 néphrectomies intracapsulaires.
Résultats
Les temps d’opération étaient de 110,9 et 130,4 minutes pour les néphrectomies intracapsulaires et les néphrectomies extra-capsulaires (p = 0,02) et la perte de sang était évaluée à 226 mL pour l’intervention intracapsulaire contre 483 mL pour l’intervention extracapsulaire (p = 0,004). Les complications peropératoires et postopératoires étaient rares avec l’une ou l’autre technique et les différences à cet égard n’étaient pas significatives sur le plan statistique. Règle générale, la variation dans le pourcentage d’activité de la rénine plasmatique entre les périodes préopératoire et postopératoire était de +2,1 % pour la néphrectomie intracapsulaire et de +1,2 % pour la néphrectomie extracapsulaire (NS) 3 à 12 mois après l’intervention (NS). Le pourcentage de patients reportés sur la liste d’attente était de 33,3 % contre 54,3 % (NS), et le pourcentage de patients ayant subi une nouvelle transplantation après leur report sur la liste était également similaire, soit 63,2 % pour la néphrectomie extracapsulaire contre 66,7 % pour la néphrectomie intracapsulaire (NS), après un suivi moyen de 4,5 et 8,4 ans, respectivement.
Conclusion
La néphrectomie intracapsulaire est une intervention moins longue et entraîne moins de pertes de sang qu’une intervention extracapsulaire. De même, cette technique chirurgicale ne semble pas affecter l’allosensibilisation et la possibilité de recourir à une nouvelle transplantation chez ces patients.
Introduction
Renal transplantation remains the optimal mode of renal replacement therapy for patients with end stage renal disease. Allograft survival at 10 years varies from 46% for deceased donor to 58% for living donor transplants.1 Fortunately, improvements in immunosuppression have led to declining rates of graft failure from acute humoral and cellular rejection. Graft loss from interstitial fibrosis and tubular atrophy, however, remains ever problematic and 10 year graft failure rates have not changed over the last decade. When the grafts fail, indications for surgical removal of the late rejecting graft include development of graft malignancy, infection, acute on chronic rejection, and the desire to wean immunosuppression.2–6 Allograft nephrectomy (AN) in this late setting is associated with surgical hazards owing to the development of desmoplastic reaction around the graft. The renal capsule is often adherent to the abdominal wall and the renal hilum is usually difficult to identify. Intracapsular allograft nephrectomy (ICAN) facilitates identification of the graft and provides reliable access to the renal hilum for safe vascular control. One theoretical disadvantage of ICAN is that a greater amount of donor tissue (capsule and urothelium) may be left in situ versus extracapsular allograft nephrectomy (ECAN), and this may lead to increased allo-sensitization of the patient and compromise the potential for re-transplantation. We hypothesized that ICAN is associated with superior surgical outcomes versus ECAN and is also associated with limited effects on further sensitization of the patient.
Materials and methods
Between January 1990 and December 2004, 96 ANs were performed at our centre by 5 different surgeons. Prior to 2000, 4 different surgeons performed ECAN in an attempt to remove the entire graft, including the renal capsule along with most of the associated urothelium. After 2000, 1 surgeon (PL) performed all procedures exclusively, and an intra-capsular approach was undertaken.
Intracapsular allograft nephrectomy
The approach to ICAN has been well described and our technique was very similar with some minor modifications.2 Briefly, the previous skin incision was opened, and the graft was identified. A capsulotomy was performed and the plane between the renal capsule and parenchyma developed by finger dissection until the hilum was identified. At this point, a vascular clamp was used to secure the entire hilum. The persistence of a robust femoral pulse was confirmed by palpation after each stage of the procedure, and the graft was excised above the clamp. The hilum was secured with a running continuous horizontal mattress 2.0 prolene suture immediately below the clamp, and the clamp was released. A second running continuous suture was then used to reinforce the initial layer.
Extracapsular allograft nephrectomy
An ECAN was performed after entering the retroperitoneal space through the old transplant incision. The entire kidney was dissected out with complete isolation of the ureter and the renal artery and vein. These vascular structures were individually ligated and divided with heavy 0.0 or 1.0 silk sutures. The ureter was dissected out to the bladder and ligated with vicryl ties and divided immediately above its insertion into the bladder.
After appropriate approval was obtained through our Institutional Review Board, a retrospective review of all hospital records of AN patients was performed. To limit the analysis to late graft failures in which fibrosis had been well-established, 29 procedures performed within 1 month of the transplant date were omitted from analysis. Forty-four ECAN and 23 ICAN procedures were compared in terms of operative times, estimated blood loss and complications. The immunologic impact of the AN was evaluated by using the change in percentage of the panel reactive antibody (%PRA) as a surrogate marker for allosensitization. The %PRA was performed using the complement-dependent cytotoxicity-based assay immediately prior to AN and 3 to 12 months post-AN. The 2 surgical approaches were also compared in terms of the percentage of patients re-listed and eventually re-transplanted.
Statistics
All proportions were compared using the Fisher exact test. Continuous variables were compared with a student t-test. The p values above 0.05 were considered statistically insignificant.
Results
The perioperative parameters of ECAN and ICAN are shown in Table 1. The operative times and estimated blood loss were significantly less with ICAN compared with ECAN. None of the patients undergoing ICAN required a blood transfusion, whereas 15.9% of ECAN patients were transfused (not statistically significant). There was 1 death in the ECAN group due to catastrophic postoperative bleeding, which could not be controlled. Although not statistically different, there were more morbidities in the ECAN group (15.9%) compared with ICAN (2.2%). Three patients in the ECAN group had inadvertent intraoperative vascular injury requiring repair. Other complications in the ECAN group included an ascitic leak, myocardial infarction, pneumonia and postoperative seizure. There was only 1 morbidity in the ICAN group. This patient developed clostridium difficile-associated toxic megacolon requiring surgical intervention.
Table 1.
Comparison of clinical outcomes for ECAN and ICAN
| ECAN | ICAN | p value | |
|---|---|---|---|
| N | 44 | 23 | |
| OR time (min) | 130.4 | 110.9 | 0.02 |
| Estimated blood loss (mL) | 483 | 226 | 0.004 |
| % Transfused | 15.9 | 0 | NS |
| Mortality | 1 (2.2%) | 0 | NS |
| Morbidity | 7 (15.9%) | 1 (2.2%) | NS |
ECAN: extracapsular allograft nephrectomy; ICAN: Intracapsular allograft nephrectomy; OR: operating room; EBL: estimated blood loss; NS: not significant.
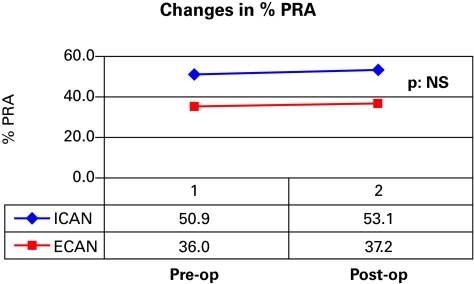
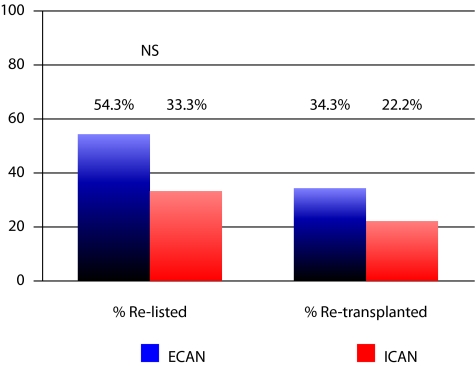
The changes in %PRA in the 2 groups were very similar (Fig. 1). In the ECAN group, the %PRA increased from 36.0% preoperatively to 37.2% between 3 and 9 months postoperatively. In the ICAN group, the %PRA increased from 50.9% to 53.1% between 3 and 9 months post-op. The difference in the percentage of patients re-listed and re-transplanted is illustrated in Fig. 2. The difference between the 2 groups, although slightly favouring ECAN, was not statistically significant. Furthermore, ECAN patients had a longer follow-up of 8.4 years versus 4.5 years for the ICAN patients. As such, the difference in the duration of follow-up may also bias re-listing rates in favour of the ECAN group. Furthermore, the percentage of patients being transplanted once re-listed was also very similar: 63.2% for ECAN versus 66.7% for ICAN.
Fig. 1.
Comparison of changes in % plasma renin activity between extracapsular allograft nephrectomy and intracapsular allograft nephrectomy.
Fig. 2.
Rates of re-listing and re-transplantation after allograft nephrectomy.
Discussion
Owing to the dense reaction around the allograft and recipient tissues, ECAN is a challenging and potentially hazardous procedure. Not surprisingly, late AN has been found to be associated with a higher complication rate than early graft removal.7,8 The mortality of late AN was found to be 1.1% during the hospital admission and 4.7% within 90 days of the date of admission.6 Medical morbidities were also found to be high within 90 days of admission, including 1.1% myocardial infarction, 7.5% congestive heart failure and 6.2% sepsis rates.
In an older study, the rate of surgical complications was found to be higher with ECAN at 16% versus ICAN at 8%.9 In another more recent series, the 2 procedures had equivalent complication rates at 17.6% for ECAN and 20% for ICAN.8 Our experience is one of the largest single centre AN series in the world. Unlike the other modern series, we found that blood loss, operative times and surgical complications (2% vs. 15%) were lower with ICAN versus ECAN. Importantly, since the desmoplastic reaction around the graft limits the ability to identify planes between the renal vasculature and pelvic vessels, there is increased risk for vascular injuries during ECAN. In fact, the rate of significant vascular injuries in one AN series was 5.6%.10 Accordingly, there were 3 intraoperative vascular injuries in our ECAN group (6.8%) versus none in our ICAN group.
The reported effect of AN on %PRA as a marker of allo-sensitization has been variable. Some have found only a transient effect11 and others have demonstrated a modest increase in %PRA after the AN.12,13 Our previously reported series12 is consistent with data from Johnston and colleagues in which the rise in %PRA post-AN is dependent upon pre-operative levels. 5 The factors driving increased allosensitization post-AN are unclear. Our series is the first to demonstrate that ICAN, despite leaving more donor tissue behind, does not adversely affect the %PRA versus ECAN. Importantly, we have also shown that ICAN does not negatively affect a patient’s chance of being re-listed or re-transplanted. As patients in the ECAN group were re-transplanted in an era that featured cyclosporine as the mainstay immunosuppressive agent and tacrolimus was used to re-transplant the ICAN group, we did not compare graft survival or functional results between the 2 groups after re-transplantation, since potential era-based biases in ICAN and ECAN groups would confound the results of re-transplant outcomes.
The authors acknowledge that this study is not a randomized controlled trial and that the ICAN and ECAN procedures were not performed contemporaneously and were performed by different surgeons. However, no new technology and no advancements in medical therapy was used to facilitate the ICAN procedures. As well, the ECAN procedures were performed by 4 highly experienced transplant surgeons, whereas the ICAN procedures were performed by a surgeon in the infancy of his career. Nevertheless, the authors acknowledge that differences in operative time, bleeding and complications between the 2 groups may be surgeon-related.
Conclusion
An ICAN can be performed with shorter operative times, less blood loss and reduced complication rates compared with an ECAN in the late failing allograft. In addition, an ICAN does not seem to affect allosensitization from the point of view of alteration of %PRA, re-listing and re-transplantation versus ECAN. In our centre, the ICAN remains the preferred treatment for late allograft failure requiring AN.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Cecka JM. The OPTN/UNOS Renal Transplant Registry. Clin Transpl. 2005:1–16. [PubMed] [Google Scholar]
- 2.Sutherland DE, Simmons RL, Howard RJ, et al. Intracapsular technique of transplant nephrectomy. Surg Gynecol Obstet. 1978;25:950–2. [PubMed] [Google Scholar]
- 3.Sharma DK, Pandey AP, Nath V, et al. Allograft nephrectomy – a 16 year experience. Brit J Urol. 1989;64:122–4. doi: 10.1111/j.1464-410x.1989.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 4.Toledo-Pereyra LH, Gordon C, Kaufmann R, et al. Role of immediate versus delayed nephrectomy for failed renal transplants. Am Surg. 1987;53:534–6. [PubMed] [Google Scholar]
- 5.Secin FP, Rovegno AR, del Rosario Brunet M, et al. Cumulative incidence, indications, morbidity and mortality of transplant nephrectomy and the most appropriate time for graft removal: only nonfunctioning transplants that cause intractable complications should be excised. J Urol. 2003;69:1242–6. doi: 10.1097/01.ju.0000050658.94353.24. [DOI] [PubMed] [Google Scholar]
- 6.Johnston O, Rose C, Landsberg D, et al. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. 2007;7:1961–7. doi: 10.1111/j.1600-6143.2007.01884.x. [DOI] [PubMed] [Google Scholar]
- 7.Grochowiecki T, Szmidt J, Galazka Z, et al. Influence of timing of transplant nephrectomy on surgical complication. Transplant Proc. 2000;32:1381. doi: 10.1016/s0041-1345(00)01265-3. [DOI] [PubMed] [Google Scholar]
- 8.Mazzucchi E, Nahas WC, Antonopoulos IM, et al. Surgical complications of graft nephrectomy in the modern transplant era. J Urol. 2003;170:734–7. doi: 10.1097/01.ju.0000080566.42381.94. [DOI] [PubMed] [Google Scholar]
- 9.Voesten HG, Slooff MJ, Hooykaas JA, et al. Safe removal of failed transplanted kidneys. Br J Surg. 1982;69:480–1. doi: 10.1002/bjs.1800690818. [DOI] [PubMed] [Google Scholar]
- 10.Eng MM, Power RE, Hickey DP, et al. Vascular complications of allograft nephrectomy. Eur J Vasc Endovasc Surg. 2006;32:212–6. doi: 10.1016/j.ejvs.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Vanrenterghem Y, Khamis S. The management of the failed renal allograft. Nephrol Dial Transplant. 1996;11:955–7. [PubMed] [Google Scholar]
- 12.Khakhar AK, Shahinian VB, House AA, et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35:862–3. doi: 10.1016/s0041-1345(02)04031-9. [DOI] [PubMed] [Google Scholar]
- 13.Sumrani N, Delaney V, Hong JH, et al. The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation. 1992;53:52–5. doi: 10.1097/00007890-199201000-00009. [DOI] [PubMed] [Google Scholar]