Abstract
Pathological hypertrophy is commonly induced by activation of protein kinases phosphorylating class II histone deacetylases (HDACs) and de-suppression of transcription factors, such as NFAT. We hypothesized that nifedipine, an L-type Ca2+ channel blocker, inhibits Ca2+ -calmodulin dependent kinase II (CaMKII) and NFAT, thereby inhibiting pathological hypertrophy. Mice were subjected to sham operation or transverse aortic constriction (TAC) for 2 weeks with or without nifedipine (10 mg/kg/day). Nifedipine did not significantly alter blood pressure or the pressure gradient across the TAC. Nifedipine significantly suppressed TAC-induced increases in left ventricular (LV) weight/body weight (LVW/BW) (5.09 ± 0.80 vs. 4.16 ± 0.29, mg/g, TAC without and with nifedipine, n=6,6, p<0.05), myocyte cross sectional area (1681 ± 285 vs. 1434 ± 197, arbitrary unit, p<0.05), and expression of fetal type genes, including ANF (35. 9 ± 6.4 vs. 8.6 ± 3.3, arbitrary unit, p<0.05). TAC-induced increases in lung weight/BW (7.7 ± 0.9 vs. 5.5 ± 0.5, mg/g, p<0.05) and decreases in LV ejection fraction (65.5 ± 3.1 vs. 75.7 ± 3.3 %, p<0.05) were attenuated by nifedipine. Nifedipine caused significant inhibition of TAC-induced activation of NFAT-mediated transcription, which was accompanied by suppression of Thr 286 phosphorylation in CaMKII. Nifedipine inhibited activation of CaMKII and NFAT by phenylephrine, accompanied by suppression of Ser 632 phosphorylation and nuclear exit of HDAC4 in cardiac myocytes. These results suggest that a subpressor dose of nifedipine inhibits pathological hypertrophy in the heart by inhibiting activation of CaMKII and NFAT, a signaling mechanism commonly activated in pathological hypertrophy.
Introduction
Despite recent progress in medical therapy, congestive heart failure is a major cause of death in western countries. Cardiac myocytes undergo hypertrophy in response to increased mechanical load, such as elevated blood pressure and increased circulatory volume, initially as an adaptive mechanism. However, the continued presence of mechanical loading leads to increases in myocardial cell death and fibrosis, accompanied by a reduction in cardiac contractility, which initiates a malignant cascade consisting of increased cell death, left ventricular (LV) dysfunction and increased mechanical loading.
Agonists stimulating cardiac hypertrophy, such as catecholamines, endothelin 1 and angiotensin II, evoke cellular signaling pathways through G-protein coupled receptors (GPCRs). Currently available medical therapy for heart failure, such as β adrenergic receptor blockers (β-blockers), angiotensin converting enzyme (ACE) inhibitors, and angiotensin II type 1 receptor blockers (ARB) function primarily by blocking the actions of GPCR agonists. Increasing lines of evidence suggest that pathological forms of cardiac hypertrophy, which are accompanied by the development of LV dysfunction, are mediated by activation of specific, but not all, signaling mechanisms downstream of GPCRs. Thus, selective attenuation of the signaling mechanism mediating pathological hypertrophy may provide us with better treatment for heart failure patients. However, an effective treatment selectively targeting the signaling mechanism mediating pathological hypertrophy remains to be established.
De-suppression of nuclear transcription factors promoting cardiac hypertrophy, including MEF2 and NFAT, through nucleo-cytoplasmic shuttling of class II histone deacetylases (HDACs), promotes pathological hypertrophy. Since this signaling mechanism is utilized by many forms of pathological hypertrophy, it is referred to as the final common pathway of cardiac hypertrophy [1]. One of the mechanisms regulating the subcellular localization of class II HDACs is phosphorylation by HDAC kinases, including Ca2+ calmodulin-dependent protein kinase II (CaMKII). Due to their involvement in many forms of pathological hypertrophy, HDAC kinases are attractive targets for treatment of heart failure.
Nifedipine is a classical Ca2+ channel blocker, which belongs to the dihydropyridine family. Nifedipine is commonly used clinically for anti-hypertensive treatment. Treatment of both experimental animals and humans with nifedipine reduces blood pressure and attenuates high blood pressure-induced cardiac hypertrophy [2,3]. Interestingly, recent evidence suggests that nifedipine treatment reduces the incidence of heart failure in stable angina patients better than conventional treatment [4], indicating that nifedipine may directly act on cardiac myocytes, thereby preventing the development of LV dysfunction. Since CaMKII, an HDAC kinase, is primarily regulated by increases in intracellular Ca2+ concentration, we hypothesized that nifedipine inhibits pathological hypertrophy through inhibition of HDAC kinases in the heart in vivo. Accordingly, we treated mice with nifedipine at a dose that does not affect blood pressure, and evaluated whether nifedipine treatment inhibits pathological hypertrophy through inhibition of the final common pathway in the heart.
Materials and methods
Materials
L-Phenylephrine (PE) and nifedipine were purchased from Sigma. Anti-calcineurin antibody was purchased from BD Biosciences. Anti-phospho-CaMKII antibody was obtained from Santa Cruz Biotechnology and Cell Signaling Technology. Anti-phospho-Akt antibody, anti-phospho-GSK-3β antibody, anti-GSK-3β antibody, and anti-HDAC4 antibody were purchased from Cell Signaling Technology. Anti-actin antibody was obtained from Sigma. Anti-phospho-HDAC antibody was obtained from Abcam.
Transgenic mice
NFAT-luciferase reporter mice generated on an FVB background [5] were kindly provided by Dr. J. D. Molkentin (University of Cincinnati). All protocols concerning animal use were approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey.
Primary culture of neonatal rat ventricular myocytes
Primary cultures of ventricular cardiac myocytes were prepared from 1-day-old Crl: (WI) BR-Wistar rats (Harlan). A cardiac myocyte-rich fraction was obtained by centrifugation through a discontinuous Percoll gradient as described [6].
Continuous infusion of nifedipine
Continuous infusion of nifedipine or control vehicle delivery was conducted using a mini-osmotic pump (model 2002, Alzet). Nifedipine was prepared at a concentration calculated to deliver an average of 10mg/kg/day during a 14-day infusion period. Control mice received pumps filled with vehicle (dimethyl sulfoxide) alone.
Aortic banding
The method to impose pressure overload in mice has been described [7]. Mice were anesthetized with a mixture of ketamine (0.065 mg/g), xylazine (0.013 mg/g), and acepromazine (0.002 mg/g) and mechanically ventilated. The left chest was opened at the second intercostal space. Aortic constriction was performed by ligation of the transverse thoracic aorta between the innominate artery and left common carotid artery with a 28-gauge needle using a 7-0 braided polyester suture. Sham operation was performed without constricting the aorta. To evaluate the pressure gradient across aortic constriction, two high-fidelity catheter tip transducers (1.4F, Millar) were used; one was inserted into the right carotid artery and the other into the left femoral artery and carefully advanced to the ascending aorta and abdominal aorta, respectively, at 2 weeks after banding under the same anesthesia as described above. The pressures in the ascending aorta and abdominal aorta were measured simultaneously. The pressure gradients between the systolic pressure in the ascending and abdominal aorta were calculated.
Echocardiography
Mice were anesthetized with an intraperitoneal injection of ketamine (0.065 mg/g), acepromazine (0.02 mg/g), and xylazine (0.013 mg/g). Echocardiography was performed using ultrasonography (Apogee CX-200; Interspec Inc., Ambler, Pennsylvania, USA) as described previously [8].
Histological analyses
The LV accompanied by the septum was cut into base, mid, and apex portions, fixed with 10% formalin and submitted for Wheat germ agglutinin (WGA) staining. Myocyte cross-sectional area was measured from images captured from WGA-stained 1-µm-thick sections as described.[9] The outline of 100–200 myocytes was traced in each section. The ImagePro system software was used to determine myocyte cross-sectional area.
Western blot
Heart homogenates and cardiac myocyte lysates were prepared with a lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% IGEPAL CA-630, 0.1% SDS, 0.5% deoxycholic acid, 10 mM Na4P2O7, 5 mM EDTA, 0.1 mM Na3VO4, 1 mM NaF, 0.5 mM AEBSF, 0.5 µg/ml aprotinin, and 0.5 µg/ml leupeptin. Equal amounts of proteins (5–30 µg) were subjected to SDS-PAGE. Proteins were transferred to a PVDF membrane.
Immunostaining
Cells were fixed with PBS containing 4% paraformaldehyde, permeabilized in PBS containing 0.2% Triton X-100, and blocked with PBS containing 3% BSA. Alexa Fluor 488 Dye or Alexa Fluor 594 Dye conjugated secondary antibody (Invitrogen) was used for detecting indirect fluorescence.
NFAT luciferase assay
Cardiac myocytes were transfected using Fugene 6 (Roche). Thirty-six hours after transfection, cells were lysed with Passive Lysis Buffer (Promega), and NFAT transcriptional activity was determined by the Luciferase Reporter Assay System (Promega) using an OPTOCOMP I luminometer (MGM instruments). For mouse samples, heart homogenates (10 µg) from NFAT-luciferase transgenic mice were lysed in Reporter Lysis Buffer (Promega) and analyzed for luciferase activity.
Analysis of cell size
Myocytes were fixed in PBS containing 4% paraformaldehyde. Immunostaining was performed using Texas Red phalloidin (Molecular Probes) and anti-α-sarcomeric actinin. Myocyte surface area was evaluated as described previously [6].
Statistical analysis
All values are expressed as mean ± SEM. Statistical analyses were performed using ANOVA or t-test with a P<0.05 considered significant.
Results
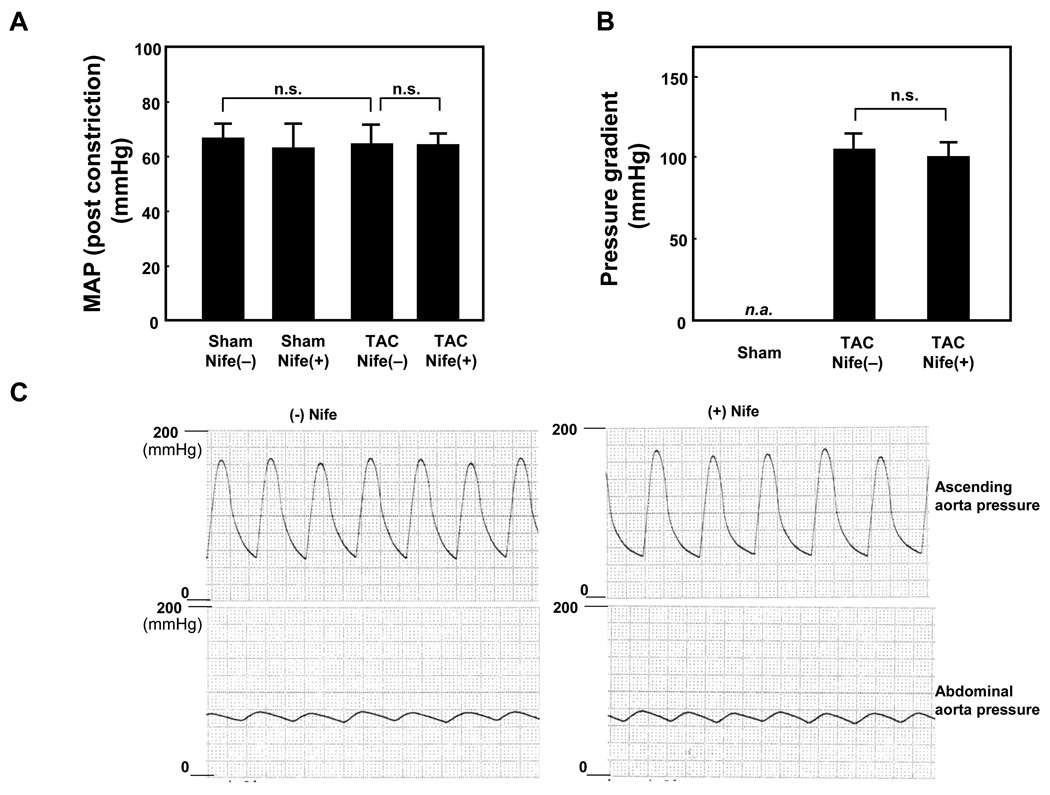
In order to examine the effect of nifedipine upon pathological hypertrophy, mice were subjected to TAC or sham operation with or without nifedipine. After 2 weeks, nifedipine did not significantly affect mean aortic pressure (MAP) in sham operated mice. In mice subjected to TAC, MAP after constriction was similar with or without nifedipine treatment (Fig. 1A). MAP in mice subjected to TAC was not significantly different from MAP in mice subjected to sham operation regardless of nifedipine treatment. The pressure gradient across the TAC after 2 weeks was similar in mice with or without TAC (Fig. 1B). The representative tracings of the pressure measurement are shown in Fig. 1C.
Figure 1. Nifedipine infusion does not affect blood pressure.
Mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. Post constriction mean arterial pressure (MAP) (A) and pressure gradient (B) were measured, using a catheter under anesthesia. Values are mean ± SEM. n=4 in each group. (C) Representative simultaneous recording of blood pressure at ascending aorta and abdominal aorta from animals subjected to TAC in the presence ((+) Nife) or absence ((−) Nife) of nifedipine.
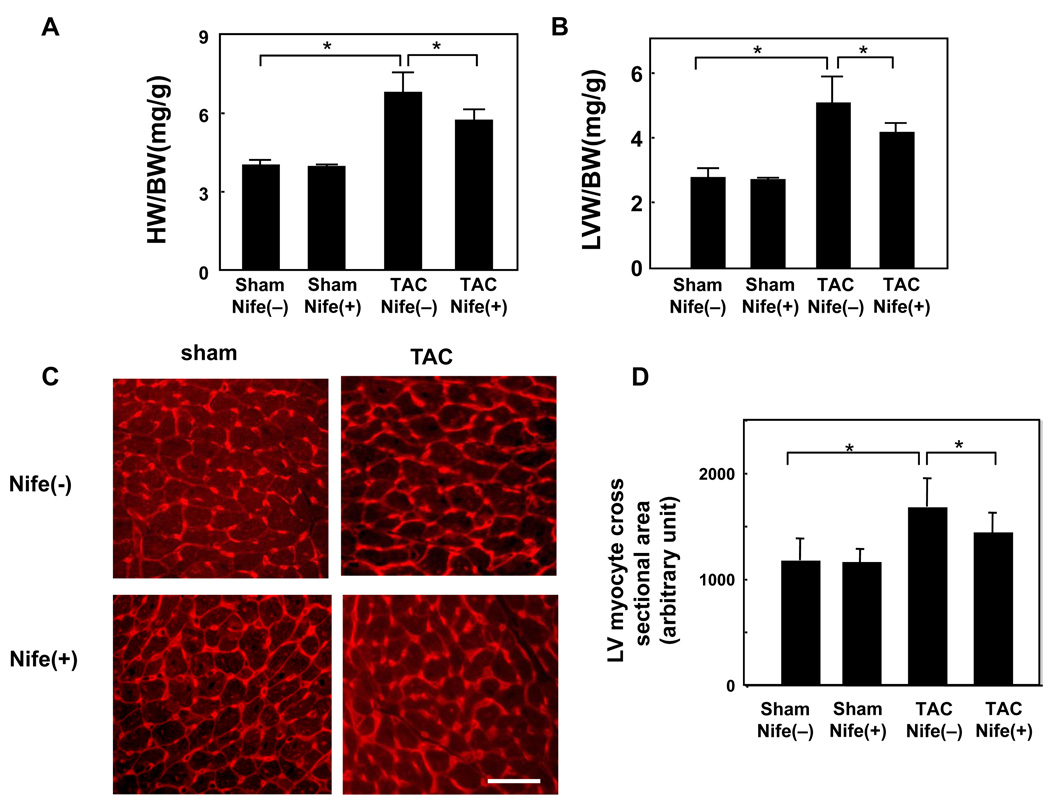
Nifedipine treatment did not significantly affect heart weight/body weight (HW/BW) or left ventricular weight/BW (LVW/BW), indexes of cardiac hypertrophy, in sham operated mice. TAC significantly increased both HW/BW and LVW/BW compared to sham, suggesting that TAC induced cardiac hypertrophy. Nifedipine treatment significantly attenuated TAC-induced increases in HW/BW and LVW/BW (Fig. 2AB). TAC also significantly increased LV cardiac myocyte cross sectional area, as determined by wheat germ agglutinin staining of LV myocardial sections. Nifedipine treatment significantly reduced TAC-induced increases in LV cardiac myocyte cross sectional area without affecting the myocyte cross sectional area in sham operated mice (Fig. 2C). These results suggest that nifedipine significantly attenuates pressure overload-induced cardiac hypertrophy.
Figure 2. Nifedipine inhibits TAC-induced cardiac hypertrophy.
Mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. Postmortem measurements of heart weight/body weight (HW/BW, mg/g) (A) and left ventricular weight/body weight (LVW/BW, mg/g) (B) are shown. (C) Representative wheat germ agglutinin (WGA)-Texas Red staining. Bar = 50 µm. (D) Myocyte cross-sectional area was determined from WGA-stained 1-µm-thick sections. The outline of 100–200 myocytes was traced in each section. The ImagePro system software was used to measure myocyte cross-sectional area. Values are mean ± SEM. n=6 in each group. * p<0.05.
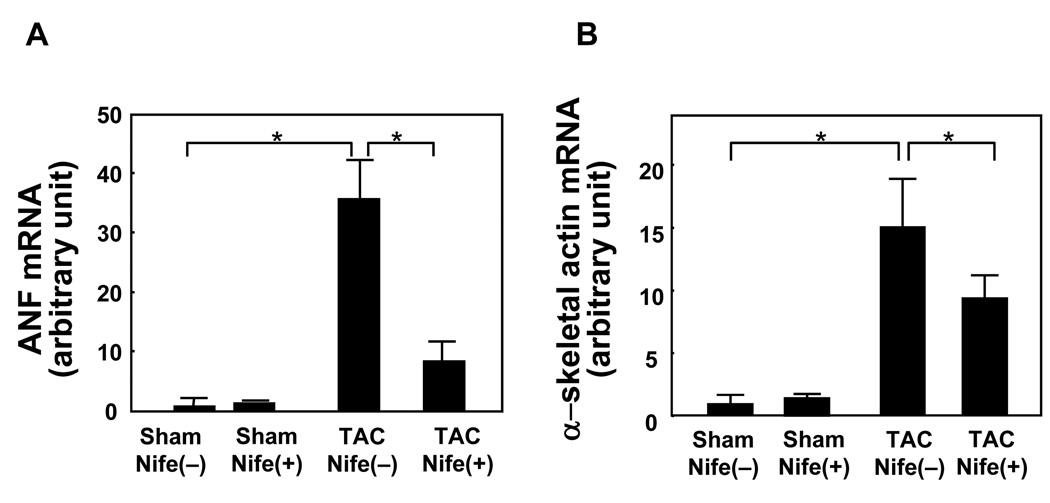
RT-qPCR analyses showed that TAC significantly increased mRNA expression of atrial natriuretic factor (ANF, Fig. 3A) and α-skeletal actin (Fig. 3B), fetal type genes. Nifedipine treatment significantly attenuated TAC-induced increases in ANF and α-skeletal actin without significantly affecting expression of ANF or α-skeletal actin in sham operated mice (Fig. 3AB).
Figure 3. Nifedipine attenuates TAC-induced upregulation of fetal type genes.
Mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. The apex portion of the LV was homogenized and total RNA was extracted. qRT-PCR analyses of ANF mRNA (A) and α-skeletal actin mRNA (B) were conducted. Values are mean ± SEM. n=6 in each group. *p<0.05.
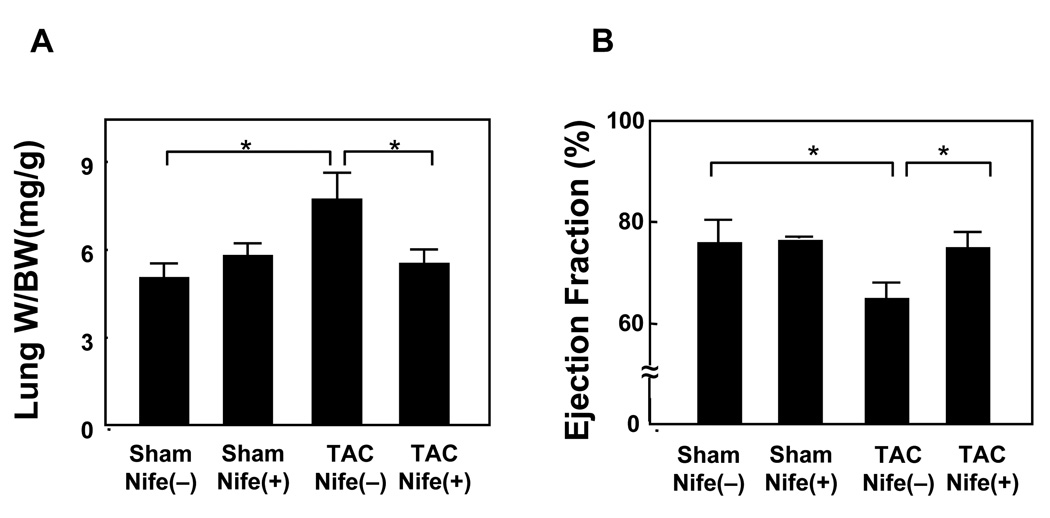
TAC significantly increased lung weight/BW, an index of lung congestion, whereas it significantly attenuated LV ejection fraction as determined by echocardiographic measurement, suggesting that TAC induces LV dysfunction (Fig. 4AB). Although nifedipine did not significantly affect lung weight/body weight or LV ejection fraction in sham operated mice, it significantly attenuated TAC-induced increases in lung weight/BW and TAC-induced decreases in LV ejection fraction (Fig. 4AB). These results suggest that nifedipine treatment significantly attenuates TAC-induced LV dysfunction.
Figure 4. Nifedipine inhibits TAC induced cardiac dysfunction.
Mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. (A) Postmortem measurement of lung weight/body weight (Lung W/BW, mg/g). (B) Echocardiographic analysis was conducted under anesthesia to evaluate ejection fraction (%), an index of LV systolic function. Values are mean ± SEM. n=6 in each group. *p<0.05.
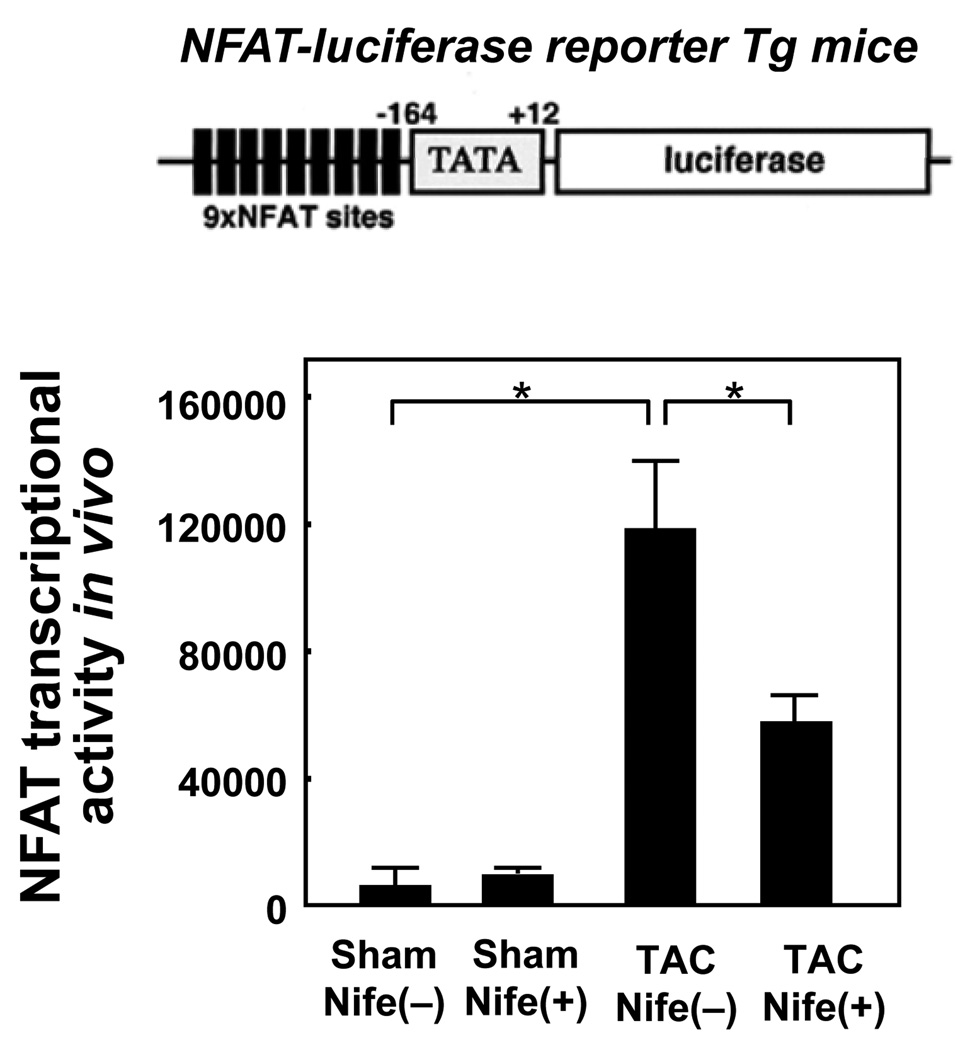
NFAT is a nuclear transcription factor which mediates many forms of pathological cardiac hypertrophy. Transcriptional activity of NFAT in the heart in vivo was evaluated using transgenic mice harboring a luciferase reporter gene driven by multimerized NFAT responsive elements (NFAT-Luc mice). TAC significantly stimulated transcription through the NFAT responsive elements, suggesting that NFAT is upregulated in the nucleus. Although nifedipine did not significantly affect the NFAT-Luc activity in sham operated mice, it significantly reduced TAC-induced increases in NFAT-Luc activity (Fig. 5). These results suggest that nifedipine significantly attenuates TAC-induced activation of NFAT in the heart.
Figure 5. Nifedipine inhibits TAC induced NFAT transcriptional activity.
NFAT-Luciferase reporter mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. (upper) A cartoon showing the NFAT-luciferase reporter construct used in transgenic mice. (lower) The luciferase activity in heart homogenates from NFAT-luciferase transgenic mice was measured and normalized by the protein content. Values are mean ± SEM. n=6 in each group. *p<0.05.
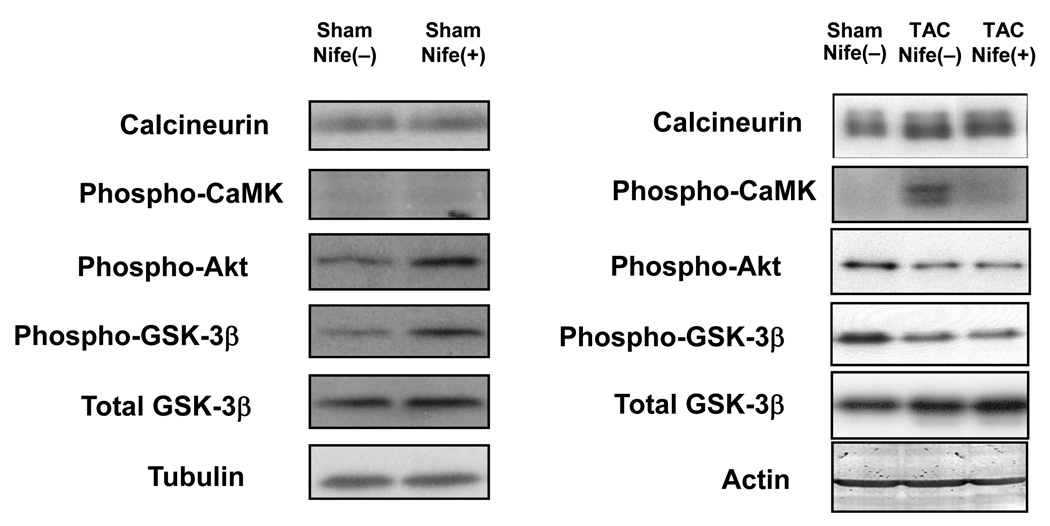
Subcellular localization of NFAT is regulated by phosphorylation. NFAT is localized in the nucleus when it is dephosphorylated by calcineurin, a positive mediator of cardiac hypertrophy, but is exported to the cytosol when it is phosphorylated by GSK-3β [10], a negative regulator of cardiac hypertrophy. In addition, NFAT-mediated transcription is inhibited in the presence of class II histone deacetylases in the nucleus [11]. Nucleo-cytoplasmic shuttling of class II HDACs is regulated by phosphorylation of their serine residues by HDAC kinases. One such kinase, CaMKII, is regulated by Ca2+-dependent mechanisms. In order to examine the mechanism by which nifedipine inhibits TAC-induced activation of NFAT, we evaluated the effect of nifedipine upon the activities of the aforementioned regulators of NFAT, including calcineurin, CaMKII, and GSK-3β. TAC slightly upregulated calcineurin and significantly increased phosphorylation of CaMKII in the heart. TAC decreased phosphorylation of GSK-3β and Akt, a serine/threonine kinase known to phosphorylate GSK-3β (Fig. 6). Since nuclear localization of NFAT is stimulated by upregulation/activation of calcineurin and CaMKII or phosphorylation of GSK-3β, our results suggest that a slight upregulation of calcineurin or activation of CaMKII may mediate TAC-induced activation of NFAT. Although nifedipine treatment did not affect TAC-induced upregulation of calcineurin or TAC-induced decreases in GSK-3β phosphorylation, it significantly reduced TAC-induced increases in CaMKII phosphorylation (Fig. 6). Nifedipine did not significantly affect expression of calcineurin or phosphorylation of CaMKII, Akt, and GSK-3β at baseline.
Figure 6. Nifedipine attenuates TAC-induced upregulation of calcium-related cardiac genes.
Mice were subjected to either sham or transverse aortic constriction (TAC) operation in the presence of continuous infusion of nifedipine (10mg/kg/day) or vehicle by osmotic pump for two weeks. Heart homogenates were prepared for immunoblot analyses. Immunoblots shown are representative of 4 experiments.
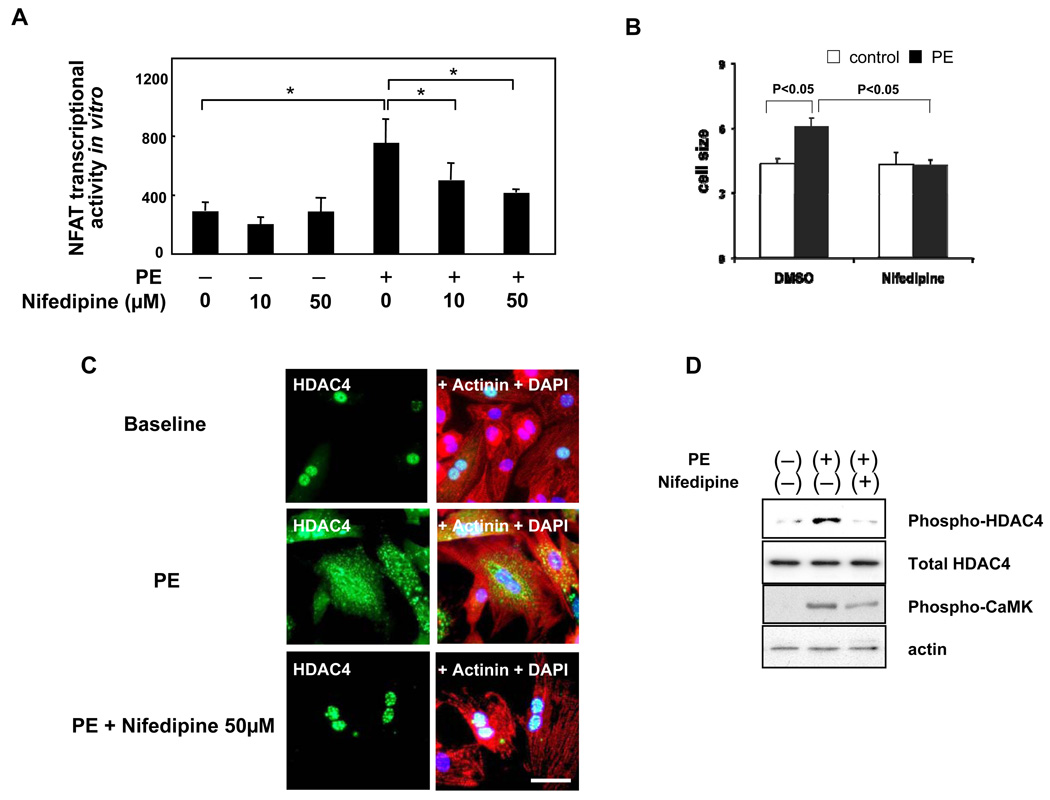
In order to evaluate whether nifedipine inhibits activation of NFAT by hypertrophic stimuli in cardiac myocytes, we examined the effect of nifedipine upon NFAT activation by phenylephrine, an agonist for the alpha1-adrenergic receptor, in cultured cardiac myocytes. As expected, phenylephrine treatment significantly increased NFAT activity in cardiac myocytes, as determined by NFAT-Luc reporter gene assays (Fig. 7A). Although nifedipine did not significantly affect NFAT-Luc activity at baseline, nifedipine dose-dependently inhibited phenylephrine-induced activation of NFAT-Luc activity (Fig. 7A). We confirmed that nifedipine significantly inhibits phenylephrine-induced increases in cardiac myocyte cell size (Fig. 7B). Since nifedipine inhibited activation of CaMKII, without affecting expression of calcineurin or phosphorylation of GSK-3β, in response to TAC in vivo, nifedipine may affect nucleo-cytoplasmic shuttling of class II HDACs. In cultured cardiac myocytes, phenylephrine induced translocation of HDAC4 from the nucleus to the cytosol, as determined by immunostaining of HDAC4. This nucleo-cytoplasmic translocation of HDAC4 by phenylephrine was inhibited in the presence of nifedipine in cardiac myocytes (Fig. 7C). Consistent with the results of immunostaining, immunoblot analyses showed that phenylephrine-induced increases in phosphorylation of HDAC4 (Ser 632) were inhibited in the presence of nifedipine. Nifedipine treatment also attenuated phenylephrine-induced increases in CaMKII phosphorylation (Thr 286) (Fig. 7D). These results indicate that nifedipine inhibits phosphorylation of CaMKII, phosphorylation and nucleo-cytoplasmic shuttling of HDAC4, and activation of NFAT in response to phenylephrine in cardiac myocytes in a cell autonomous fashion.
Figure 7. Nifedipine attenuates phenylephrine-induced activation of NFAT transcription, nuclear exit of HDAC4, and Ser 632 phosphorylation of HDAC4 in cultured cardiac myocytes.
(A) Cultured neonatal rat cardiac myocytes were subjected to transient transfection with NFAT-Luc reporter gene. Twenty-four hours after transfection, cardiac myocytes were treated with phenylephrine (PE) and/or nifedipine for 36–48 hours and harvested for the NFAT luciferase activity measurement. n=9 in each group. *p<0.05. (B) Myocytes were treated with phenylephrine in the presence or absence of nifedipine. After forty-eight hours, cell surface area was evaluated as described in the Method section. n=4 in each group. (C,D) Cultured cardiac myocytes were treated with PE in the presence or absence of nifedipine for 4 hours either for either immunostaining using anti-HDAC4 antibody (green), anti-α-actinin (red) and DAPI (blue) (C) or for immunoblotting with antibodies as indicated (D). The results are representative of 4 experiments. Bar = 50 µm.
Discussion
Our results suggest that nifedipine inhibits cardiac hypertrophy and LV dysfunction in response to pressure overload in mice in vivo. Furthermore, nifedipine inhibits the final common pathway mediating pathological hypertrophy, namely the nuclear export of class II HDACs and subsequent activation of NFAT, in part through inhibition of CaMKII, an HDAC kinase.
Previous studies have shown that nifedipine treatment in SHR rats suppresses cardiac hypertrophy induced by hypertension, accompanied by a significant reduction in blood pressure. Since mechanical loading is a major mechanism promoting hypertrophy, it has been difficult to determine whether or not nifedipine has direct anti-hypertrophic actions in vivo. Since the dose of nifedipine used in this study did not significantly affect MAP or the pressure gradient across the TAC, the beneficial effect of nifedipine against hypertrophy is likely to be blood pressure-independent. The fact that nifedipine suppresses phenylephrine-induced activation of NFAT and CaMKII in cultured cardiac myocytes indicates that nifedipine acts cell autonomously to inhibit “the final common pathway” of cardiac hypertrophy. Taken together, our results suggest that nifedipine may be able to inhibit cardiac hypertrophy by acting directly on cardiac myocytes in the heart.
Although insufficient hypertrophy in the presence of pressure overload could induce systolic dysfunction [12], modification of some signaling mechanisms allows the heart to maintain or even exhibit better function against increased afterload with less hypertrophy [13]. Our results suggest that nifedipine not only inhibits cardiac hypertrophy but also attenuates the progression of LV dysfunction in response to pressure overload. Increasing lines of evidence suggest that cardiac hypertrophy with progressive LV dysfunction, termed pathological hypertrophy, and cardiac hypertrophy with well maintained LV function, termed compensated/physiological hypertrophy are mediated through distinct signaling mechanisms [14]. It has been suggested that increased activity of the final common pathway, leading to the nuclear exit of class II HDACs and subsequent transcription through NFAT, positively correlate with the development of pathological hypertrophy [15]. The fact that nifedipine inhibits LV dysfunction during pressure overload, accompanied by suppression of the final common pathway and NFAT, supports the notion that nifedipine inhibits pathological hypertrophy. According to the results of the ACTION trial, a double blinded study, nifedipine was more effective in preventing heart failure with de novo onset than a β adrenergic receptor blocker in patients with stable angina pectoris [4]. Although nifedipine could prevent the development of heart failure through the traditional blood pressure lowering effect, it may prevent LV dysfunction by directly acting on the signaling mechanism mediating pathological hypertrophy and LV dysfunction in cardiac myocytes.
Our results suggest that nifedipine treatment inhibits activation of CaMKII in response to TAC in vivo and phenylephrine in vitro. Since the mouse heart expresses primarily δ and γ isoforms CaMKII [16], judging from the molecular weights of the doublet shown in Fig. 6, we speculate that both δ and γ isoforms are activated by TAC and their activation after TAC is attenuated in the presence of nifedipine. There are two subtypes in the δ isoform, namely CaMKIIδB and CaMKIIδC. CaMKIIδB is primarily localized in the nucleus, whereas CaMKIIδC is in the cytosol. CaMKIIδB phosphorylates HDAC5, a class II HDAC, in the nucleus, thereby inducing nuclear exit of HDAC5. On the other hand, CaMKIIδC phosphorylates HDAC5 in the cytoplasm and prevents HDAC5 from re-entering the nucleus [17]. Thus, suppression of CaMKII activation may in part mediate the suppression of HDAC phosphorylation by nifedipine. In addition, CaMKIIδC affects the progression of heart failure through phosphorylation of Ca2+ regulatory proteins, including ryanodine receptor [18]. The effect of nifedipine upon CaMKIIδB and CaMKIIδC in distinct subcellular compartments and the downstream targets of CaMKII isoforms remains to be elucidated.
It should be noted that other kinases besides CaMKII also phosphorylate class II HDACs. For example, PKD is activated by novel protein kinase C (nPKCs) and phosphorylates HDAC5 [19]. Recent evidence suggests that G protein coupled receptor kinase-5 (GRK5), which is localized in the nucleus, phosphorylates HDAC5 and promotes cardiac hypertrophy [20]. Other kinases, including PKCδ, Mark2 and SIK1, also phosphorylate class II HDACs [21,22]. The effect of nifedipine upon these kinases remains to be elucidated.
CaMKII is activated by increases in intracellular Ca2+ concentration. Increases in intracellular Ca2+ concentration, which could lead to cardiac hypertrophy, may come from multiple sources, including L type Ca2+ channels coupled with Ca2+-induced Ca2+ release from the ryanodine receptors, T type Ca2+ channels, transient receptor potential channels, and IP3 receptor channels [23]. Although nifedipine is known as a classical L type Ca2+ channel blocker, it could affect other signaling molecules, including these intracellular regulators of Ca2+ or even CaMKII or NFAT themselves, to reduce pathological hypertrophy.
We have recently shown that HDAC4 is subjected to redox modification through an intramolecular disulfide bond formation at Cys-667 and Cys-669 [24]. Hypertrophic stimuli induce HDAC4 oxidation, which triggers nucleo-cytoplasmic translocation of HDAC4 without affecting its deacetylase activity. The cysteine residues subjected to oxidation are conserved in all class II HDACs. Thus, the class II HDACs are regulated not only by phosphorylation but also by oxidation. Furthermore, CaMKII can be directly activated by ROS through methionine oxidation [25], independently of Ca2+/calmodulin. Increasing lines of evidence suggest that oxidative stress directly and independently regulates the final common pathway leading to pathological hypertrophy. This issue is relevant to this study because nifedipine reduces oxidative stress in some cell types [9]. The cysteine residues in HDAC4 oxidized by hypertrophic stimuli are reduced by thioredoxin1 (Trx1), a 12 kD anti-oxidant [24]. CaMKII is reduced by methionine sulfoxide reductase A [25], whose redox status is regulated by Trx1. Thus, testing whether or not nifedipine positively affects either expression or reduction of Trx1 would be of great interest.
In summary, our results suggest that nifedipine has the potential to attenuate the development of cardiac hypertrophy and LV dysfunction by acting directly on the signaling mechanisms in cardiac myocytes. Since the final common pathway, involving regulation of class II HDACs and NFAT, is utilized by many forms of pathological hypertrophy, we speculate that nifedipine may be useful for the prevention of heart failure in patients with cardiac hypertrophy with various etiologies.
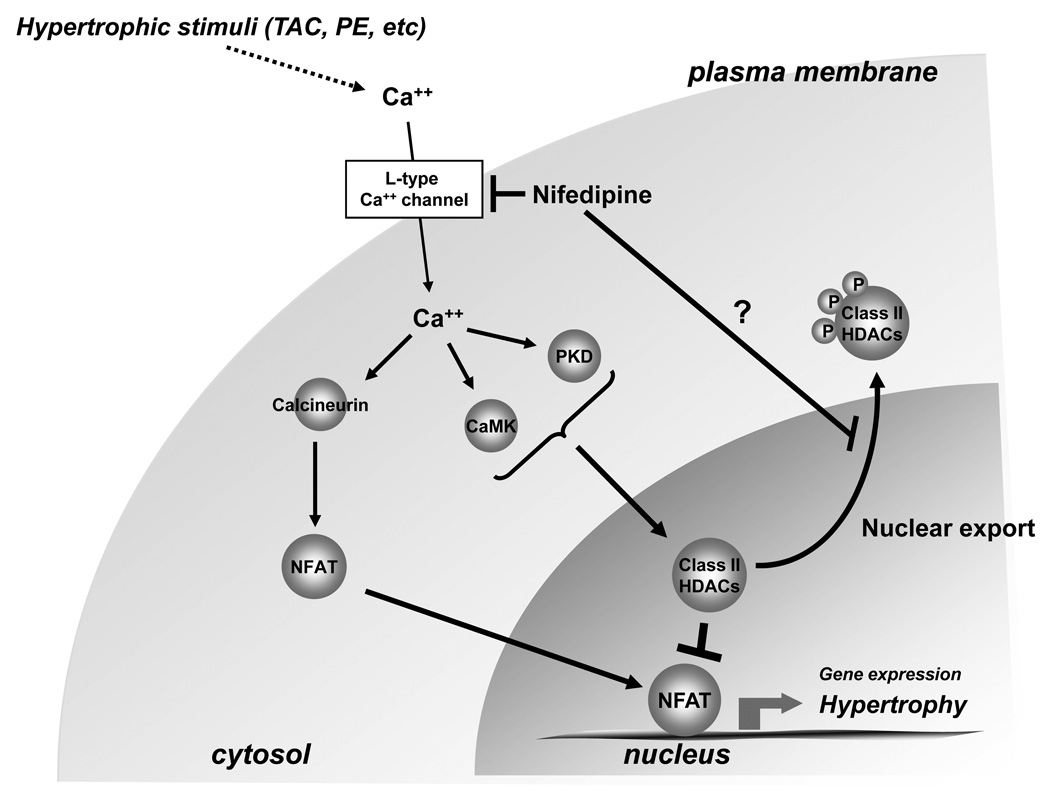
Figure 8. Nifedipine inhibits cardiac hypertrophy.
A cartoon demonstrating the pathways by which nifedipine inhibits cardiac hypertrophy. The current hypothesis is that nifedipine inhibits activation of Ca2+/calmodulin kinase (CaMK), thereby preventing nuclear exit of class II HDACs. Since recent evidence suggests that nucleo-cytoplasmic shuttling of class II HDACs is also regulated by other mechanisms, including oxidation of cysteine residues, it is possible that nifedipine induces nuclear localization of class II HDACs through unknown mechanisms.
Acknowledgement
We thank Daniela Zablocki for critical reading of the manuscript. We also thank Dr. Jeffery D Molkentin for NFAT-Luc mice.
Sources of Funding
This work was suported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL73048, HL91469, AG 23039, AG27211, and HL102738.
Footnotes
Disclosures
None
References
- 1.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98(1):15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 2.Strauer BE, Atef Mahmoud M, Bayer F, Bohn I, Motz U. Reversal of left ventricular hypertrophy and improvement of cardiac function in man by nifedipine. Eur Heart J. 1984;5 Suppl F:53–60. doi: 10.1093/eurheartj/5.suppl_f.53. [DOI] [PubMed] [Google Scholar]
- 3.Zou Y, Yamazaki T, Nakagawa K, Yamada H, Iriguchi N, Toko H, et al. Continuous blockade of L-type Ca2+ channels suppresses activation of calcineurin and development of cardiac hypertrophy in spontaneously hypertensive rats. Hypertens Res. 2002;25(1):117–124. doi: 10.1291/hypres.25.117. [DOI] [PubMed] [Google Scholar]
- 4.Lubsen J, Wagener G, Kirwan BA, de Brouwer S, Poole-Wilson PA. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with symptomatic stable angina and hypertension: the ACTION trial. J Hypertens. 2005;23(3):641–648. doi: 10.1097/01.hjh.0000160223.94220.29. [DOI] [PubMed] [Google Scholar]
- 5.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. Embo J. 2003;22(19):5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276(30):28586–28597. doi: 10.1074/jbc.M103166200. [DOI] [PubMed] [Google Scholar]
- 7.Sadoshima J, Montagne O, Wang Q, Yang G, Warden J, Liu J, et al. The MEKK1-JNK pathway plays a protective role in pressure overload but does not mediate cardiac hypertrophy. J Clin Invest. 2002;110(2):271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui Y, Nakano N, Shao D, Gao S, Luo W, Hong C, et al. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103(11):1309–1318. doi: 10.1161/CIRCRESAHA.108.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagishi S, Nakamura K, Matsui T. Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr Med Chem. 2008;15(2):172–177. doi: 10.2174/092986708783330557. [DOI] [PubMed] [Google Scholar]
- 10.Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2001;276(5):3666–3673. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- 11.Dai YS, Xu J, Molkentin JD. The DnaJ-related factor Mrj interacts with nuclear factor of activated T cells c3 and mediates transcriptional repression through class II histone deacetylase recruitment. Mol Cell Biol. 2005;25(22):9936–9948. doi: 10.1128/MCB.25.22.9936-9948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morisco C, Sadoshima J, Trimarco B, Arora R, Vatner DE, Vatner SF. Is treating cardiac hypertrophy salutary or detrimental: the two faces of Janus. Am J Physiol Heart Circ Physiol. 2003;284(4):H1043–H1047. doi: 10.1152/ajpheart.00990.2002. [DOI] [PubMed] [Google Scholar]
- 13.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105(1):85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 14.Diwan A, Dorn GW., 2nd Decompensation of cardiac hypertrophy: cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94(1):110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63(3):476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, et al. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282(48):35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92(8):912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 19.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24(19):8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martini JS, Raake P, Vinge LE, DeGeorge B, Jr, Chuprun JK, Harris DM, et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105(34):12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berdeaux R, Goebel N, Banaszynski L, Takemori H, Wandless T, Shelton GD, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13(5):597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 22.Chang S, Bezprozvannaya S, Li S, Olson EN. An expression screen reveals modulators of class II histone deacetylase phosphorylation. Proc Natl Acad Sci U S A. 2005;102(23):8120–8125. doi: 10.1073/pnas.0503275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116(3):623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133(6):978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 25.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]