Abstract
Purpose
While previous evidence has shown increased likelihood for survival in cancer patients who have social support, little is known about changes in social support during illness and their impact on survival. This study examines the relationship between social support and survival among women diagnosed with breast carcinoma, specifically assessing the effect of network size and changes in social contact post-diagnosis.
Methods
A population-based sample of 584 women was followed for up to 12.5 years (median follow-up =10.3 years). The mean age at diagnosis was 44 years, 81% were married, and 29% were racial/ethnic minorities. Cox regression analysis was used to estimate survival as a function of social support (changes in social contact and the size of social support), disease severity, treatment, health status, and socio-demographic factors.
Results
Fifty-four-percent of the women had local and 44% had regional stage disease. About 53% underwent mastectomy, 68% received chemotherapy, and 55% had radiation. Regression results showed that disease stage, estrogen receptor status, and mastectomy were associated with greater risk of dying. Although network size was not related to survival, increased contact with friends/family post-diagnosis was associated with lower risk of death, with a hazard ratio of 0.31 (95% CI, 0.17-0.57).
Conclusion
Findings from this study have identified an important aspect of a woman’s social network that impacts survival. An increase in the amount of social contact, representing greater social support, may increase the likelihood of the women’s survival by enhancing their coping skills, providing emotional support, and expanding opportunities for information-sharing.
Keywords: Breast cancer, oncology, social support, social network, survivorship
BACKGROUND
Over the past decade, social epidemiologists have become increasingly aware of associations between psychosocial factors and cancer survival in addition to that between physical health status and survival. In their Alameda County study, Berkman and Syme (1979) first reported that one’s social network was predictive of mortality [1]. Reynolds et al. (1994) found that social network size and sources of emotional support were related to breast cancer survival [2]. In the 1980s, a group of researchers looked at the effect of marital status on breast cancer survival and found that being married had a protective effect [3-5]. Their work follows from Emile Durheim’s (1951) seminal work in which he considers the importance of family and work roles as regulative influences integrating the individual into society [6]. In the 1990s, researchers began to evaluate characteristics of one’s social network, using the concept of social support [7-10].
Social support is defined generally as information, advice, or tangible aid provided through contact with one’s social network that has beneficial effects on the recipient [11, 12]. To further dissect the concept of social support, a synthesis of current literature renders the consensus that there are at least two different aspects of the social support system that are conceptually distinct: Structural and functional support [13, 14]. Structural support consists of a network of relationships that exist between people, such friends and relatives, co-workers, etc. that bind an individual to her community. Participating in a system of mutual obligations and reciprocal relationships with individuals who share common interest and concerns is the essence of a social network [14-18]. Functional support includes but is not limited to information, tangible, and emotional support [14, 19-21]. Informational support refers to the provision of knowledge relevant to the individual’s experience. Specific to cancer patients, helpful information may include getting a reference of an oncology specialist, low-cost medical care, or access to the tumor board [14, 20]. Instrumental or tangible support is consisted of resources the individual may receive from her network. Examples of instrumental support include a loan, transportation to medical visits, child care, etc. Emotional support refers to the individual’s feeling or perception that she is loved, esteemed, valued and cared for regardless of achievement [14, 20-24]. In many incidences, the perception of available emotional or instrumental support is more important than their actual utility [13, 25]. In fact, the actual use of instrumental support tends to play a greater role among those who are in poorer physical health [14]. In terms of age, social support may play a larger role for younger breast cancer patients as the burden of disease may be greater for younger women than for their older counterparts. The treatment is often more aggressive, which heightens their needs for both emotional and instrumental support. Moreover, younger breast cancer patients are in the midst of their productive years, family and work demands are already great in addition to their illness, thus making the needs for emotional support even more critical [14].
Social support is thought to maintain or sustain the individual by promoting biologic or behavioral adaptation in the face of stress and threats to health [26-28]. Specifically, meta analyses by Piquart et al. (2009) and Falagas et al. (2007) as well as studies by Waxler-Morrison et al. (1991) and Ell et al. (1992) examined the amount of social support, defined by network size, frequency of contact with family and friends, and adequacy of emotional support received by cancer patients [9, 10, 18, 29]. They reported significant impact of these factors on survival. Maunsell et al. (1995) took a step further and captured the effect on survival of the types of confidant the woman had within her social network and the manner in which the confidant was utilized [12]. However, literature on the relationship between psychosocial factors and survival has largely concentrated on methods of coping with the initial illness in lieu of an assessment of general emotional health and how social support buffers the individual from environmental threats [13].
To date, the literature documenting the impact of social support on the survival of younger women with localized and regional breast cancer has been limited. Many of the studies reported mixed findings, making relationships between psychosocial factors and survival difficult to ascertain [3, 5, 12, 16, 18, 30-32]. Some studies have methodological limitations, in particular a lack of multivariate analytic methods, and measures of psychosocial characteristics have been relatively poor [12]. There is also little information about the way social support changes during the course of the woman’s illness and how these changes impact survival.
The main objective of this study is to examine the relationship between social support, in both social network and contact, and the overall survival following a breast cancer diagnosis in a cohort of women aged 50 or younger. Specifically, this study assessed how social support affected long-term survival, when disease severity, treatment, sociodemographic factors, and health status were taken into account. More importantly, this study focused on the structural and emotional aspects of social support by examining the effect of changes in social contact on survival in the early months after diagnosis, capturing a snapshot at a point of time that may be the most vulnerable for breast cancer patients.
METHODS
Sample
The initial population-based cohort consisted of 723 women who were diagnosed with breast cancer at age 50 years or younger in the Greater San Francisco Bay Area between 1994 and 1997. All were participants in a two-phase project, Breast Cancer in Younger Women, aimed to develop and test a psycho-educational intervention tailored to the needs of younger women in a multi-ethnic population in the early months after diagnosis, funded by the National Cancer Institute. Participants in the first phase completed an in-person interview designed to identify areas of concern. Based on these results, a ten-session psycho-educational support group intervention was designed and implemented. The sessions included topics on resources, employment, body image, diet and nutrition, social functioning, future planning, etc. Participants recruited for the second phase were randomized into the intervention or control group and were asked to complete three surveys: an in-person interview at baseline and telephone interviews at three-month and six-month post baseline interview. We observed no differences in survival rates by the phase of enrollment (p=0.49) and surgical intervention (p=0.22).
All subjects were identified via Rapid Case Ascertainment (RCA) by the Greater Bay Area Cancer Registry, which is a part of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program and the California Cancer Registry. RCA is employed when patient contact is needed more quickly post diagnosis than is possible through the usual SEER reporting time frame. The RCA system identifies eligible cases via histologically confirmed tumors through searches of the pathology files in surveyed institutions. Rapid case finding for this study was conducted once every one or two weeks at the area’s larger hospitals and once a month at the smaller facilities. RCA abstracts were then forwarded to the study coordinator, who in turn contacted treating physicians and then the women were invited to participate. The surveys were administered at two to seven months post diagnosis.
In Phase One, of 700 cases identified through RCA, 137 were ineligible, 51 could not be located, and 88 were not reached by the study deadline. Of the 424 eligible women contacted, 61 (14%) refused, 31 (7%) had scheduling problems, and 332 (78%) were interviewed. In Phase Two, of 1659 cases identified through RCA, 192 were found to be ineligible before screening, 133 could not be located, and 255 were not reached by the study deadline. Of the 1,079 potentially eligible women contacted, 91 (8%) refused, 43 (4%) had scheduling problems, and 945 (88%) completed the screener interview. Among those screened, 238 were ineligible for the study and four could not be reached. Of the 703 eligible women contacted, 288 (41%) refused, 24 (3%) had scheduling problems, and 391 (56%) were interviewed. The recruiting efforts during Phase Two yielded a lower response rate compared to the response rate from Phase One. This difference may be attributed to the fact that women recruited for Phase Two were obligated to three waves of data collection, which was a larger time commitment, versus one-time participation requested of those participated in Phase One [7]. We found no difference between participants and non-participants in Phase Two in terms of age at diagnosis (p=0.28), race (p=0.95), and education (p=0.95).
This study included 584 of 719 possible study participants for whom we had data at the end of 2006. For this analysis, we excluded those who were in at least one of the following categories: (1) had a diagnosis of carcinoma in-situ as none had died within the time under examination; (2) had unknown stage disease; or (3) did not receive surgery. Our post-hoc power analyses indicated sufficient power for our sample size. Date of diagnosis, age at diagnosis, and disease and treatment data were obtained from the cancer registry; additional demographic information, self-reported health status, and social characteristics were obtained from in-person interviews conducted within seven months of diagnosis by the Northern California Cancer Center.
Measures
Four types of measures were used in this survival analysis: (1) socio-demographic characteristics; (2) disease and treatment characteristics; (3) health status; and (4) social support. Demographic characteristics include age at diagnosis, as reported to the cancer registry, and self-reported ethnicity, education, marital status, and employment status. Other than disease and treatment information, which was obtained from the SEER Cancer Registry, all measures used in the analysis were information collected at baseline interviews following diagnosis. Age was entered in the model as a linear term, with marital status (married/partner vs. single), employment status (full-/part-time vs. none), and education (high school or less vs. some college or more) entered as binary variables. The ethnic groups were categorized as African American, Latina, Asian, with White serving as the reference group.
Disease and treatment characteristics included stage of disease, estrogen receptor status (ERS), and type of surgical and adjuvant treatment received. Consistent with the tumor registry, stages of disease were categorized as local, regional, and remote. ERS was entered in the model as a binary variable (negative vs. positive/unknown). Positive and unknown ERS were combined because survival results for both groups are the same. Treatment variables included in the analysis were surgery (mastectomy vs. breast conserving surgery) and receipt in the first course of treatment of each type of adjuvant therapy--chemotherapy, radiation therapy, and hormone therapy (Tamoxifen). There is good agreement between treatment recorded in the cancer registry and the reported treatment plan.
Baseline health status was measured using two scales from the Medical Outcomes Study (MOS SF-36) scale [33, 34]. General health is a scale measuring the personal evaluation of health, including five items that assess current health, health outlook, and resistance to illness (reliability=0.81). The mental health measure is a four-item scale measuring general mental health, including depression, anxiety, behavioral-emotional control, and general positive affect (Reliability=0.84) [33, 34]. These variables were entered as linear terms in the model.
We explored the effects of social support by considering both the amount of social contact, and quality of such contacts. Social contact factors include the number of contacts and changes in social contact with relatives and friends since diagnosis. Social contact was measured using a sub-scale from the Berkman-Syme Social Network Index (SNI) [1, 15, 35]. Specifically, it is an ordinal variable that determines sociability as quantified by contacts with extended family and close friends. The number of close friends and relatives one sees at least once a month was entered in the model as a linear term, coded as follows: 1, No friends or relatives; 2, 1 or 2; 3, 3 to 5; 4, 6 to 9; and 5, 10 or more friends and relatives. Although we used one of the SNI subscales, the index has been well validated, and found useful in predicting utilization of screening services, morbidity and mortality in community samples [36]. This scale was selected for its brevity and consistent cultural meaning [1, 15, 35]. In addition, the women surveyed were asked whether the number of close friends and relatives seen at least once a month was more, fewer, or the same since diagnosis. This item was included as a categorical variable in the model. The quality of social support is examined via the emotional support scale. The emotional support scale contains items measuring the perceived availability of caring individuals with whom one can share thoughts and feelings. The scale consists of 11 items, with a Cronbach’s alpha of 0.92 [37-39]. Principal components analysis found that all of the items loaded on one factor, indicating its unidimensionality [14]. This variable was entered into the model as a linear term.
The dependent variable, survival, is the survival status of study participants at the end of year 2006. Survival Status was extracted from the SEER Cancer Registry.
Analysis
Associations between various social support indicators and hazard rates were estimated using the Cox proportional hazards regression model [40]. The regression analysis was conducted to calculate risk ratios and 95% confidence intervals (CIs) for social support variables, as well as for modifying factors such as demographic characteristics, disease severity, treatment, and baseline physical and mental health status, controlling for the phase of enrollment and time since diagnosis. The risk ratio represents the instantaneous risk of death among women who had exposure to the factors of interest (e.g., social support, type of treatment) during the follow-up period versus that of women in the reference group who had no exposure to the factors. All statistical analyses were conducted using SAS v.9.1 [SAS Institute, Cary, NC].
RESULTS
Table 1 presents the sample characteristics of the study population. The mean age at diagnosis was 44 years. Over 80% of the women were married or partnered, and 30% identified themselves as racial/ethnic minorities, including 7% African American, 9% Latina, and 14% Asian Americans. Eighty-two-percent of the women received some education post high school.
Table 1.
Sample Characteristics of Young Women with Breast Cancer
| Young Women with Invasive Breast Cancer (n=584) | ||||
|---|---|---|---|---|
| n | % | Mean (SD) | ||
| Demographics | ||||
| Age at Diagnosis | 43.9 (5.3) | |||
| Race | White | 413 | 71 | |
| African American | 42 | 7 | ||
| Latina | 50 | 9 | ||
| Asian/Pacific Islander | 79 | 14 | ||
| Education | High School or Less | 108 | 18 | |
| Some College or more | 476 | 82 | ||
| Marital Status | Married/Stable Partner | 475 | 81 | |
| Single | 109 | 19 | ||
| Employment | Yes | 451 | 77 | |
| None | 132 | 23 | ||
| Disease and Treatment Status | ||||
| Stage of Disease | Localized | 317 | 54 | |
| Regional | 258 | 44 | ||
| Remote | 9 | 2 | ||
| Estrogen Receptor Status | Negative | 151 | 26 | |
| Positive | 368 | 63 | ||
| Unknown | 65 | 11 | ||
| Surgery | Mastectomy | 311 | 53 | |
| Lumpectomy | 273 | 47 | ||
| Adjuvant Therapy | Chemotherapy | 390 | 68 | |
| Radiation | 320 | 55 | ||
| Hormone Therapy | 169 | 29 | ||
| Months since diagnosis | 111.5 (35.8) | |||
| Duration of follow-up (years) | 9.3 (3.0) | |||
| Health and Survival Status | ||||
| Baseline General Health | 71.6 (21.0) | |||
| Baseline Mental Health | 70.8 (18.2) | |||
| Alive at end of 2006 | Yes | 449 | 77 | |
| No | 135 | 23 | ||
| Social Support Characteristics | ||||
| Number of Relatives/Friends Seen at Least Once a month | More than before diagnosis | 108 | 18 | |
| Fewer than before diagnosis | 49 | 8 | ||
| Same as always | 427 | 73 | ||
| Frequency of contact with relatives and friends per month | None | 6 | 1 | |
| 1 or 2 | 42 | 7 | ||
| 3 to 5 | 171 | 29 | ||
| 6 to 9 | 116 | 20 | ||
| 10 or more | 249 | 43 | ||
| Emotional Support | 40.9 (4.3) | |||
The disease and treatment status of the sample indicated that 54% of the women had local stage disease and 44% had regional stage disease. Sixty-three percent of the women had positive ERS while 26% were ERS negative. In our sample, more than half (53%) of the women had a mastectomy, two-thirds received chemotherapy, 55% had radiation therapy, and 29% were prescribed Tamoxifen as a part of the first course of treatment. Tamoxifen use is known to have been under-reported by the registry because most women do not receive it until after first course of treatment.
With respect to social support, we examined the amount of social contact the women had with relatives and friends. Eighteen percent of the women indicated that they had contact with more relatives and friends since their breast cancer diagnosis while 8% reported decreased contact. About half of the women reported seeing three to nine while another 43% reported seeing 10 or more friends and relatives at least once a month. As of December 31, 2006, 23% of the women had died during the follow-up period of up to 9.3 years post diagnosis. The median follow up time for the women in the sample was 10.3 years, ranging from 6 months to 12.5 years.
The Cox regression results (Table 2) demonstrated that one of the indicators of social network contacts was significantly associated with survival among younger women with breast cancer. While the size of social network did not make a difference, women who reported increased contact with their social support network post diagnosis had better survival rates, with a hazard ratio of 0.31 (95% CI, 0.17-0.57) at up to 12.5 years, compared to those who maintained the same level of contact with relatives and friends. There was no difference in risk for mortality between women who maintained the same level and those who decreased their social contact. Figure 1 illustrates the survival curves as they vary by the amount of social contact. The level of emotional support was also associated with improved survival, with a hazard ratio of 0.96 (95% CI, 0.92-1.00) per unit increase in the emotional support scale.
Table 2.
Predictors of Risk of Mortality
| Proportional Hazards Regression Results Predictors of Survival as of 12/31/2006 (n=584) | |||
|---|---|---|---|
| Risk Ratio | 95% Confidence Interval | ||
| Age at Diagnosis | Per Year | 0.98 | (0.95, 1.01) |
| Race/Ethnicity | African American | 1.48 | (0.80, 2.80) |
| Latina | 1.06 | (0.56, 2.03) | |
| Asian | 0.86 | (0.50, 1.48) | |
| White | 1.00 | ||
| Education | High School or Less | 1.53 | (0.90 2.59) |
| Some College or More | 1.00 | ||
| Marital Status | Married/Partner | 1.04 | (0.65, 1.66) |
| Single | 1.00 | ||
| Employment | None | 1.21 | (0.81, 1.82) |
| Full-/Part-time | 1.00 | ||
| Stage of Disease | Remote | 17.57*** | (7.37, 41.90) |
| Regional | 2.05** | (1.32, 3.18) | |
| Local | 1.00 | ||
| Estrogen Receptor | Negative | 1.75** | (1.18, 2.61) |
| Status | Unknown | 1.50 | (0.84, 2.67) |
| Positive | 1.00 | ||
| Surgery | Mastectomy | 2.24** | (1.41, 3.56) |
| Lumpectomy | 1.00 | ||
| Chemotherapy | Yes | 1.52 | (0.89, 2.59) |
| No | 1.00 | ||
| Radiation Therapy | Yes | 1.50 | (0.98, 2.29) |
| No | 1.00 | ||
| Tamoxifen | No | 1.11 | (0.74, 1.67) |
| Yes | 1.00 | ||
| General Health | Per unit | 0.99* | (0.98, 1.00) |
| Mental Health | Per unit | 1.00 | (0.99, 1.02) |
| Emotional Support | Per unit | 0.96* | (0.92, 1.00) |
| Relatives/Friends† | Per unit | 1.12 | (0.93, 1.36) |
| Relatives/Friends | More | 0.31*** | (0.17, 0.57) |
| Seen Since | Fewer | 0.90 | (0.48, 1.68) |
| Diagnosis | Same | 1.00 | |
Number sees at least once a month: 1= None; 2= 1 or 2; 3= 3 to 5; 4= 6 to 9; 5= 10 or more
p<0.0001
p<0.01
p<0.05
Figure 1.
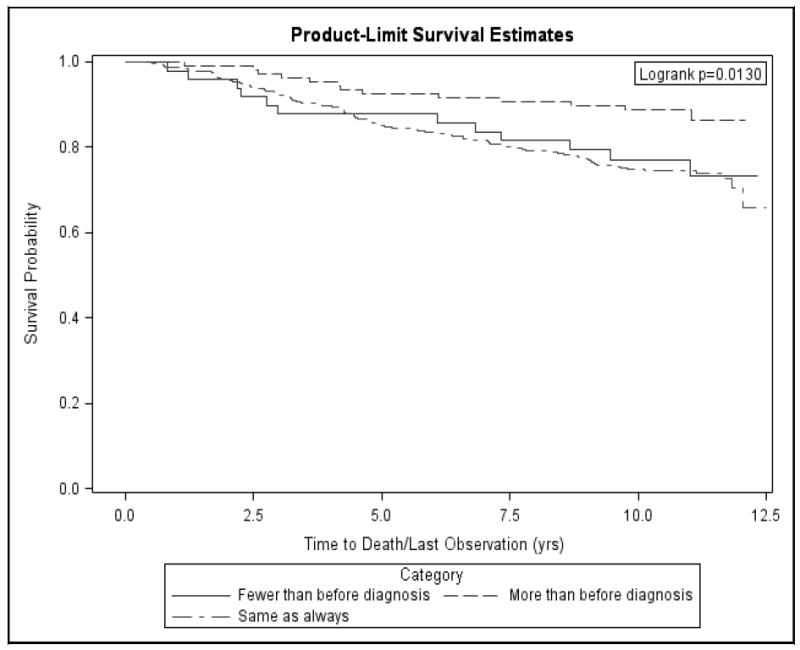
Survival curve of young women by the amount of social contact
These regression results also indicated that disease and health status significantly affected survival, given the type of treatment received. Compared to those with localized disease, women who were initially diagnosed with regional or remote stage disease had a 2.05- and 17.57- fold greater risk of mortality, respectively. Among women who had negative ERS, a 1.77-fold increase in risk of mortality was observed compared to those with positive ERS. Furthermore, women with better general health at diagnosis had better survival rates. Having had a mastectomy was associated with a lower likelihood for survival, with a hazard ratio of 2.24 (95% CI, 1.41-3.56). Demographic factors, such as age, marital status, or ethnicity, and mental health status were not associated with survival in our sample.
DISCUSSION
Findings from this study have identified an important aspect of a woman’s social network that may impact survival. Although our study did not demonstrate an association between survival and size of the social support network, the reported change in social contact with one’s network and level of emotional support were significantly related to survival. While we did not collect information on the way in which social support expanded and the type of new contacts that women made in increasing their social exchange, we do know that an increase in contact with a network of relationships tend to engender greater support [14]. There are several plausible explanations for how changes in social contact affected survival. With the diagnosis and treatment of breast cancer, women may experience emotional distress, physical symptomatology, disruption in marital relationships, and fears about pain, disease progression, and mortality [41-45]. One’s social network serves as a key resource for dealing with these reactions to cancer by enhancing the patient’s self-esteem, and increased contact with the network would help alleviate or support the burden of decision-making and problem-solving responsibilities [46].
Greater social exchange and emotional support after a cancer diagnosis may benefit the women’s survival by enhancing their coping skills, providing additional support, and increasing opportunities for obtaining cancer-related information. Being a part of a network of supportive relationships can foster a sense of community necessary for successful coping with the disease and provide opportunities to learn from one another. These networks can range from formal ones such as support groups to an informal type consisting of family, friends, fellow patients with cancer, and medical providers. Taylor and Lobel (1989) have indicated that cancer patients who have proactively sought interactions with other patients have either overcome their illness or adjusted well [47]. This can possibly be attributed to the fact that social contacts help the women manage anxiety, stress, and anger, especially with fellow patients who may be in similar situations, thus enabling women to adopt more positive coping styles. In a theoretical analysis, Folkman (1984) described the relationship between coping and control, and how believing one has control in a stressful transaction can affect adaptational outcomes of such stressful encounters [48]. Reliance on the social network and increased contact may be coping strategies which breast cancer patients pursue, and women who do so may more likely to have lower level of anxiety associated with their illnesses [49], report satisfactory encounter outcomes [50], and show better survival outcomes [51].
Social “connectedness” functions as an informal network where information is exchanged and decisions are supported. For example, a woman can learn about benefits and harms of a certain treatment option through sharing experiences with members of the informal network. The informal network not only facilitates sociability but also strengthens information-processing abilities among women with breast cancer. Women who are well connected also deal more effectively with their physicians, families, friends, and colleagues, and navigate through the crisis successfully by managing negative emotions and obtaining information [14]. These social connections also provide much of the emotional support, where an increased level of emotional support impacted likelihood of survival in our findings. Bloom et al. have found that emotional support was a significant predictor of mental well-being. This may be particularly relevant to our study population as it has been described that the severity of the disease would be greater for younger women and they often require greater emotional support because of their family and work demands [14].
On the other hand, we did not observe a difference between those whose level of social contact remained the same and those who had a decrease in social exchange. This lack of difference has been described in literature where the decline in social relationships has often been reported in individuals with a serious illness. We have learned from the Bloom and Spiegel (1984) study that a woman’s social network is likely to contract when she becomes ill because the illness limits her social functioning and opportunities for social exchange [52]. Two recent prospective studies by Bloom and colleagues reported smaller social network among cancer survivors as time post-treatment increases [7, 53]. Further, the process by which the constriction in the network occurs is explained by Waxler-Morrison and colleagues. The Waxler-Morrison study reported that many women with breast cancer choose to change their lives following a diagnosis of cancer and withdraw from social activities including leaving jobs that they find unappealing or stressful, thereby reducing the size of their networks and actual social contact with others [18]. It is possible that women in our sample recognized or chose to decrease social contact with their network. They may have identified other mechanisms to cope with the disease and therefore, less social support did not significantly affect their survival in comparison to that of those whose social activities remained the same.
The lack of association between survival and network size can also be explained in part by available literature. Schaefer et al. (1981) found that network size can be empirically separable from social support, where it had a weaker relationship to outcomes than did social support [20]. The decrease in network size may be attributed to the fact that relationships and social support often deteriorate significantly following a stressful life event, such as a cancer diagnosis. Individuals may distance themselves from those under stress due to their own inability to cope [54]. In particular to cancer, the literature suggests that social support may change for women who were diagnosed with cancer due to the stigma associated with cancer [55, 56]. Moreover, restrictions are often imposed on one’s activities by the illness itself [36]. In a follow-up interview of this study cohort five years post diagnosis, despite improvement in most physical, mental, and social measures, the women reported a reduction in the size of their social network over time [53]. However, this did not mean that the amount of social support they received was unimportant. Some of these ties may have actually strengthened over time.
What distinguishes the current study from previous analyses is that we examined social support and survivorship, focusing on a population more homogeneous by age (women 50 years of age or younger at diagnosis). While social support has been demonstrated to have a stronger association with survival in younger patients in general, current literature remains limited in examining survivorship specific to younger women with breast cancer [10]. As the five-year survival rate continues to improve, women who are diagnosed with breast cancer at a younger age will have to deal with residual problems and symptoms for a longer period of time. It is important to identify factors that will enhance their survival as well as their quality of life and general health. In addition, this study had a large sample size and utilized population-based sampling. The findings are also generalizable to English-speaking ethnic minority women, including African American, Asian American, and Latina women, who are well represented in the sample (approximately 30%). Furthermore, while the focus of this study was on the effects of social support, it accounted for personal, psychological, and medical aspects of breast cancer and its treatment, giving a fairly complete “picture” of the role of social support for women with breast cancer. The study also introduced a measure that provided further insights into how change in social contact affected survival. Our data lend to the development of a measure of the women’s reintegration into society following the initial diagnosis and treatment for breast cancer: change in the number of social contacts. In addition, our results demonstrated a positive relationship between this measure and survival, and the effect persisted over time.
Finally, this study is not without limitations. First, it is possible that the increase in social contact served as a proxy for other indicators of better health that were not captured in our data. For example, women in better health may have made an effort to reach out to make social contact and may have had fewer limitations to do so [36]. Moreover, women who have completed their treatment may be more likely to engage in activities and therefore contact with their social network. Since we controlled for disease severity and baseline health status in our analyses, the decline in contact with one’s network due to health is less probable. Second, we were not able to evaluate the effect of psycho-educational intervention on survival in our study population as the intervention was only available to women enrolled during Phase Two. It is possible that contents from the intervention would facilitate better coping strategies, which may in turn translate into improved physical or mental health that may affect survival. Third, while we asked the women to assess changes in their social contact compared to that pre-diagnosis, we only interviewed the women after diagnosis and therefore cannot quantify with precision the level of social contact prior to diagnosis. In addition, it would be important to explore the functions of various types of support, such as instrumental support (e.g., information), and tangible support (e.g., a loan of money, a ride to the physicians, child care, etc.) to survival. While there is no specific research indicating a relationship between these types of support and survival, they are important components of social support. In their 1981 study, Schaefer et al. examined the informational, tangible and emotional components of social support [20]. Schaefer et al. detected no relationship between these aspects of social support and physical health, but found that low tangible support and emotional support along with certain life events, were related to depression and negative morale [20].
Future research directions should include a closer examination of the effects of social support from a variety of sources (e.g., health providers) and the development of new measures to explore mechanisms through which social support affects survival. In addition to that from friends, relatives, and partner, social support by providers may prove to be an area that warrants more exploration as the current practice trend in medicine emphasizes more interaction and dialogue between providers and patients. Patients, in general, appreciate information and advice from care providers [12, 21, 57, 58]. Medical personnel may play an important role in providing objective support during the time of diagnosis, treatment and adaptation to cancer as family and friends may be dealing with the same stress as the newly diagnosed patients and therefore lack the capacity to provide support [12]. Understanding the relationship between social support from providers and survival is important as it has implications on physician practice and the psychosocial follow-up for breast cancer patients. However, while women report providers as a major source of support early in the diagnostic treatment sequence, providers may become less central to the women’s support system following the completion of their initial treatment. Neuling and Winefield (1988) noted that lower levels of anxiety and depression were related to satisfaction with support from physicians at one-month post-surgery but support from family members had a more significant effect in reducing anxiety and depression over time [59]. Longitudinal studies will also be helpful in measuring the relative importance of social support along the course of illness and recovery following diagnosis and treatment completion.
Acknowledgments
We are very grateful to all the women who participated in this survey. We would like to acknowledge the expertise and support of Merrilee Morrow, Subo Chang, and Priscilla Banks at the Northern California Cancer Center. This study was approved by Institutional Review Boards at the University of California and Northern California Cancer Center and was supported by NIH Grant 78951 from the National Cancer Institute.
References
- 1.Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds P, Boyd PT, Blacklow RS, et al. The relationship between social ties and survival among black and white breast cancer patients. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1994;3(3):253–259. [PubMed] [Google Scholar]
- 3.Goodwin J, Hunt W, Key C, Samet J. The effect of martial status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3120–3125. [PubMed] [Google Scholar]
- 4.Le Marchand L, Kolonel LN, Nomura A. Relationship of ethnicity and other prognostic factors to breast cancer survival patterns in Hawaii. J Natl Cancer Inst. 1984;73(6):1259–1265. [PubMed] [Google Scholar]
- 5.Funch D, Marshall J. The role of stress, social support and age in survival from breast cancer. J of Pscychosom Res. 1983;27:77–83. doi: 10.1016/0022-3999(83)90112-5. [DOI] [PubMed] [Google Scholar]
- 6.Durheim E. Suicide: A Study in Sociology. Toronto: Free Press; 1951. [Google Scholar]
- 7.Bloom JR, Stewart SL, D’Onofrio C, et al. The effects of a psycho-educational group intervention on the quality of life for young women with breast cancer. In: Patterson JA, Lipschitz IN, editors. Psychological Counseling Research Focus. Nova Science Publishers, Inc.; 2008. pp. 23–45. [Google Scholar]
- 8.Bloom JR, Stewart SL, D’Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change? J Cancer Surviv. 2008;2(3):190–204. doi: 10.1007/s11764-008-0058-x. [DOI] [PubMed] [Google Scholar]
- 9.Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI. The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast Cancer Res. 2007;9(4):R44. doi: 10.1186/bcr1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom JR. Surviving and thriving? Psychooncology. 2002;11(2):89–92. doi: 10.1002/pon.606. [DOI] [PubMed] [Google Scholar]
- 12.Maunsell E, Brisson J, Deschenes L. Social support and survival among women with breast cancer. Cancer. 1995;76(4):631–637. doi: 10.1002/1097-0142(19950815)76:4<631::aid-cncr2820760414>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Thoits PA. Stress, coping, and social support processes: where are we? What next? J Health Soc Behav. 1995:53–79. Spec. [PubMed] [Google Scholar]
- 14.Bloom JR, Stewart SL, Johnston M, Banks P, Fobair P. Sources of support and the physical and mental well-being of young women with breast cancer. Soc Sci Med. 2001;53(11):1513–1524. doi: 10.1016/s0277-9536(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 15.Bloom J. Social support and adjustment to breast cancer. New York: Springer Verlag; 1986. [Google Scholar]
- 16.Spiegel D, Bloom J, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–91. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 17.Cutrona C, Russell D. Social Support: An Interactional View. New York: Wiley; 1990. Type of Social Support and Specific Stress: Toward a Theory of Optimal Matching. [Google Scholar]
- 18.Waxler-Morrison N, Hislop T, Mears B, Kan L. Effects of social relationships on survival for women with breast cancer: A prospective study. Soc Sci Med. 1991;33(2):177–183. doi: 10.1016/0277-9536(91)90178-f. [DOI] [PubMed] [Google Scholar]
- 19.House J, Robbins C, Metzner H. The Association of Social Relationships and Activities with Mortality: Perspective Evidence From the Tecumseh Community Health. Am J Epidemiol. 1982;116:123–140. doi: 10.1093/oxfordjournals.aje.a113387. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer C, Coyne JC, Lazarus RS. The health-related functions of social support. J Behav Med. 1981;4(4):381–406. doi: 10.1007/BF00846149. [DOI] [PubMed] [Google Scholar]
- 21.Wortman CB. Social Support and the cancer patients:conceptual and methodological issues. Cancer. 1984;53(suppl):2339–2362. doi: 10.1002/cncr.1984.53.s10.2339. [DOI] [PubMed] [Google Scholar]
- 22.Cobb S. Presidential Address-1976. Social support as a moderator of life stress. Psychosom Med. 1976;38(5):300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 23.House J. Work, Stress, and Social Support. Reading, PA: Addison-Wesley; 1981. [Google Scholar]
- 24.Bloom J. Social support, accommodation to stress and adjustment to breast cancer. Soc Sci Med. 1982;16:1329–1338. doi: 10.1016/0277-9536(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 25.Seeman TE, Syme SL. Social networks and coronary artery disease: a comparison of the structure and function of social relations as predictors of disease. Psychosom Med. 1987;49(4):341–354. doi: 10.1097/00006842-198707000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb B, Selby P. Social support and mental health: a review of the literature: Review funded by National Health Research and Development Programme, Health and Welfare Canada. 1989 [Google Scholar]
- 27.Cohen M, March J, Olsen J. A garbage can model of organizational choice. New York: Blackwell; 1988. [Google Scholar]
- 28.House J, Landis K. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 29.Ell K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. J Pscychosom Res. 1992;36:531–541. doi: 10.1016/0022-3999(92)90038-4. [DOI] [PubMed] [Google Scholar]
- 30.Michael L, Berkman LF, Graham A, Holmes D, Kawachi I. Social networks and health related quality of life in breast cancer survivors-a prospective study. J of Psychosom Res. 2001;52:285–93. doi: 10.1016/s0022-3999(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 31.Neale AV, Tilley BC, Vernon SW. Marital status, delay in seeking treatment and survival from breast cancer. Soc Sci Med. 1986;23(3):305–12. doi: 10.1016/0277-9536(86)90352-7. [DOI] [PubMed] [Google Scholar]
- 32.Cassileth BR, Lusk EJ, Miller DS, Brown LL, Miller C. Psychosocial correlates of survival in advanced malignant disease. New Eng J Med. 1985;312:1551–1555. doi: 10.1056/NEJM198506133122406. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 34.Ware J. SF-36 Health Survey. Boston: The Health Institute of the New England Medical Center; 1993. [Google Scholar]
- 35.Berkman LF. Social networks, support and health: Taking the next step forward. Am J Epidemiol. 1986;123:559–562. doi: 10.1093/oxfordjournals.aje.a114276. [DOI] [PubMed] [Google Scholar]
- 36.Bloom J, Kessler L. Risk and timing of counseling and support interventions for younger women with breast cancer. J Natl Cancer Inst Monogr. 1994;16:199–206. [PubMed] [Google Scholar]
- 37.Bloom JR, Stewart S, Johnston M, Banks P. Intrusiveness of illness and quality of life in young women with breast cancer. Psychooncology. 1998;7:89–100. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<89::AID-PON293>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.BCSG. Psychological response to mastectomy. A prospective comparison study. Psychological aspects of Breast Cancer Study Group. Cancer. 1987;59(1):189–96. doi: 10.1002/1097-0142(19870101)59:1<189::aid-cncr2820590136>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Flamer DP. Perceived social support scale. San Francisco: West Coast Cancer Foundation; 1977. [Google Scholar]
- 40.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 41.Stead ML. Sexual Dysfunction after treatment for gynaecologic and breast malignancies. Curr Opin Obstet Gynecol. 2003;15(1):57–61. doi: 10.1097/00001703-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Allen SM, Shah AC, Nezu AM, et al. A problem-solving approach to stress reduction among younger women with breast carcinoma: a randomized controlled trial. Cancer. 2002;94(12):3089–3100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- 43.Griffin IS, Fentiman M. Psychosocial problems following a diagnosis of breast cancer. Int J Clin Pract. 2002;56(9):672–675. [PubMed] [Google Scholar]
- 44.Nosarti C, Roberts JV, Crayford T, McKenzie K, David AS. Early psychological adjustment in breast cancer patients: a prospective study. J Psychosom Res. 2002;53(6):1123–1130. doi: 10.1016/s0022-3999(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 45.Rabinowitz B. Psychologic issues, practitioners’ interventions, and the relationship of both to an interdisciplinary breast center team. Surg Oncol Clin N Am. 2000;9(2):347–365. [PubMed] [Google Scholar]
- 46.Rose JH. Social support and cancer: adult patients’ desire for support from family, friends, and health professionals. Am J Community Psychol. 1990;18(3):439–464. doi: 10.1007/BF00938117. [DOI] [PubMed] [Google Scholar]
- 47.Taylor S, Lobel M. Social comparison activity under threat: Downward evaluation and upward contacts. Psychol Rev. 1989;96:569–575. doi: 10.1037/0033-295x.96.4.569. [DOI] [PubMed] [Google Scholar]
- 48.Folkman S. Personal control and stress and coping processes. J Pers Soc Psychol. 1984;46(4):839–852. doi: 10.1037//0022-3514.46.4.839. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro M. Anxiety and patient participation in clinical decision-making: The case of patient with Ureteral Calculi. Soc Sci Med. 1997;45(3):419–427. doi: 10.1016/s0277-9536(96)00357-7. [DOI] [PubMed] [Google Scholar]
- 50.Folkman S, Lazarus R. Stress-processes and depressive symptomatology. J Abnorm Psychol. 1986;95:107–113. doi: 10.1037//0021-843x.95.2.107. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds P, Hurley S, Torres M, Jackson J, Boyd P, Chen V. Use of coping strategies and breast cancer survival: results from the Black/White Cancer Survival Study. Am J Epidemiol. 2000;152:940–949. doi: 10.1093/aje/152.10.940. [DOI] [PubMed] [Google Scholar]
- 52.Bloom J, Spiegel D. Relationship of two dimensions of social support to psychological well-being and social functioning of women with advanced breast cancer. Soc Sci Med. 1984;19:831–837. doi: 10.1016/0277-9536(84)90400-3. [DOI] [PubMed] [Google Scholar]
- 53.Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13(3):147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 54.Turner-Cobb JM, Sephton SE, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62(3):337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Else-Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychology & health. 2009;24(8):949–964. doi: 10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 56.Holland JC, Kelly BJ, Weinberger MI. Why psychosocial care is difficult to integrate into routine cancer care: stigma is the elephant in the room. J Natl Compr Canc Netw. 2010;8(4):362–366. doi: 10.6004/jnccn.2010.0028. [DOI] [PubMed] [Google Scholar]
- 57.Rowland JH. Interpersonal resources :social support. New York: Oxford University Press; 1989. [Google Scholar]
- 58.Peters-Golden H. Breast cancer :varied perceptions of social support in the illness experience. Soc Sci Med. 1982;16:482–491. doi: 10.1016/0277-9536(82)90057-0. [DOI] [PubMed] [Google Scholar]
- 59.Neuling SJ, Winefield HR. Social support and recovery after surgery for breast cancer :frequency and correlates of supportive behaviors by family,friends and surgeon. Soc Sci Med. 1988;27:385–390. doi: 10.1016/0277-9536(88)90273-0. [DOI] [PubMed] [Google Scholar]


