Abstract
RNA is an attractive biopolymer for nanodesign of self-assembling particles for nanobiotechnology and synthetic biology. Here, we experimentally characterize by biochemical and biophysical methods the formation of thermostable and ribonuclease resistant RNA nanorings previously proposed by computational design. High yields of fully programmable nanorings were produced based on several RNAI/IIi kissing complex variants selected for their ability to promote polygon self-assembly. This self-assembly strategy relying on the particular geometry of bended kissing complexes has potential for developing siRNAs delivery agents.
Keywords: RNA architectonics, bionanotechnology, silencing RNA, supramolecular assembly, RNA interaction, RNA motif
Introduction
The current advancement of nanotechnology bears witness to the fact that nucleic acids remain the premiere building block for the programmable construction of objects on the nanometer scale 1–7. RNA is an attractive candidate for nanoparticle design because it offers a number of structural motifs that can be used to generate RNA units that can assemble into complex 1D, 2D or 3D nano-architectures via inter molecular interactions such as loop-loop, loop-receptor interactions or pairings between single stranded overhangs 8–14. RNA’s modularity allows researchers to treat well-defined structural regions as interchangeable parts that can be swapped in and out from a particular molecular context without inducing dramatic perturbations on the structure as a whole 2, 6, 8, 15–17. In this manner, structural motifs like hairpins, junctions, and loops can be isolated, exchanged, and grafted onto one another to build predefined artificial RNA nanostructures 8–13, 16, 18–21. Operating under this concept called RNA architectonics2, it becomes apparent that the discovery and characterization of new motifs or ‘parts’ can lead to new RNA nanostructures with novel geometries and functionalities.
In addition to its potential in the broader field of nanotechnology, RNA holds great promises for the emerging sub-discipline of nanobiotechnology 22–24 because of its essential roles in gene expression and regulation as small interfering siRNAs, antisense RNAs, nucleic acid aptamers and ribozymes. In this manner, RNA has been shown to have tremendous potential to down regulate specific gene expression in cancerous and virus-infected cells through the RNA interference (RNAi) pathway 25–28. Current limitations related to the systemic delivery of short interfering RNAs (siRNAs), however, continue to present real obstacles to its more widespread clinical development 29, 30. The all-RNA packaging system described herein provides an attractive biomaterial for stabilizing and delivering multiple siRNAs for RNAi-based therapeutics.
RNAI and RNAII are sense and antisense plasmid-encoded transcripts that control the replication of the ColE1 plasmid of E. coli 31, 32. Initial NMR and subsequent theoretical analysis determined that the inverted ColE1 loop-loop interaction based on the inverse of the RNAI and RNAII loop sequences, forms a bend of approximately 120° between adjacent helices 33. This type of interaction is commonly referred to as a kissing-loop complex 34, 35. Based on its unique geometry, Yingling and Shapiro proposed its incorporation into the design of a hexagonal RNA nanoparticle 36 (Figure 1a). Molecular dynamics simulations suggested that the nanoring was quite stable 37. Although computational analysis of the RNAI/RNAII inverse (RNAI/IIi) kissing complex and its identification as a possible tool in RNA assembly constitute an integral component of RNA architectonics and design 2, experimental analysis provides the means to validate and refine previous theoretical models. Herein, we report the characterization of RNA nanoparticles based on RNAI/IIi kissing complexes, demonstrate their preferential assembly into pentameric and hexameric RNA nanorings, and report the design of a fully programmable nanoring employing five additional artificially designed loop-loop interactions selected for their ability to adopt similar geometry to the wild-type RNAI/IIi interaction. These nanorings have increased thermal stability and increased resistance toward ribonucleases from human blood serum. The nanorings can be functionalized with siRNA sequences and are capable of being processed by dicer, suggesting their potential use as siRNA delivery systems.
Figure 1.
RNA self-assembly mediated through kissing-loop complexes. (a) Front and side views of the 3D model of the hexagonal nanoring based on RNAI/IIi kissing complex. (b) Schematic of the assembly of S7–8 units with RNAI/II inverse (top) and HIV (bottom) kissing-loop complexes leading to nanoring (top) or linear (bottom) assemblies. The two assembly strategies used are generically referred to as AB and SM systems. The AB system employs two building blocks (A and B units), each housing only one of the two interacting loops: AB units can only assemble once mixed. The SM system uses a single unit containing both interacting loop sequences—allowing the SM unit to self-assemble. The first system leads to even numbered multimers, while the second allows an unbiased formation of both odd and even numbered multimers. Atomic structures for RNAI/IIi and HIV kissing complexes are from PDB_ID: 2BJ2 and PDB_ID: 2FCX, respectively. (c) Schematic of RNA units used in this study. Sequence variations within the central helical region allow modulation of the rigidity and flexibility of these units. C15 units are rigid circular dumbbell RNA units of 15 bp; S7–8, S6–9 and S5–10 units vary by the positioning of their 5' and 3' end: they are formed of two continuous stem loops of different length, each unit totaling 15 bp (7+8, 6+9 or 5+10 bp); −1nt, −2nt and −3nt units vary by a 3'-5’ gap of 1, 2, and 3- nucleotides separating the two stem-loops, respectively; the 4WJ unit uses an A-minor motif to rigidify the two-helix stack.
Results and Discussion
Nanoring design and self-assembly
The initial nanoring design proposed by Yingling and Shapiro was based on the assumption that RNA self-assembly could be directed by the 120° geometry afforded by the RNAI/IIi kissing-loop complex (Figure 1a,b) 36. The nanoparticle essentially consists of monomer RNA units formed from two self-assembling loops separated by a central helical region typically consisting of 15+11xN base pairs (bp) (N=0,1,2…). According to three-dimensional modeling, hexamers can be formed when the loops correspond to those of the RNAI/IIi kissing-loop complex. Herein, three assembly systems were used to characterize and assess the formation of RNA nanorings. In the AB system, assemblies are formed from two analogous RNA units, each of them with a single loop sequence on either end of the central helical region (Figure 1b). As these loops sequences are complementary to one another, the two units self-assemble by forming specific kissing-loop complexes when mixed together. In the SM system, a single RNA unit designed with both complementary loop sequences has the ability to assemble with other SM units to form multimers. The AB system can potentially form even numbered polygons such as dimers, tetramers, hexamers, etc., while the SM system allows an unbiased formation of both odd and even numbered polygons. A third approach, called the FP system, takes advantage of fully programmable RNA units based on variant RNAI/IIi kissing-loops (see below). Most characterizations of RNA nanorings were performed using units with stem regions totaling 15 bps in length (Figure 1c). These monomer units differ with respect to their structural rigidity (C15 and 4WJ), relative positioning of the 5’-3’ nick (S7–8, S6–9, S5–10), and their flexibility (−1nt, −2nt, and −3nt). As detailed in the legend of Figure 1c, their names are essentially intended to reflect the variations in the unit's central helical region.
Structural characterization of nanoring assembly
Initial experiments were conducted with the S7–8 RNA unit, designated for its 7 and 8 bp helical stems adjacent to the 3’ and 5’ ends, respectively. S7–8 RNA units assembling through RNAI/IIi kissing-loop complex expected to form 120° bend, were compared to similar units assembling through a previously characterized HIV kissing-loop complex known to adopt a co-linear 180° stacking geometry (Figure 1b) 16, 17, 38. At 2mM Mg2+ 1xTB buffer, native polyacrylamide gel electrophoresis (PAGE) analysis reveals that assemblies resulting from the formation of RNAI/IIi interactions form discrete molecular species (Figure 2a). In contrast, S7–8 assemblies containing HIV kissing-loop complexes result in smeared bands corresponding to RNA multimers of high molecular weights. Atomic force microscopy (AFM) characterization of both AB assembly systems were performed at 200 nM RNA concentration and buffer conditions identical to those used in the native PAGE experiments. AFM images confirm the formation of well-defined discrete particles from units assembling through RNAI/IIi kissing-loops (Figure 2b and e), while long fibers are observed from units assembling through HIV kissing-loops (Figure 2c). These results clearly demonstrate the influence of the geometry of a particular kissing-loop complex on the resulting supra-molecular assembly.
Figure 2.
Comparison of RNA self-assemblies mediated through RNAI/IIi and HIV kissing-loop complexes at 2 mM Mg(OAc)2 and 10°C. (a) Native PAGE of the assemblies obtained from RNAI/IIi and HIV kissing-complex S7–8 units visualized by total staining with SYBR Green II on 7% native gel. For each type of kissing-loop complexes, lanes M indicate migration of monomeric S7–8 RNA units (unit A of the AB bi-molecular system). Lanes AB and SM indicate migration of AB systems and SM systems of S7–8 units, respectively. (b–c) AFM image of RNAI/IIi nanorings (b) and HIV nanofibers (c) obtained by assembly of their corresponding AB S7–8 units at a total RNA concentration of 200 nM. (d) Statistical distribution of RNAI/IIi nanorings obtained by analysis of multiple AFM images of RNA nanoparticles (see Materials and Methods). (e) AFM magnification of individual and clustered nanorings observed in (b).
Cross-sections of the discrete particles based on the RNAI/IIi interaction were measured to estimate their diameters (see Materials and Methods section). Statistical analysis of particle diameters reveals the presence of three species with size distributions corresponding to squares (10.6 nm), hexagons (15.0 nm), and octagons (18.9 nm) (Figure 2d). Nearly 80% of the particles measured in two separate 1.2 × 1.2 micron grids have diameters consistent with the theoretical 3D model corresponding to the hexagonal nanoring 36 (Figure 1a). The relative distribution of RNA particles imaged by AFM is also in agreement with the distribution of molecular species observed by native PAGE experiments. At 200 nM of RNA, hexamer and octamer assemblies are formed in a relative ratio of ~5:1.
AFM magnification of some of these hexagonal particles reveals edges of 6.5 nm (Figures 1a and 2e). Interestingly, when hexameric nanorings are found next to one another on the mica surface, they tend to arrange through these edges in a honey-combed fashion (Figure 2e). This suggests that electronegative repulsion between adjacent negatively charged ribose-phosphate backbones can be easily neutralized by magnesium.
Exploring structural flexibility versus structural rigidity on nanoring assembly
The intrinsic degree of structural stress associated with a given self-assembling system can influence the types of assemblies formed 16, 39. The effect that rigidifying the RNA units had on RNAI/IIi nanoring assemblies was investigated in the SM and AB systems by native PAGE analysis (Figure 3a). Monomers were rigidified by either creating circular RNA units, called C15, which present a fully continuous stem separating the two interacting loops, or by creating four way junction units, called 4WJ, containing an A-minor junction motif involving an A-minor interaction between a GNRA tetaloop and its corresponding helical receptor 17, 40–42 (Figure 1c).
Figure 3.
Biochemical and biophysical characterizations of nanoring assemblies obtained from various RNAI/IIi units. (a) Native PAGE analysis of RNAI/IIi SM and AB assemblies resulting from S7–8, C15 and 4WJ units at various RNA concentrations, 2 mM Mg(OAc)2 and 10°C as described in the Materials and Methods. Data illustrate the effect of structural rigidity on nanoring formation and size distribution. "m" stands for the monomeric A unit. (b) Normalized distribution profiles of various SM assemblies at RNA concentration of 200 nM and 2000 nM, 2 mM Mg(OAc)2 and 10°C obtained from native PAGE. (c) Melting curves obtained by TGGE analysis (see Materials and Methods) for the isolated hexamer and pentamer SM assemblies of the C15, S7–8, and 4WJ monomers as well as the hexamer and pentamer FP assemblies.
In the AB system, the predominant products observed upon assembly of S7–8, C15 and 4WJ units are hexamers at all RNA concentrations tested. The extent of formation of hexameric nanorings is however found to be concentration dependent, as small amounts of other assembly species such as tetramers and octamers can be observed at low and high RNA concentrations, respectively (Figure 3a and b). By contrast, in the SM system, introducing rigidity favors the formation of pentamers over hexamers. For instance, at all RNA concentrations tested, the predominant products observed upon assembly of C15 and 4WJ units are pentamers. However, the S7–8 units preferentially assemble into pentamers only at concentration below 500 nM.
In order to determine whether the formation of pentamers is driven by kinetic or thermodynamic processes, the thermal stability of pentamer and hexamer SM assemblies isolated from native PAGE were compared by thermal gradient gel electrophoresis (TGGE) at 2mM MgOAc2. For all the constructs tested (S7–8, C15 and 4WJ) the hexameric rings are determined to be 5–10°C more stable than their pentameric-ringed counterparts, with hexamers formed of C15 units being the most stable of all assembly products characterized (Figure 3c and Table 1). Partial conversion of pentamers into hexamers could be achieved by incubating these assembly products above the melting temperature (Tm) of the pentamers as determined by TGGE (data not shown). This clearly demonstrates that the formation of pentamers is kinetically driven.
Table 1.
Melting temperature (Tm) values measured by TGGE for the disassembly transition of various nanorings at 2 mM Mg(OAc)2 (see Materials and Methods and Figure S1).
| Monomer Unit | Polygon | TmTGGE (°C) | |
|---|---|---|---|
| SM Assembly | C15 | Hexamer | 52.7 (±1.6) |
| C15 | Pentamer | 42.4 (±2.3) | |
| S7–8 | Hexamer | 41.0 (±1.5) | |
| S7–8 | Pentamer | 35.6 (±2.3) | |
| 4WJ | Hexamer | 42.0 (±0.1) | |
| 4WJ | Pentamer | 37.0 (±1.6) | |
| AB Assembly | C15A, C15B | Hexamer | 49.8 (±2.6) |
| S7–8A, S7–8B | Hexamer | 42.7 (±1.9) | |
| 4WJA, 4WJB | Hexamer | 41.3 (±0.9) | |
| S6–9A, S6–9B | Hexamer | 33.6 (±0.1) | |
| S5–10A, S5–10B | Hexamer | 29.9 (±.06) | |
| −1ntA, −1ntB | Hexamer | 27.7 (±0.5) | |
| −2ntA, −2ntB | Hexamer | 27.9 (±0.4) | |
| −3ntA, −3ntB | Hexamer | 27.0 (±0.6) | |
| C26A, C26B | Hexamer | 39.2 (±1.1) | |
| C27A, C27B | Hexamer | 26.9 (±1.4) | |
| FP Assembly | C15 (c3FP) | Hexamer | 50.2 (±1.2) |
| C15 (c6FP) | Hexamer | 48.1 (±1.1) | |
| C15 (c5FP) | Pentamer | 45.2 (±2.3) | |
| C15 (c4FP) | Tetramer | 30.2 (±2.5) | |
| C15 (c4FP) | Octamer | <25° | |
The positioning of the 3' and 5' end at the helical junction can also have an affect on nanoring assembly by changing the way the two interacting hairpins can bend with respect to one another (Figure 1c and Supporting Information (SI) Figure S2a). In both AB and SM systems, offsetting the position of the 5'-3' nick provides a way to tune the assembly toward the formation of higher order assembly species. In the AB system, shifting the 5’-3’ nick by one bp (S6–9) or 2 bps (S5–10) lead to a significant amount of octamers when compared to the S7–8 units (Figure S2a). In the SM system, the hexamer to pentamer ratio increased at all concentrations tested for S6–9 while S5–10 precluded pentamer assembly altogether (Figure 3b). Comparison of the thermal stability of the AB hexamers of S7–8, S6–9 and S5–10 at 2mM MgOAc2 indicates a decrease of stability with respect of the shortening of the stem of one of the interacting hairpins. This suggests that decreasing the stability of the secondary structure of the stem associated with the kissing-loop complex can significantly contribute to the destabilization of the kissing-loop interaction and the resulting assembly as a whole.
Finally, −1nt, −2nt, and −3nt assembling units with increased flexibility were designed by introducing 1, 2, or 3 nucleotide gaps at the level of their inter-helical junction, respectively (Figure 1c). In the SM system, the gap broadened the distribution of assembly species formed for all units (Figure 3b). Unit −1nt effectively favored the formation of hexamer and higher ordered assembly species. However, −2nt and −3nt shifted the assemblies toward the formation of lower-ordered species (Figure 3b and Figure S2b). This suggests that the increased relative mobility of the two interacting hairpins with respect of one another favor bending to allow formation of rings as small as trimers. In the AB system, the size of the gap has a less dramatic effect on the preferential formation of hexamers. However, TGGE analysis reveals that all three hexamers formed of −1nt, −2nt, and −3nt units show similar Tms, 22°C below the Tm of the C15 hexamer (Table 1). This suggests that the overall stability of hexamer assembly is significantly affected by the disruption of the helical stack at the inter-helical junction.
Design and characterization of programmable nanorings
The degree of programmability afforded by the AB system provided a minimal level of control over hexamer formation—both in terms of mitigating kinetic traps and showing remarkable tolerance toward stem mutations. Complete control over assembly, however, requires using six specific kissing-loop interactions that need to assemble selectively in concert and form the same 120° bend geometry as the RNAI/IIi kissing-complex. The 7 bps RNAI/IIi kissing-loop complex, originally studied as a substitute for the wild-type ColE1 RNAI/RNAII interaction, assembles with a Kd over 300 times lower than its natural counterpart 43; and their respective thermodynamic stability does not correlate with the theoretical stability of RNA duplexes of corresponding sequences 44, 45.
Consequently, in order to generate fully programmable (FP) nanorings, we designed a scheme, aiming at selecting within a set of 40 variants of the RNAI/IIi kissing-complex (Table S2), those able to promote nanoring assemblies (Figure S3). Kissing-loop variants were first encoded within the context of S7–8 SM constructs and checked for their ability to assemble into polygonal species like those obtained from RNAI/IIi S7–8 SM units (Figure 4a and Figure S4). Out of the 40 constructs tested at 2 mM MgOAc2 and 1 µM RNA, 19 constructs assemble according to the assembly pattern of the RNAI/IIi S7–8 SM unit (Figure S5) while all the others are either forming undefined, smeared assemblies (3 constructs, Table S2) or are unable to assemble in these conditions (18 constructs, Table S2).
Figure 4.
Characterization of RNAI/RNAIIi kissing-loop complex variants and fully programmable nanorings. (a) The three different migration patterns observed by native PAGE for the various SM S7–8 units designed to assemble through RNAI/IIi kissing-loop complex variants (Table S2). n: no assembly; y: polygonal assembly similar to the one from SM S7–8 unit with RNAI/IIi kissing-loops; s: smearing assembly. (b) Sequence signature of RNAI/IIi like complexes and resulting polygon formations as observed by native PAGE at 2 mM MgOAc2, 10°C and 1 µM [RNA]. −: kissing-loop sequence signature not tested. (c) 2-D diagram of the fully programmable (FP) hexameric nanoring, c6FP, generated from circularized C15 units α, β, γ, δ, ε, and ζ (see Table S3). Kds values are those corresponding to each RNAI/IIi kissing-loop complex in isolation. (d) Native PAGE visualization of several "open" and "closed" FP assemblies generated from programmable C15 units at 2 mM at 2 mM MgOAc2, 10°C and 600 nM total [RNA]. The composition of each "open" (o) and "closed" (c) FP assembly tested is given Tables S3 and S4. The number of different units entering is specified for each assembly complex (e.g: c6FP indicates that the assembly complex is a closed (c) fully programmable (FP) assembly formed of 6 units). Each closed assembly is programmed with kissing-loop complexes allowing closed ring structures to form while each open assembly contains one unit preventing one kissing complex to form. The three control lanes on the left indicate the position of an unassembled C15 monomer (M) and AB and SM assemblies of C15 units assembling through RNAI/IIi kissing complexes.
Sequence comparative analysis of all the kissing-loop variants indicates that polygonal assembly is highly favored by the two sets of complementary loops 5'GN5U3':5'AN5C3' and 5'GN5G3':5'CN5C3', as well as by high G/C content (Figure 4b). These two sequence signatures are the only ones to provide assembly with three G:C bps. Sequence variations at the first and last positions of the kissing-loops are tolerated far less than those within the five other loop positions. For instance, the formation of GC, CG or UA Watson-Crick bps between these two nucleotide positions totally prevents polygon assembly irrespective of the G/C content of the loops (Figure 4b and Table S2). However, increasing the G/C content of the other kissing-complex signatures to four or five bps may still promote efficient polygonal assembly. Interestingly, swapping the two kissing-loops within a particular S7–8 SM unit has no effect on the resulting assemblies, indicating that the stem closing base pairs do not significantly contribute to the stability of the adjacent kissing-loop base pairing (data not shown).
Out of the 19 kissing-loop complexes, eight complexes (16 loops) were chosen and checked for their selectivity and potential compatibility within the context of a fully programmable assembly (Figure S6). In addition to the RNAI/IIi kissing-loops, none of the new 16 loops tested have the ability to form self-dimers and all but four are remarkably selective for only with their cognate partner at RNA concentration up to 2 µM, 2 mM MgOAc2 and 30°C. Their equilibrium constants of dissociation (Kd) were found to range from 6 to 84 nM (Figure S7). Again, no correlation is found between the measured stability of each kissing-loop complex and the theoretical stability of duplex made of identical sequences 44, 45. However, within kissing complex of sequence signature 5'GN5U3':5'AN5C3', it becomes apparent that loops able to form at least one intramolecular Watson-Crick bp between their two first and two last nucleotide positions have slightly higher apparent Kds than those that avoid it.
The fully programmable (FP) hexagonal nanoring was built from six C15 RNA units that, in addition to the RNAI/IIi kissing complex, incorporate five of the new kissing-loop complexes (aa', cc', ee', gg', ll'). These kissing-loops were chosen because of their low Kds and their specificity for their cognate (Table S3, Figure 4c). At 2 mM MgOAc2 and 12°C, the FP nanoring can be produced in yields higher than 90 % (Figure 4d). The FP nanoring’s apparent Tm of 48.2±1 °C is comparable to the "closed" hexamers generated from SM or AB C15 units (Table 1). Additional programmable "closed" and "open" assemblies of various sizes were synthesized and compared by native PAGE to the hexameric nanoring. "Open" dimer, trimer, tetramer and pentamer assemblies were generated from two, three, four or five C15 units entering into the composition of the FP hexamer (Table S3). The “closed” hexamer nanoring migrates faster than an "open" hexamer missing one of the kissing complexes (Figure 4d).
Furthermore, the potential to form programmable "closed" trimeric, tetrameric, and pentameric rings was also investigated at 2 mM MgOAc2. While all "open" assemblies can be easily obtained, only the "closed" FP pentamer can be produced in high yield as anticipated (Figure 4d). The pentamer’s Tm is three degrees lower than the FP hexamer (Table 1). By contrast, the C15 units expected to form "closed" FP tetramers assemble also into octamers (Figure 4d). While the tetramer has a Tm of 30°C, the Tm of the octamer is below 25°C, indicative of a kinetic trap (Table 1). Interestingly, C15 units programmed to form a "closed" trimer assemble instead into a hexameric nanoring with an apparent Tm of 50.2 ±1 °C (Figure 4d and Table 1). Overall, full programmability of RNA nanorings through RNAI/RNAIIi kissing-loop variants clearly corroborate the fact that the optimal geometry favored by this class of kissing-loops is the hexagon. While pentagon assemblies can still be induced with minimal structural adjustments, the structural penalty for forming tetramers and octamers is much greater to overcome and affect significantly the stability of the resulting assemblies. In the case of the trimer, the geometrical constraints imposed by RNAI/RNAIIi kissing-loop variants simply prevent its formation.
Nanorings with increased resistance towards ribonuclease degradation in blood serum
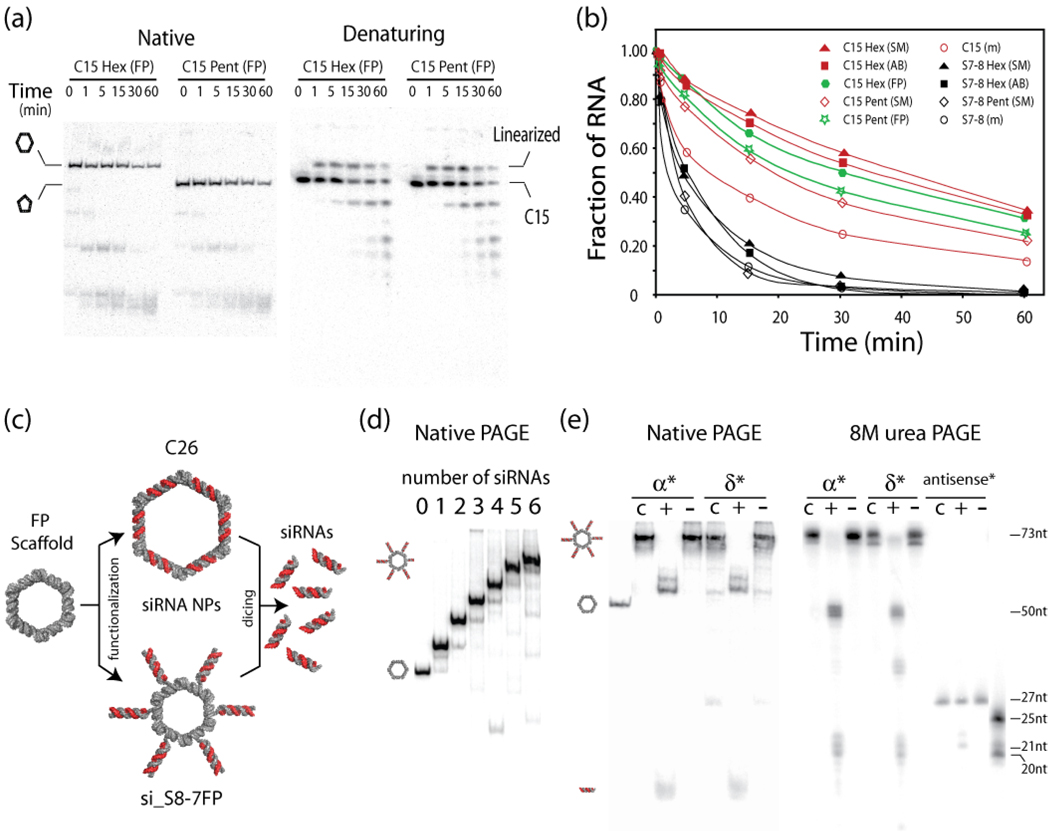
Nanoring assemblies based on circular RNA units (like C15) are particularly attractive because of their perceived potential as a siRNA packaging and stabilizing agent 36. In a biological context linear RNA molecules can be degraded by both exonucleases and endonucleases. Endonucleases are typically less prevalent than exonucleases but nonetheless present a substantial threat to RNA especially by targeting single stranded RNAs 46. Interestingly, short circularized dumbbell RNAs similar to C15 have been shown to be resistant to exonuclease activity and able to carry siRNAs 47. Through kissing complex formation, C15 nanoring assemblies can therefore provide further chemical stability by shielding the loops from single-strand RNA endonucleolytic cleavage. In order to investigate the added resistance to ribonuclease degradation afforded by nanoring assembly, RNA degradation time courses were performed on S7–8 and C15 monomers and their corresponding SM, AB and FP assemblies in 2% (v/v) human blood serum at 37°C (Figure 5ab). Overall, S7–8 constructs are much less ribonuclease resistant than C15 constructs, corroborating the significant protection offered by circularized RNAs versus linear ones 47. While only 5–10 % of the linear S7–8 RNA units assembling into pentamer and hexamer nanorings remain intact after 30 minutes, C15 units involved into the formation of pentamer and hexamer nanorings display a dramatic increase of resistance toward degradation (Figure 5b). More than 30% of the circular C15 units involved in hexamers are still intact after incubation of 1 hour in 2% (v/v) human blood serum (Figure 5ab). Therefore, the involvement of circular C15 units into nanoring assemblies offers a significant additional protection by at least a factor of two when compared to unassembled C15 monomers (Figure 5b). Interestingly, all hexamers tested are more resistant than pentamers toward ribonuclease degradation (Figure 5b). This can either result from an increased thermodynamic stability of hexamers versus pentamers (Table 1) or a more ideal geometrical protection of the kissing-loops in hexagonal versus pentagonal nanorings.
Figure 5.
Increased ribonuclease resistance and siRNA functionalization of nanorings. (a) Native (left) and denaturing (right) PAGE indicating RNA degradation time courses for C15 FP monomers assembled into pentamers and hexamers in presence of human blood serum 2% (v/v) at 37°C. (b) RNA degradation time courses of S7–8 and C15 monomers and corresponding SM, AB and FP assemblies in 2% (v/v) human blood serum at 37°C. The total final concentration of RNA (as monomer or in RNA assembly) is always 200 nM. (c) The two strategies employed to functionalize nanoring scaffolds with six siRNAs. (top) Double stranded siRNAs (anti-sense strands are shown in red) are encoded within C26 units that are assembled into hexamers prior to dicing. (bottom) Nanoring monomers are extended at their 3' end with 29-nt tails, each tail comprising a 2nt linker and a 27-nt sense strand complementary to a 25-nt anti-sense siRNA strand (shown in red). After assembly, the resulting particles (si_S8–7FP) are diced. (d) Native PAGE illustrating the assembly of modified S8–7 units with an increasing number of siRNAs attached. (e) Dicing of si_S8–7FP nanorings modified with six siRNAs. Native and denaturing PAGE gels were used to identify the dicing products corresponding to the processed hexagonal si_S8–7FP nanoring (with remaining 3' overhang tails of 8 nt) and siRNA products formed of 21-nt complementary strands using appropriate molecular RNA markers of known length. α* and δ* indicate the unit that is 32P radio-labeled within si_S8–7FP assemblies (see Table S1g). "c": control nanorings without incubation at 37°C; "+": nanorings incubated with Dicer for 18 hours at 37 °C;"−": nanorings incubated at 37°C for 18 hours in the dicing buffer without the Dicer enzyme..
Nanoring functionalization with siRNAs
The nanoring design was initially proposed as a possible delivery system of multiple siRNAs 36. Two orthogonal strategies were used to functionalize programmable nanorings with siRNAs and both resulting siRNA nanoparticles were tested for their ability to be processed by human recombinant Dicer (from Invitrogen Life Technologies) (Figure 5c). Each strategy allows up to six siRNA duplexes to be incorporated into hexamer assemblies. The first strategy involves encoding siRNA sequences within the helical stems of the nanoring units. Native gel shift assays and thermal stability analysis were carried out to determine the optimal stem length necessary for hexamer assembly and compatible with the length requirement for siRNAs 48, 49. As anticipated from 3D models, circular C26 units, with helical stems of 26 bp, lead to the most stable hexamers at 2 mM MgOAc2 (Table 1). By contrast, C27 units, with stems of 27 bp, form hexamers with greatly reduced thermal stability (Table 1) and monomers with stems of 24, 25 and 28 bp are unable to assemble into closed hexamers. In the second strategy, siRNAs are appended to the nanoring by extending the 3’ end of S8–7 RNA units (Table S1g), allowing step-wise addition of up to six siRNA duplexes after annealing of the corresponding anti-sense strands (Figure 5d). Both strategies led to siRNA nanoparticles able to be processed by human recombinant dicer (Figure 5e and Figure S8). After incubation with dicer at 37°C, expected products of ~21-nts in length are identified by denaturing PAGE for both C26 and S8–7FP modified hexamers. Dicer is slightly more effective at processing siRNAs when attached to si_S8–7FP because only one cut is required to release each siRNA (Figure 5d). However, this apparent advantage might eventually be overridden by the fact that C26 nanorings offer an increased resistance toward ribonucleases from blood serum without needing to introduce chemical modifications 50–52. Thus, both strategies are able to be recognized and processed by dicer while also offering the advantage to precisely control the stoichiometry of multiple siRNAs by packaging them into single all-RNA nanoparticles. As such, they might offer combinatorial targeting of six different sequences through RNAi pathways (co-RNAi) in order to knockout genes more efficiently 53, 54.
Conclusion
We have demonstrated herein that the particular geometry of the RNAI/IIi kissing-loop complex can be used to efficiently build self-assembling polygonal nanorings. These RNA nanorings constitute a new class of multifunctional all-RNA nanoparticles for possible applications in nano-medicine and synthetic biology. In previous RNA self-assembly systems 13, 16, 17, several RNA nanoparticles have been constructed using tertiary structure motifs embedded at the core of the assembling monomer units. By contrast to these systems, the RNAI/IIi nanoring’s overall geometry is dictated by the geometry of the kissing-loop complex at the periphery of their constituent subunits. As a result, the RNAI/IIi nanoring does not require the use of complex structure motifs or folding strategies to ensure proper assembly while also leaving the stem region the versatility to encode virtually any sequence signature.
Assemblies of RNA subunits using the RNAI/IIi kissing complex alone result in a distribution of species that is concentration dependent. The formation of lower-ordered and less thermo-stable species like tetramers and pentamers can be mitigated by increasing the RNA assembly concentration, but this strategy results in an increased formation of other undesired and less stable species like septamers and octomers. While subtle modifications about the helical stem region can tune nanoring assemblies toward the thermodynamically more stable hexamer products, only the design and incorporation of six specific kissing-loop complexes are able to provide complete thermodynamic control over RNA self-assembly to form hexagonal RNA nanorings and fully counteract the concentration dependency and kinetic factors associated to the assembly of AB and SM systems. Our results highlight that even "rigid" RNA units can be kinetically trapped in assemblies that are not the most thermodynamically stable, suggesting that RNA has an intrinsic flexibility that could potentially be harnessed for kinetically controlling assembly formation in future applications 55, 56. Furthermore, the definite hexagonal shape and planar geometry of the FP nanoring gives it the potential to serve as a tile for 2D architectures or as an interesting scaffold for additional 3D assemblies.
Nanorings may prove to be a versatile tool used to package multiple siRNAs or other functionalities within the stem region of their constituent subunits. We have demonstrated that RNA nanoring assemblies confer increased resistance toward ribonucleases present in human blood serum. While circularized subunits were shown to be the most stable and may not require the usage of chemically more stable nucleotide analogs, nanoring assemblies as a whole conferred increased resistance when compared to unassembled subunits, showing that assembly factors offer a significant contribution toward the protection of each constituent subunit. Finally, we have demonstrated that the fully programmable nanoring offers two robust strategies to provide stoichiometric control over the incorporation of multiple siRNAs that can be processed by human recombinant Dicer in vitro. By contrast to other methods used to target cells 57, 58, our strategy has the potential to incorporate up to six siRNAs or other functional RNAs (i.e. aptamers or ribozymes) with stiochiometric control. We therefore anticipate that our programmable RNA nanoring system will be able to offer new approaches for the simultaneous delivery of multiple siRNAs and/or other functionalities for cellular therapeutics, in a similar fashion to strategies based on trimeric pRNA nanoparticles 1.
Materials and Methods
RNA structural and sequence design
Sequence design of nanoring monomers was based on the previous 3D computer model reported by Yingling and Shapiro 36. The 3D model was used to generate consensus secondary structures specifying invariant nucleotide positions. Sequences were optimized with the MFOLD program 59 to maximize thermodynamic stability and minimize the occurrence of alternative secondary structure folds. The full list of RNA sequences used is available in Table S1. Additionally, the FP nanoring sequences were optimized to minimize noncognate cross-pairings as described below.
RNA synthesis
RNA constructs were synthesized by in-vitro transcription with T7 RNA polymerase from PCR generated double stranded DNA template, followed by 8 M urea/ 8% poly-acrylamide gel electrophoresis (PAGE) purification as previously described 13, 16, 17. To obtain circular RNA molecules (C15, C24–27), T7 RNA transcription was performed in vitro with an excess of GMP compared GTP to ensure the transcription of RNA having a 5’-monophosphate. After gel purification, these RNAs were circularized by 3'-5' intra-molecular ligation with T4 RNA ligase 2 (New England Biosciences) (see Extended Materials and Methods in Supporting Information). Linear RNA transcripts were typically labeled at their 3' end with [32P]pCp while circular RNAs were body-labeled with [32P]αATP.
RNA assembly, native PAGE and TGGE experiments
RNA nanorings were assembled in one pot from various sets of RNA units at concentrations specified in the text. Typically, RNA unit transcripts were heated to 95°C for two minutes, snap cooled to 4°C on ice, and assembled at 30°C by addition of MgOAc2 buffer to a final concentration (89 mM tris-borate (pH 8.3), 2 mM Mg(OAc)2, 50 mM KCl, and 50 mM NaCl) and incubation for 30 minutes at 30°C. Native PAGE and TGGE experiments were performed as described 13, 16, 17. Typically, assembly experiments reported were analyzed at 12°C on 7% (29:1) native polyacrylamide gels in presence of 89 mM Tris-borate, pH 8.3, 2 mM Mg(OAc)2. A Typhoon phosphoimager was used to visualize 32P labeled or SYBR® Green II stained RNA. For TGGE analysis, a linear temperature gradient applied perpendicular to the electric field was used. Total monomer RNA concentration was 200 nM for all assembly systems. The fluctuation of the temperature gradient during migration was generally less than 1 to 2°C.
AFM characterization
RNA architectures were assembled in solution, deposited on a mica surface in the 2 mM MgOAc2 buffer (see composition above) and dried under nitrogen before AFM imaging in air. AFM images were acquired at room temperature in tapping mode using a Multimode microscope equipped with a Nanoscope IIIa controller (Veeco, Santa Barbara). Silicon probes (model NSC12 from MikroMesch) with resonance frequency ~150–250 kHz and spring constant ~4–8 N/m (Nanodevices, Santa Barbara, CA) were used. Images were processed by NanoScope® (DI) and leveled by a first order plane fit in order to correct for sample tilt. Diameters of nanorings were obtained by measuring orthogonal cross sections of each individual particle. These two measurements were fitted to an elliptical equation to obtain a circumference. This was accomplished in order to compensate for any distortions resulting from contact between the RNA nanorings and the mica surface. The equation for the circumference of a circle was then used to determine the corresponding diameter of a given particle.
Specificity study and determination of equilibrium constant of dissociation (Kd)
Kissing-loop complex sequences were incorporated into two complementary hairpin constructs with 8 and 12 bps hairpin stems (Table S1). Equimolar amounts of each hairpin at various concentrations (1 nM-5 µM final) were mixed and subjected to the assembly protocol described above in the presence of 2 mM Mg(OAc)2 at 30°C. Analysis of RNA migration on native PAGE was accomplished by monitoring one of the hairpin constructs which contained a fixed amount of 3' end [32P]pCp-labeled RNA (0.25– 0.5 nM final).
Human blood serum degradation studies
Aliquots of pooled human blood serum obtained frozen (Bioreclamation) were thawed and immediately used for each new study. 32P body-labeled RNA molecules assembled into nanorings (as described above) were kept on ice prior to incubation with 2% (v/v) human blood serum at 37°C for various time periods. Final RNA concentration was 200 nM. Prior to immediate loading on native PAGE, degradation time courses were quenched on ice. Prior to loading on 8M urea/10% of poly-acrylamide gels, RNA samples were quenched by addition of ELIMINase (Decon Laboratories, Inc.), placed on ice, and followed by the addition of 4M urea blue.
Recombinant Human Dicer assays
Modified antisense-sense body-labeled nanorings and C26 nanorings were prepared as described above to a final concentration of 3 µM. Samples were incubated for 18 hours at 37°C in 1x dicer Buffer (Invitrogen) in presence of BLOCK-iT™ dicer enzyme (BLOCK-iT™ Dicer RNAi Kit (Invitrogen)). Recombinant human turbo dicer enzyme kit (Genlantis), containing an ultra-active form of human recombinant dicer enzyme, was also used in order to increase the processing rate of C26 nanorings. Samples were incubated for 4 hours at 37°C in the supplied dicer reaction buffer (Genlantis) according to the manufacturer’s suggested protocol. Dicing reactions were quenched by adding dicer stop solution (provided by the manufacturers) prior to analysis on 2 mM Mg(OAc)2 native 7% PAGE or 8M Urea /10% PAGE as described above and in Supporting Information.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Isil Severcan for her early involvement in the project and Jannine Tuttle for her assistance acquiring AFM images. This research was supported by NIH R01GM079604 (to L.J.). This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (to B.A.S.). L.J. wishes to dedicate this paper to St. Giuseppe Moscati and St. Gianna Beretta Molla.
Footnotes
Author contributions: WG, BS, and LJ designed research; WG, PZ, and KA performed biochemical and biophysical experiments; AC performed AFM; WG, PZ, BS, and LJ analyzed data; WG and LJ wrote the paper.
Supporting Information Available. File (Grabow_et_al(2011)SI.pdf) with extended Materials and Methods section, Tables S1 to S4 and additional Figures S1 to S8.
References
- 1.Guo P. RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy. J Nanosci Nanotechnol. 2005;5(12):1964–1982. doi: 10.1166/jnn.2005.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaeger L, Chworos A. The architectonics of programmable RNA and DNA nanostructures. Curr Opin Struct Biol. 2006;16(4):531–543. doi: 10.1016/j.sbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 4.Aldaye FA, Palmer AL, Sleiman HF. Assembling materials with DNA as the guide. Science. 2008;321(5897):1795–1799. doi: 10.1126/science.1154533. [DOI] [PubMed] [Google Scholar]
- 5.Douglas SM, Dietz H, Liedl T, Hogberg B, Graf F, Shih WM. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature. 2009;459(7245):414–418. doi: 10.1038/nature08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito H, Inoue T. Synthetic biology with RNA motifs. Int J Biochem Cell Biol. 2009;41(2):398–404. doi: 10.1016/j.biocel.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Seeman NC. Nanomaterials based on DNA. Annu Rev Biochem. 2010;79:65–87. doi: 10.1146/annurev-biochem-060308-102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeger L, Westhof E, Leontis NB. TectoRNA: modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 2001;29(2):455–463. doi: 10.1093/nar/29.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chworos A, Severcan I, Koyfman AY, Weinkam P, Oroudjev E, Hansma HG, Jaeger L. Building programmable jigsaw puzzles with RNA. Science. 2004;306(5704):2068–2072. doi: 10.1126/science.1104686. [DOI] [PubMed] [Google Scholar]
- 10.Shu D, Moll WD, Deng ZX, Mao CD, Guo PX. Bottom-up assembly of RNA arrays and superstructures as potential parts in nanotechnology. Nano Letters. 2004;4(9):1717–1723. doi: 10.1021/nl0494497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasalean L, Baudrey S, Leontis NB, Jaeger L. Controlling RNA self-assembly to form filaments. Nucleic Acids Res. 2006;34(5):1381–1392. doi: 10.1093/nar/gkl008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afonin KA, Cieply DJ, Leontis NB. Specific RNA self-assembly with minimal paranemic motifs. J Am Chem Soc. 2008;130(1):93–102. doi: 10.1021/ja071516m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severcan I, Geary C, Chworos A, Voss N, Jacovetty E, Jaeger L. A polyhedron made of tRNAs. Nat Chem. 2010;2(9):772–779. doi: 10.1038/nchem.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonin KA, Bindewald E, Yaghoubian AJ, Voss N, Jacovetty E, Shapiro BA, Jaeger L. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat Nanotechnol. 2010;5(9):676–682. doi: 10.1038/nnano.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westhof E, Masquida B, Jaeger L. RNA tectonics: towards RNA design. Fold Des. 1996;1(4):R78–R88. doi: 10.1016/S1359-0278(96)00037-5. [DOI] [PubMed] [Google Scholar]
- 16.Severcan I, Geary C, Verzemnieks E, Chworos A, Jaeger L. Square-shaped RNA particles from different RNA folds. Nano Lett. 2009;9(3):1270–1277. doi: 10.1021/nl900261h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geary C, Chworos A, Jaeger L. Promoting RNA helical stacking via A-minor junctions. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq748. published online September, 2010 (doi:10.1093/nar/gkq847). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeger L, Leontis NB. Tecto-RNA: One-Dimensional Self-Assembly through Tertiary Interactions This work was carried out in Strasbourg with the support of grants to N.B.L. from the NIH (1R15 GM55898) and the NIH Fogarty Institute (1-F06-TW02251-01) and the support of the CNRS to L.J. The authors wish to thank Eric Westhof for his support and encouragement of this work. Angew Chem Int Ed Engl. 2000;39(14):2521–2524. doi: 10.1002/1521-3773(20000717)39:14<2521::aid-anie2521>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Ikawa Y, Fukada K, Watanabe S, Shiraishi H, Inoue T. Design, construction, and analysis of a novel class of self-folding RNA. Structure (Camb) 2002;10(4):527–534. doi: 10.1016/s0969-2126(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 20.Bindewald E, Hayes R, Yingling YG, Kasprzak W, Shapiro BA. RNAJunction: a database of RNA junctions and kissing loops for three-dimensional structural analysis and nanodesign. Nucleic Acids Res. 2008;36(Database issue):D392–D397. doi: 10.1093/nar/gkm842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bindewald E, Grunewald C, Boyle B, O'Connor M, Shapiro BA. Computational strategies for the automated design of RNA nanoscale structures from building blocks using NanoTiler. J Mol Graph Model. 2008;27(3):299–308. doi: 10.1016/j.jmgm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled A, Guo S, Li F, Guo P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 2005;5(9):1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Famulok MH, J S, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem. Rev. 2007;107:3715–3743. doi: 10.1021/cr0306743. [DOI] [PubMed] [Google Scholar]
- 24.Hess H, Jaeger L. Nanobiotechnology. Curr Opin Biotechnol. 2010;21(4):373–375. doi: 10.1016/j.copbio.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Lee JF, Stovall GM, Ellington AD. Aptamer therapeutics advance. Curr Opin Chem Biol. 2006;10(3):282–289. doi: 10.1016/j.cbpa.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Scherer L, Rossi JJ, Weinberg MS. Progress and prospects: RNA-based therapies for treatment of HIV infection. Gene Ther. 2007;14(14):1057–1064. doi: 10.1038/sj.gt.3302977. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Rossi JJ. The therapeutic potential of cell-internalizing aptamers. Curr Top Med Chem. 2009;9(12):1144–1157. doi: 10.2174/156802609789630893. [DOI] [PubMed] [Google Scholar]
- 28.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manjunath N, Dykxhoorn DM. Advances in synthetic siRNA delivery. Discov Med. 2010;9(48):418–430. [PubMed] [Google Scholar]
- 30.Lares MR, Rossi JJ, Ouellet DL. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010;28(11):570–579. doi: 10.1016/j.tibtech.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eguchi Y, Tomizawa J. Complex formed by complementary RNA stem-loops and its stabilization by a protein: function of CoIE1 Rom protein. Cell. 1990;60(2):199–209. doi: 10.1016/0092-8674(90)90736-x. [DOI] [PubMed] [Google Scholar]
- 32.Tomizawa J. Control of ColE1 plasmid replication. Interaction of Rom protein with an unstable complex formed by RNA I and RNA II. J Mol Biol. 1990;212(4):695–708. doi: 10.1016/0022-2836(90)90231-a. [DOI] [PubMed] [Google Scholar]
- 33.Lee AJ, Crothers DM. The solution structure of an RNA loop-loop complex: the ColE1 inverted loop sequence. Structure. 1998;6(8):993–1005. doi: 10.1016/s0969-2126(98)00101-4. [DOI] [PubMed] [Google Scholar]
- 34.Paillart JC, Westhof E, Ehresmann C, Ehresmann B, Marquet R. Non-canonical interactions in a kissing loop complex: the dimerization initiation site of HIV-1 genomic RNA. J Mol Biol. 1997;270(1):36–49. doi: 10.1006/jmbi.1997.1096. [DOI] [PubMed] [Google Scholar]
- 35.Brunel C, Marquet R, Romby P, Ehresmann C. RNA loop-loop interactions as dynamic functional motifs. Biochimie. 2002;84(9):925–944. doi: 10.1016/s0300-9084(02)01401-3. [DOI] [PubMed] [Google Scholar]
- 36.Yingling YG, Shapiro BA. Computational design of an RNA hexagonal nanoring and an RNA nanotube. Nano Lett. 2007;7(8):2328–2334. doi: 10.1021/nl070984r. [DOI] [PubMed] [Google Scholar]
- 37.Paliy M, Melnik R, Shapiro BA. Molecular dynamics study of the RNA ring nanostructure: a phenomenon of self-stabilization. Phys Biol. 2009;6(4):046003. doi: 10.1088/1478-3975/6/4/046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ennifar E, Walter P, Ehresmann B, Ehresmann C, Dumas P. Crystal structures of coaxially stacked kissing complexes of the HIV-1 RNA dimerization initiation site. Nat Struct Biol. 2001;8(12):1064–1068. doi: 10.1038/nsb727. [DOI] [PubMed] [Google Scholar]
- 39.He Y, Mao C. Balancing flexibility and stress in DNA nanostructures. Chem Commun (Camb) 2006;(9):968–969. doi: 10.1039/b513962g. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger L, Michel F, Westhof E. Involvement of a GNRA tetraloop in long-range RNA tertiary interactions. J Mol Biol. 1994;236(5):1271–1276. doi: 10.1016/0022-2836(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 41.Nissen P, Ippolito JA, Ban N, Moore PB, Steitz TA. RNA tertiary interactions in the large ribosomal subunit: the A-minor motif. Proc Natl Acad Sci U S A. 2001;98(9):4899–4903. doi: 10.1073/pnas.081082398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lescoute A, Westhof E. Topology of three-way junctions in folded RNAs. Rna. 2006;12(1):83–93. doi: 10.1261/rna.2208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eguchi Y, Tomizawa J. Complexes formed by complementary RNA stem-loops. Their formations, structures and interaction with ColE1 Rom protein. J Mol Biol. 1991;220(4):831–842. doi: 10.1016/0022-2836(91)90356-b. [DOI] [PubMed] [Google Scholar]
- 44.Freier SM, Kierzek R, Jaeger JA, Sugimoto N, Caruthers MH, Neilson T, Turner DH. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner DH, Mathews DH. NNDB: the nearest neighbor parameter database for predicting stability of nucleic acid secondary structure. Nucleic Acids Res. 2010;38(Database issue):D280–D282. doi: 10.1093/nar/gkp892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136(4):763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Abe N, Abe H, Ito Y. Dumbbell-shaped nanocircular RNAs for RNA interference. J Am Chem Soc. 2007;129(49):15108–15109. doi: 10.1021/ja0754453. [DOI] [PubMed] [Google Scholar]
- 48.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15(2):188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342(3):919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 51.Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18(4):305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 52.Hoerter JA, Walter NG. Chemical modification resolves the asymmetry of siRNA strand degradation in human blood serum. Rna. 2007;13(11):1887–1893. doi: 10.1261/rna.602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu YP, von Eije KJ, Schopman NC, Westerink JT, ter Brake O, Haasnoot J, Berkhout B. Combinatorial RNAi against HIV-1 using extended short hairpin RNAs. Mol Ther. 2009;17(10):1712–1723. doi: 10.1038/mt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm D, Kay MA. Combinatorial RNAi: a winning strategy for the race against evolving targets? Mol Ther. 2007;15(5):878–888. doi: 10.1038/sj.mt.6300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Hashimi HM, Walter NG. RNA dynamics: it is about time. Curr Opin Struct Biol. 2008;18(3):321–329. doi: 10.1016/j.sbi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lubrich D, Green SJ, Turberfield AJ. Kinetically controlled self-assembly of DNA oligomers. J Am Chem Soc. 2009;131(7):2422–2423. doi: 10.1021/ja807765v. [DOI] [PubMed] [Google Scholar]
- 57.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24(8):1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 58.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, 2nd, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27(9):839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.