Abstract
Background
The effects of immunologic and virologic factors on AMI rates in HIV patients are unclear.
Methods
HIV-infected patients in a U.S. health care system were assessed for AMI.
Results
Of 6,517 HIV patients, 273 (4.2%) had an AMI. In a model adjusting for cardiovascular risk factors, antiretroviral medications, and HIV parameters, CD4 count less than 200/mm3 (OR 1.74, 95% CI 1.07-2.81, P=0.02) predicted AMI. Increased HIV viral load was associated with AMI accounting for CVD risk factors and antiretroviral medications but was not significant when CD4 count was considered.
Conclusions
Immunologic control appears to be the most important HIV-related factor associated with AMI.
Keywords: HIV, myocardial infarction, immune function, cardiovascular risk factors
Introduction
HIV infection confers an increased risk of cardiovascular disease1, 2 but the etiology does not appear to be explained in full by traditional cardiovascular disease (CVD) risk factors.2, 3 Recent data suggest that impaired immune function may be associated with markers of preclinical atherosclerosis and vascular dysfunction in HIV patients.4, 5 Whether these changes translate into increased CVD event rates, however, is unclear. Several studies have shown conflicting results with respect to the association of CD4 count3, 6-10 or HIV viral load3, 7, 11 with cardiovascular disease, but methodology was not uniform. In this study, we employ a large U.S. clinical cohort to investigate the relationship between CD4 cell count and HIV viral load with AMI, specifically assessing whether these clinical immunologic and virologic parameters are risk factors for cardiovascular disease, independent of traditional CVD risk factors and effects of antiretroviral drugs.
Methods
Data Source and Study Sample
The patients in the study received care at two large tertiary care hospitals and their affiliated outpatient clinics, Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH), located in Boston, Massachusetts. The study period began on December 17, 1998 and ended on February 4, 2008. Eligible patients were identified from the Research Patient Data Registry (RPDR), a comprehensive clinical database including all inpatient and outpatient encounters for the Partners HealthCare System based on billing codes and containing data on more than 2.7 million patients. All patients with at least two encounters (inpatient or outpatient) with a diagnosis of HIV (ICD-9-CM codes 042 and all subtypes, 043 and all subtypes, 044.9, 079.53, and V08) during the study period were included. All data were censored at the end of the study period, on the date of the last encounter, or on the date of first AMI if one occurred, whichever was earliest. The study was approved by the Partners Human Research Committee.
Outcome Ascertainment
We classified patients as having the primary outcome of AMI if they had at least one documented code of ICD-9-CM of 410.xx (acute myocardial infarction) occuring after the first HIV code and within the observation period. The outcome definition has been validated in a previous study of RPDR data that showed this ICD code to have a sensitivity of 98% and specificity of 85% for clinically defined AMI.12
Clinical Exposure Definitions
Clinical exposures were identified using ICD-9-CM codes 401 for hypertension (HTN), 250 for diabetes mellitus (DM), 272 for dyslipidemia, 585-586 for chronic kidney disease (CKD), and 410-414 for coronary heart disease (CHD). Previous validation studies of RPDR data have shown both sensitivity and specificity of more than 85 percent for ICD-based definitions of HTN, DM, and dyslipidemia.12 Antiretroviral therapy (ART) use was characterized by receipt of the drug during the study period and prior to the censor date. HIV viral load and CD4 cell count were identified as the most recent laboratory value prior to the censor date.
Smoking Ascertainment
Smoking status was not available as a coded field in the RPDR but was routinely recorded in free text fields. We used natural language processing software, developed by a collaborative BWH/MGH team, to interpret the free text in the electronic medical record and assign a smoking status. This software has been previously validated with RPDR data and found to have an accuracy of 90 percent.13 We applied this software to the text for each patient during the last 12 months of observation.
Statistical Analysis
In the primary analysis, CD4 count and HIV viral load data were represented as dichotomous variables with breakpoints for clinically relevant cutoffs. In sensitivity analyses, recent and nadir CD4 count were expressed as continuous variables in increments of 50/mm3. For all analyses using continuous HIV RNA data, laboratory values were log transformed.
We used logistic regression modeling to test the hypothesis that CD4 count and HIV viral load are independently associated with AMI, accounting for demographic factors, CVD risk factors, any individual ART significantly associated with AMI risk in univariate analysis, and years since first ART use. The models included patients with complete data for each covariate included. CVD risk factors included hypertension, diabetes, dyslipidemia, and chronic kidney disease. A series of models was performed representing CD4 count and viral load as dichotomous versus continuous variables, as recent versus nadir or peak laboratory values, and in combination or individually included in the model.
Statistical analysis was conducted using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC) and a P value less than 0.05 was considered to indicate statistical significance.
Results
A total of 6,517 patients met criteria for the study, with AMI occurring in 273 (4.2%). Demographic characteristics, cardiovascular risk factors, HIV-related factors, antiretroviral medication use, and encounter history are shown in Table 1. The median time from laboratory measurement to either AMI or last encounter was 55 days for CD4 (interquartile range [IQR] 161) and 55 days for HIV viral load (IQR 144). Patients with an AMI had significantly higher rates of hypertension, diabetes, chronic kidney disease, and coronary heart disease and higher rates of dyslipidemia and smoking. Among patients with AMI, a significantly increased proportion had CD4 counts less than 200/mm3 or HIV viral loads greater than 100,000 copies/ml. Patients with AMI were more likely to be African-American and had more encounters but a shorter overall duration of follow-up in the health care system. Antiretroviral medication use patterns according to AMI status are shown in the table.
Table 1.
Patient Characteristics
| All | AMI | No AMI | |
|---|---|---|---|
| N=6517 | N=273 | N=6244 | |
| Demographics | |||
| Female Gender - N (%) | 1993 (30.6) | 85 (31.1) | 1908 (30.6) |
| Age - Mean (SD) | 46.0 (11.7) | 53.7 (11.9) | 45.7 (11.6) |
| Race | |||
| African-American - N (%) | 1432 (23.7) | 91 (34.6) | 1341 (23.2) |
| Caucasian - N (%) | 3324 (55.0) | 122 (46.4) | 3202 (55.4) |
| Hispanic - N (%) | 1064 (17.6) | 45 (17.1) | 1019 (17.6) |
| Other - N (%) | 220 (3.6) | 5 (1.9) | 215 (3.7) |
| CVD-Related Factors | |||
| Hypertension - N (%) | 1700 (26.1) | 155 (56.8) | 1545 (24.7) |
| Diabetes - N (%) | 1053 (16.2) | 77 (28.2) | 976 (15.6) |
| Dyslipidemia - N (%) | 1898 (29.1) | 91 (33.3) | 1807 (28.9) |
| Smoking - N (%) | 1643 (49.9) | 124 (54.6) | 1519 (49.5) |
| Chronic Kidney Disease - N (%) | 455 (7.0) | 37 (13.6) | 418 (6.7) |
| Coronary Heart Disease - N (%) | 673 (10.3) | 95 (34.8) | 578 (9.3) |
| HIV-Related Factors | |||
| VL Available | 3424 (52.5) | 111 (40.7) | 3313 (53.1) |
| VL<400 copies/ml - N (%) | 2126 (62.1) | 53 (47.8) | 2073 (62.6) |
| VL>100,000 copies/ml - N (%) | 342 (10.0) | 22 (19.8) | 320 (9.7) |
| CD4 Available | 3887 (59.6) | 191 (70.0) | 3696 (59.2) |
| CD4<200/mm3 - N (%) | 1018 (26.2) | 79 (41.4) | 939 (25.4) |
| Antiretroviral Medications | |||
| Any ART | 3237 (49.7) | 155 (56.8) | 3082 (49.4) |
| NRTI - No. (%) | 3108 (47.7) | 150 (55.0) | 2958 (47.4) |
| Abacavir - No. (%) | 1018 (15.6) | 58 (21.3) | 960 (15.4) |
| Didanosine - No. (%) | 595 (9.1) | 54 (19.8) | 541 (8.7) |
| Emtricitabine - No. (%) | 1199 (18.4) | 19 (7.0) | 1180 (18.9) |
| Lamivudine - No. (%) | 2182 (33.5) | 109 (39.9) | 2073 (33.2) |
| Stavudine - No. (%) | 848 (13.0) | 60 (22.0) | 788 (12.6) |
| Tenofovir - No (%) | 1727 (26.5) | 36 (13.2) | 1691 (27.1) |
| Zidovudine - No. (%) | 1381 (21.2) | 51 (18.7) | 1330 (21.3) |
| NNRTI - No. (%) | 1859 (28.5) | 82 (30.0) | 1777 (28.5) |
| Efavirenz - No. (%) | 1525 (23.4) | 56 (20.5) | 1469 (23.5) |
| Nevirapine - No. (%) | 493 (7.6) | 35 (12.8) | 458 (7.3) |
| PI - No. (%) | 1969 (30.2) | 84 (30.8) | 1885 (30.2) |
| Atazanavir - No. (%) | 727 (11.2) | 15 (5.5) | 712 (11.4) |
| Indinavir - No. (%) | 339 (5.2) | 21 (7.7) | 318 (5.1) |
| Lopinavir-Ritonavir - No. (%) | 918 (14.1) | 37 (13.6) | 881 (14.1) |
| Nelfinavir - No. (%) | 496 (7.6) | 38 (13.9) | 458 (7.3) |
| Ritonavir - No. (%) | 1514 (23.2) | 50 (18.3) | 1464 (23.5) |
| Saquinavir - No. (%) | 169 (2.6) | 15 (5.5) | 154 (2.5) |
| Clinical History | |||
| Duration in years - Median (IQR) | 5.1 (7.4) | 2.9 (5.3) | 5.2 (7.5) |
| Encounters - Median (IQR) | 42 (98) | 66 (125) | 41 (97) |
CD4 and VL proportions are calculated using patients with the relevant laboratory data available as denominator. ARV proportions are calculated using all patients (not those who received ART) as the denominator.
SD, standard deviation; CVD, cardiovascular disease; VL, viral load; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; IQR, interquartile range.
In univariate regression models, CD4 count less than 200/mm3 (OR 2.00, 95% CI 1.48-2.71; P<0.0001) and viral load greater than 100,000 copies/ml (OR 2.23, 95% CI 1.37-3.65; P=0.001) were associated with an increased risk of AMI. Conversely, viral load less than 400 copies/ml (OR 0.56, 95% CI 0.38-0.82; P=0.003) was associated with a decreased risk of AMI.
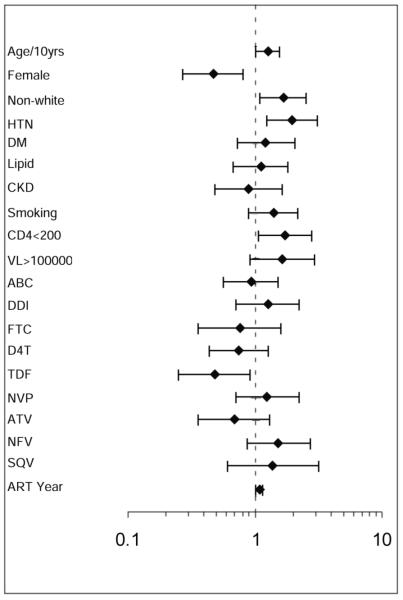
In a multivariate regression model adjusting simultaneously for CD4 count, viral load, age, gender, race, hypertension, diabetes, dyslipidemia, chronic kidney disease, smoking, years since first ART use, and antiretroviral medications individually associated with AMI, CD4 count less than 200/mm3 was significantly associated with an increased risk of AMI (OR 1.74, 95% CI 1.07-2.81; P=0.02). Having a viral load greater than 100,000 was also a predictor of AMI, but this effect did not reach statistical significance (OR 1.63, 95% 0.91-2.93, P=0.10). We tested for an interaction and did not find evidence of a statistically significant interaction between CD4 count and viral load as dichotomous variables (P=0.47). Other covariates significantly associated with an increased risk of AMI included age, male gender, non-Caucasian race, and hypertension. Tenofovir, but not other individual antiretroviral medications, was associated with AMI (OR 0.48, 95% CI 0.25-0.92, P=0.03). The odds ratios and 95 percent confidence intervals for all covariates in the model are shown in Figure 1.
Figure 1.
Odds Ratio for AMI in a Multivariate Analysis
Odds ratios and 95% confidence intervals are shown for each cardiovascular risk factor with respect to AMI risk. The odds ratios for all covariates in the model are shown. Medications were included in the model if they were significantly associated with AMI in univariate analyses. Age is represented in 10 year increments. ABC indicates abacavir; DDI, didanosine; FTC, emtricitabine; D4T, stavudine; TDF, tenofovir; NVP, nevirapine; ATV, atazanavir; NFV, nelfinavir; SQV, saquinavir; ART, antiretroviral therapy.
A series of models was constructed to further explore the relationships between CD4 and viral load with AMI. When both CD4 count and viral load were represented as continuous variables, a CD4 count increase of 50/mm3 was associated with a decreased risk of AMI (OR 0.93, 95% CI 0.89-0.97; P=0.002). Similarly, when CD4 nadir and peak viral load were evaluated in the same model, a CD4 nadir count increase of 50/mm3 was associated with a somewhat decreased risk of AMI, although this result did not reach statistical significance (OR 0.95, 95% CI 0.89-1.01, P=0.09). Viral load did not significantly predict AMI when included with CD4 count as a continuous variable or as peak level.
When included individually in the multivariate model without viral load, the effects of the CD4 count on AMI were similar, with CD4 count less than 200/mm3 conferring an increased risk (OR 1.53, 95% CI 1.08-2.16, P=0.02) and a CD4 count increase of 50/mm3 conferring a decreased risk (OR 0.95, 95% CI 0.92-0.98, P=0.001). An increase of CD4 nadir count did not demonstrate a significant effect. In multivariate models including viral load parameters but not CD4 count, a higher viral load was consistently and significantly associated with AMI (for viral load greater than 100,000 copies/ml, OR 2.16, 95% CI 1.26-3.69, P=0.01; for each log10 increase in viral load, OR 1.23, 95% CI 1.04-1.44, P=0.01; for each log10 increase in peak viral load, OR 1.23, 95% CI 1.04-1.44, P=0.02). Viral load less than 400 copies/ml significantly predicted a decreased risk of AMI (OR 0.60, 95% CI 0.38-0.93, P=0.02).
Discussion
Using data from a large U.S. health care system clinical cohort, we demonstrate that decreased CD4 count is significantly associated with an increased risk of myocardial infarction, and is second only to hypertension in terms of its effect size as a risk factor. Furthermore, having a CD4 count less than 200/mm3 was a more important factor that any individual antiretroviral medication with respect to increased risk of AMI. Increased viral load was also a predictor of AMI risk, although it was not an independent risk factor when included concurrently in a model with CD4 count. The finding that immunologic and virologic parameters are related to AMI independent of CVD risk factors and antiretroviral medications suggests that immune dysfunction and inflammation may be important etiologic factors for CVD among HIV patients.
In multiple analyses, we demonstrated that CD4 count was associated with AMI risk, independent of CVD risk factors and antiretroviral medications. Confirmatory analyses showed that increases in CD4 count confer a reduction of AMI risk. Several studies have demonstrated a lower CD4 count to be associated with surrogate markers of atherosclerosis or vascular disease, with a recent CD4 count less than 200/mm3 correlated with an increased prevalence of carotid artery lesions4 and a nadir CD4 count less than 350/mm3 independently associated with increased arterial stiffness.5
Studies specifically investigating the effect of CD4 count on myocardial infarction, however, are limited. Initial data from one study showed a CD4 count of less than 200/mm3 in virologically suppressed patients to be associated with a significantly increased rate of a combined cardiovascular outcome,10 and several other studies showed decreased CVD outcomes or CVD mortality with increased CD4 count, but these latter results failed to reach statistical significance.6, 7 Several additional studies have failed to show an association of CD4 count with CVD outcome,3, 8, 9 but only one assessed the specific outcome of myocardial infarction and adjusted for many relevant CVD risk factors.3 Our analyses, specifically designed to investigate the outcome of AMI, account for specific CVD risk factors including chronic kidney disease, recently shown to be an independent CVD risk factor in HIV patients,14 as well as individual antiretroviral medications which might have proatherogenic effects. Our findings therefore offer strong support for an independent role of immunosuppression as a contributing factor for AMI in HIV patients.
While the effects of immunosuppression appear to outweigh those of increased viremia in our dataset, HIV viral load was a significant predictor of AMI in multivariate analyses when immune parameters were not concurrently considered. Its effect was independent of traditional CVD risk factors and of individual antiretroviral medications, and highlights the possibility that active viremia with possible accompanying inflammation might heighten CVD risk. Prior data on the effect of HIV viral load on CVD outcomes is conflicting, with one study showing a nearly four-fold increase in cardiovascular mortality with higher HIV RNA7 and another showing no association between peak HIV RNA and risk of myocardial infarction.3 While the SMART study demonstrated increased CVD event rates for patients in the drug conservation arm with a hazard ratio of 1.57, there was not a specific association of viral load with CVD events.11
Our data reinforce the emerging hypothesis that treatment of HIV infection decreases the risk of cardiovascular disease. Our study is one of the first to specifically and rigorously assess the relationship between immunologic and virologic indices and AMI, adjusting for potential confounding factors including traditional CVD risk factors and individual antiretroviral medications, some of which are thought to confer increased CVD risk.15, 16 Cardiovascular risk reduction might therefore be an additional benefit of earlier initiation of ART, as endorsed by the most recent U.S. Department of Health and Human Services HIV treatment guidelines.17
We additionally assessed other factors which might confer cardiovascular risk and showed demographics and traditional risk factors, including age, male gender, non-Caucasian race, and hypertension, to be significant predictors of AMI. Patients with AMI tended to have higher rates of dyslipidemia, but this relationship was not significant in multivariate analysis accounting for other traditional risk factors and ART. Finally, individual antiretroviral medications that were significant in univariate analyses were assessed and with the exception of tenofovir, none contributed significantly to AMI risk when accounting for traditional CVD risk factors and HIV-related indices. The protective effect of tenofovir should be interpreted with caution as the potential for preferential prescribing to patients of perceived low cardiovascular risk during the study period might have resulted in confounding by indication.
Our study was limited by several factors intrinsic to clinical care cohorts. Data on certain HIV indices were limited for a number of patients, but the association of CD4 count with AMI was significant in a final model in which all covariates were available for all patients, suggesting our data set was large enough to perform a highly rigorous analysis. Furthermore, the fact that the median time from both CD4 count and HIV viral load to event or last encounter was 55 days provided reassurance that these laboratory values with potential for relatively rapid change were measured proximally to event dates. Our results reflect treatment practices in a large U.S. health care system during the modern era of ART. Different results may be seen resulting from analyses in other settings with differing prescribing practices.
Our data suggest that immunologic status is the most important HIV-related factor associated with AMI risk and confers a greater risk than any individual medication. We demonstrate a relationship between elevated viral load and increased rates of AMI which is only attenuated when CD4 count is also considered. Our finding that overall CVD risk is lower in patients with improved immune function suggests an overall global benefit of ART on CVD risk, despite varying risk associated with individual drugs. Treatment of HIV infection to improve immunologic function is likely to be an important component of cardiovascular prevention for HIV patients.
Acknowledgements
The authors are grateful for support from the Harvard University CFAR biostatistics core (Heather Ribaudo) for statistical consultation and Shawn Murphy, MD, PhD (Massachusetts General Hospital Laboratory of Computer Science) and the Partners HealthCare Research Patient Data Registry group for facilitating use of their database and natural language processing tool.
Funded in part by K01 AI073109 (VAT); K24 DK080140 (JBM); K24 DK064545 (SKG)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2003;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008 Aug 20;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho J, Deeks S, Hecht F, et al. Earlier Initiation of ART in HIV-Infected Individuals Is Associated with Reduced Arterial Stiffness. Paper presented at: Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- 6.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. Aids. 2008 Apr 23;22(7):841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. Aids. 2009 Aug 24;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. Aids. 2010 Jun 19;24(10):1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet F, Chene G, Thiebaut R, et al. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000-2004. HIV Med. 2007 Nov;8(8):547–554. doi: 10.1111/j.1468-1293.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 10.van Lelyveld S, Gras L, Kesselring A, et al. Incomplete Immune Recovery on HAART Is Associated with Significantly More Cardiovascular Events and a Trend Towards More Non-AIDS-related Malignancies in Dutch ATHENA Cohort. Paper presented at: Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- 11.Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 12.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009 Jul 1;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng QT, Goryachev S, Weiss S, Sordo M, Murphy SN, Lazarus R. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. BMC Med Inform Decis Mak. 2006;6:30. doi: 10.1186/1472-6947-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George E, Lucas GM, Nadkarni GN, Fine DM, Moore R, Atta MG. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. Aids. 2009 Dec 17; doi: 10.1097/QAD.0b013e3283359253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010 Feb 1;201(3):318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 16.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. Aids. 2008 Sep 12;22(14):F17–24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panel on Antiretroviral Guidelines for Adults and Adolescents . Department of Health and Human Services; [Accessed July 14, 2010]. Dec 1, 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents; pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]