Abstract
Backgrounds
Although most patients with EoE have mucosal and structural changes that could potentially explain their symptoms, it is unclear whether EoE is associated with abnormal esophageal motor function. The aims of this study were to evaluate the esophageal pressure topography (EPT) findings in EoE and to compare them with controls and patients with gastro-esophageal disease (GERD).
Methods
EPT studies in 48 EoE patients, 48 GERD patients and 50 controls were compared. The esophageal contractile pattern was described for ten 5-ml swallows for each subject and each swallow was secondarily characterized based on the bolus pressurization pattern: absent, pan-esophageal pressurization, or compartmentalized distal pressurization.
Key Results
37% of EoE patients were classified as having abnormal esophageal motility. The most frequent diagnoses were of weak peristalsis and frequent failed peristalsis. Although motility disorders were more frequent in EoE patients than in controls, the prevalence and type were similar to those observed in GERD patients (p=0.61, Chi square test). Pan-esophageal pressurization was present in 17% of EoE and 2% of GERD patients while compartmentalized pressurization was present in 19% of EoE and 10% of GERD patients. These patterns were not seen in control subjects.
Conclusions & Inferences
The prevalence of abnormal esophageal motility in EoE was approximately 37% and was similar in frequency and type to motor patterns observed in GERD. EoE patients were more likely to have abnormal bolus pressurization patterns during swallowing and we hypothesize that this may be a manifestation of reduced esophageal compliance.
Keywords: eosinophilic esophagitis, esophageal pressure topography, weak peristalsis, high resolution manometry
Introduction
Intermittent dysphagia and food impaction are the most common symptoms in adult patients with eosinophilic esophagitis (EoE) (1). The typical findings on endoscopy include mucosal rings, linear furrows, and white papules. In some patients the endoscopic pattern may explain the cause for dysphagia, especially in cases of esophageal strictures or small caliber esophagus (2). However mucosal rings are often widely patent in EoE patients or dysphagia may occur without any significant endoscopic findings.
Esophageal dysmotility has been proposed as an alternative explanation for dysphagia in EoE patients outside of the overt mechanical obstruction associated with rings and stenosis. Conventional manometric studies assessing esophageal motor function in EoE have reported varied results ranging from normal peristalsis to absent peristalsis as well as motility disorders including achalasia, diffuse esophageal spasm, nutcracker esophagus, high amplitude contractions, ineffective peristalsis, and non specific motor disorders. In a previous review using conventional manometry, Nurko et al noted that abnormal motility findings may be seen in 41% of patients (3) and that non-specific disorders were the most frequent abnormalities encountered (71% of patients with abnormal manometric findings).
Although the histopathology of EoE is primarily defined by the infiltration of the mucosa with eosinophils, there are other features that may promote esophageal dysmotility. Increased contraction of fibroblasts has been observed with eosinophils and fibroblasts in co-culture (4) and axonal necrosis has been described in EoE (5). Additionally, the control of esophageal contraction could potentially be modified as the binding between eosinophil major protein and muscarinic acetylcholine receptors will promote smooth muscle contraction (6). Finally, eosinophils have profibrogenic properties and chronic inflammation with eosinophils may induce a secondary fibrosis (7). Although fibrosis may be responsible for the stiffness of the esophageal wall encountered in many EoE patients, it could also potentially disrupt esophageal contractile function.
High resolution manometry (HRM) displayed as esophageal pressure topography (EPT) is becoming a widely accepted technique for evaluating and categorizing esophageal motility disorders in clinical practice because of its technical advantages over conventional manometry (8). However, HRM and EPT findings in eosinophilic esophagitis have not yet been reported other than in small case series or case reports (9–10). Our preliminary findings using EPT suggest that EoE patients exhibit abnormalities in pressurization patterns consistent with reduced esophageal compliance and functional outflow obstruction. Thus, the aim of this study was to evaluate both the contractile and pressurization patterns observed in EPT studies of EoE patients and to compare the results with controls and subjects with gastro-esophageal reflux disease (GERD).
Patients and Methods
Subjects
We identified 48 patients diagnosed with EoE who had active disease at the time of manometry from a consecutive series of 2,500 clinical high resolution EPT studies conducted between August 2004 and May 2010. The diagnosis of EoE was based on esophageal symptoms suggestive of EoE and endoscopic biopsies with histopathology confirming EoE (>15 eos/hpf; magnification 0.196mm2 ) (11). Reflux monitoring was not consistently performed to exclude gastro-esophageal reflux disease in these patients. From the same series, we identified 48 consecutive patients with gastroesophageal reflux disease (GERD) verified by symptom profile and ambulatory reflux testing. GERD patients were defined by either esophageal acid exposure >5% (n= 44) while not taking a PPI (12–13) or greater than 73 reflux events in 24 hours detected on impedance monitoring (n=4) (14). Subjects with previous upper gastrointestinal surgery were excluded from the study. These groups were compared to 50 healthy volunteers randomly selected from a database of 75 healthy volunteers.
The study protocol for healthy volunteers was approved by the Northwestern University Institutional Review Board (PJK) and informed consent was obtained from each subject. The study protocol for the retrospective analysis of EPT findings in the EoE and GERD patients was also approved by the Northwestern University Institutional Review Board (JEP).
EPT protocol
EPT studies were done with a 4.2 mm outer diameter solid-state assembly with 36 circumferential sensors spaced at 1-cm intervals (Sierra Scientific Instruments, Los Angeles, CA). Before recording, transducers were calibrated at 0 and 300 mmHg using externally applied pressure. Studies were done in a supine position after at least a 6-hr fasting period. The manometry assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about 3 intra-gastric sensors. The catheter was fixed in place by taping it to the nose. The manometric protocol included at least a 30-s period to assess basal sphincter pressure and 10 5-ml water swallows. In some subjects two 10-ml and one 20-ml water swallows were performed.
EPT Analysis
EPT data were analyzed using ManoView™ analysis software (Sierra Scientific Instruments, Los Angeles). Esophago-gastric junction (EGJ) morphology was classified as type I when separation between the highest pressure of the lower esophageal sphincter (LES) and crural diaphragm was indiscernible (<1 cm), type II when LES-crural diaphragm separation was > 1 cm and < 2 cm and type III when LES-crural diaphragm separation was >2 cm. End-expiratory and end-inspiratory EGJ pressure and intragastric pressure were measured during the basal period without swallowing. EGJ relaxation pressure was assessed with the 4-s integrated relaxation pressure (4-s IRP) for the ten 5-ml swallows (15).
Each swallow was further characterized using the isobaric contour tool to determine the location and length of breaks in peristalsis if present. The distal contractile integral (DCI), the contractile front velocity (CFV) between the transition zone and the contractile deceleration point, and the maximal intra bolus pressure (IBP) were also measured for each swallow (16–17). The individual swallow contractile patterns were categorized using the above parameters into 5 patterns as specified in Table 1.
Table 1.
Contraction patterns using EPT
| Contraction pattern | Esophageal pressure topography characteristics |
|---|---|
| Failed | Minimal (<3 cm) integrity of the 20-mmHg isobaric contour distal to the proximal pressure through |
| Weak peristalsis | Presence of a break in the 20-mmHg isobaric contour greater than 2 cm |
| • Subdivided into small (2–5 cm) and large (>5 cm) breaks | |
| Hypertensive | Normal CFV with DCI > 5,000 mmHg.cm.s |
| Rapidly propagated | CFV ≥ 10 cm/s |
| Normal | Not achieving any of the above diagnostic criteria |
Elevated IBP was defined as exceeding 25 mm-Hg referenced to atmospheric pressure. This threshold was chosen based on data assessing flow permissive pressures through the EGJ and confirmed by assessing the 95th percentile range of IBP in asymptomatic control subjects (17–18). The presence of elevated IBP was further characterized as i) pan-esophageal pressurization when it spanned the length of the esophagus, or ii) compartmentalized distal esophageal pressurization when the pressurization extended distally from the contractile front to the EGJ (Figure 1). Moreover, IBP was measured for each swallow 2 cm above the proximal border of the EGJ and defined as the greatest pressure for a 3-s period (contiguous or non-contiguous) within the same temporal boundaries used to calculate the IRP (19). The resultant maximal IBP for each subject was calculated as the mean of the 3 greatest single swallow values as this was previously established as optimal discriminator (19).
Figure 1.
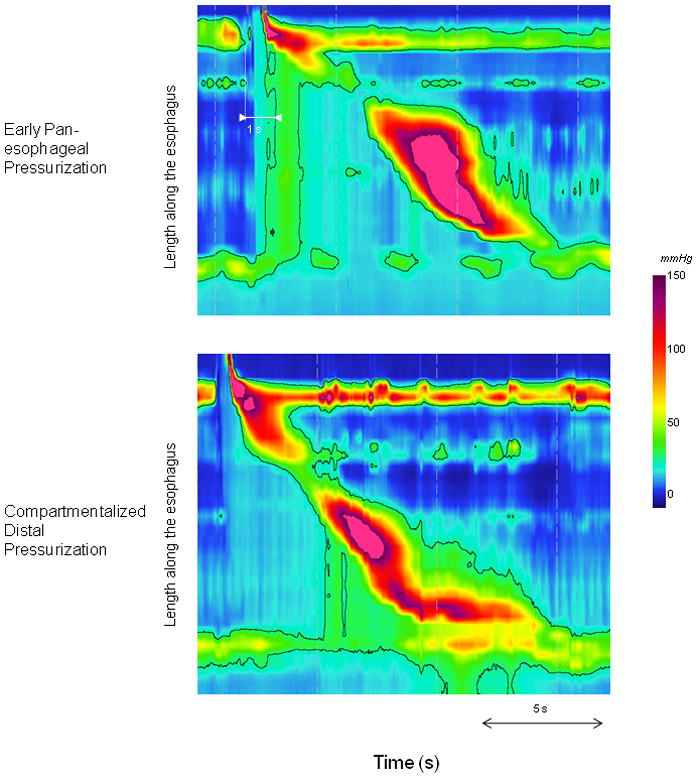
Examples of different pressurization patterns at the 25-mmHg isobaric contour in patients with eosinophilic esophagitis. The early pan-esophageal pressurization occurred in the context of normal peristalsis and normal IRP. The compartmentalized distal pressurization occurred in a patient with normal peristalsis and normal mean IRP (9.5 mmHg). For this particular swallow, the IRP is slightly elevated (16.6 mmHg).
Based on the different metrics and the pattern of the contractile activity for the ten 5-ml swallows, esophageal motility disorders were diagnosed and categorized using the most recent published version of the Chicago classification (16) modified for the diagnosis of weak peristalsis (>20% swallows with large breaks or >30% of small breaks) and frequent failed peristalsis (40 to 90% of failed swallows) (20).
Statistical Analysis
Data were expressed as median (interquartile range) unless otherwise specified. Among the 3 groups, categorical data were compared using Chi square test and continuous data using Mann Whitney or Kruskal Wallis tests. A p-value less than 0.05 was considered as significant.
Results
Demographic, clinical and endoscopic characteristics of the subjects
The demographic and clinical characteristics of the subjects are summarized in Table 2. The control subjects were significantly younger than the EoE and GERD patients (p<0.01) and the EoE patients were also younger than the GERD patients (p=0.049). All of the EoE patients but 2 reported having dysphagia at the time of the HRM study.
Table 2.
Demographic, clinical and endoscopic characteristics of the subject groups.
| Control subjects | EoE patients | GERD patients | |
|---|---|---|---|
| Number | 50 | 48 | 48 |
| Mean age (range) yrs | 28 (19–48) | 42 (19–77) | 48 (20–83) |
| Gender (male/female) | 29/21 | 28/20 | 23/25 |
| Symptoms | |||
| Dysphagia | 0 | 46 (96%) | 4 (8%) |
| Food impaction | 0 | 28 (58%) | 0 |
| Heartburn ± Regurgitation | 0 | 22 (46%) | 36 (75%) |
| Atypical GERD symptoms | 0 | 1 (2%) | 12 (25%) |
| Endoscopy* | |||
| Rings | NA | 26 (54%) | 0 |
| Furrows | NA | 29 (60%) | 0 |
| Stricture | NA | 7 (15%) | 0 |
| Schatzki ring | NA | 9 (19%) | 2 (4%) |
| Hiatus hernia | NA | 1 (2%) | 12 (25%) |
| Esophagitis (Los Angeles classification) | NA | 0 | 7 (15%) grade A, 3 (6%) grade B, 1 (2%) grade D |
NA not available
Endoscopic examination revealed esophageal rings associated with furrows in 18 EoE patients; 7 were judged to have a dominant ring. Ten EoE patients had no rings or furrows: 2 had mucosal exudates, 2 had a distal Schatzki ring, 2 had a distal stricture, 1 had narrowing of the distal esophagus and 4 had a normal endoscopy. One GERD patient had esophagitis dessicans superficialis; in this case the biopsies were negative for eosinophils. Additionally, 20 of the GERD patients had esophageal biopsies that were negative for eosinophils, while 27 did not have biopsies for evaluation.
EPT in the three subject groups
The majority of subjects had type I EGJ morphology. One control, 1 EoE and 4 GERD patients had type II EGJ morphology and 2 EoE and 9 GERD patients had type III EGJ morphology. As shown in Table 3, baseline EGJ pressure was statistically similar among the three groups; however, the GERD patients had a numerical trend toward lower end-expiratory EGJ pressure. The mean IRP was normal in all but 1 control (IRP = 16 mmHg) and elevated in 5 EoE patients (17–23 mmHg) and 3 GERD patients (15 to 22).
Table 3.
Manometric data in the different groups of subjects. Data are expressed as median (interquartile range) unless otherwise specified.
| Controls | EoE patients | GERD patients | p (Kruskal Wallis test) | |
|---|---|---|---|---|
| Expiratory EGJ pressure (mmHg) | 15 (9–22) | 18 (8–31)† | 10 (5–18)* | 0.04 |
| Inspiratory EGJ pressure (mmHg) | 21 (16–28) | 23 (14–40) | 20 (11–28) | 0.24 |
| IRP (mmHg) | 8.4(6.1–10) | 7.8 (6.2–10.9) | 7.3 (4.4–9.4) | 0.17 |
| Normal peristalsis (n) | 10 (9–10) | 8 (3–10)* | 7 (2–9)* | <0.01 |
| Failed peristalsis (n) | 0 (0–0) | 0 (0–2)* | 0 (0–1)* | <0.01 |
| Weak peristalsis (n) | 0 (0–1) | 1 (0–2)*† | 2 (1–6)* | <0.01 |
| Pressurization | <0.01** | |||
| • Pan-esophageal | ||||
| Number of subjects (%) | 0 (0%) | 8 (17%)¶‡ | 1 (2%)¶ | |
| • Compartmentalized distal | ||||
| Number of subjects (%) | 0 (0%) | 9 (19%)¶‡ | 5 (10%)¶ | |
EGJ Esophagogastric junction; IRP Integrated Relaxation Pressure
p<0.05 vs healthy volunteers (Mann Whitney test)
p<0.05 vs GERD patients (Mann Whitney test)
Chi square test
p<0.05 vs healthy volunteers (Chi square test)
p<0.05 vs GERD patients (Chi square test)
Figure 2 depicts the distribution of EPT diagnoses among the controls, EoE, and GERD patients. Motility disorders were significantly more frequent in EoE and GERD patients than in controls (p=0.02) but the occurrence and the type of motility disorders did not differ between GERD patients and EoE patients (p=0.61). Weak peristalsis and frequent failed peristalsis were the most common diagnoses observed in EoE and GERD patients. This was consistent with the observation that EoE and GERD patients had significantly more swallows with failed and weak peristalsis than controls (Table 3). The subgroup of GERD patients with esophageal biopsies negative for eosinophils was also not different from the EoE patients (p=0.25).
Figure 2.
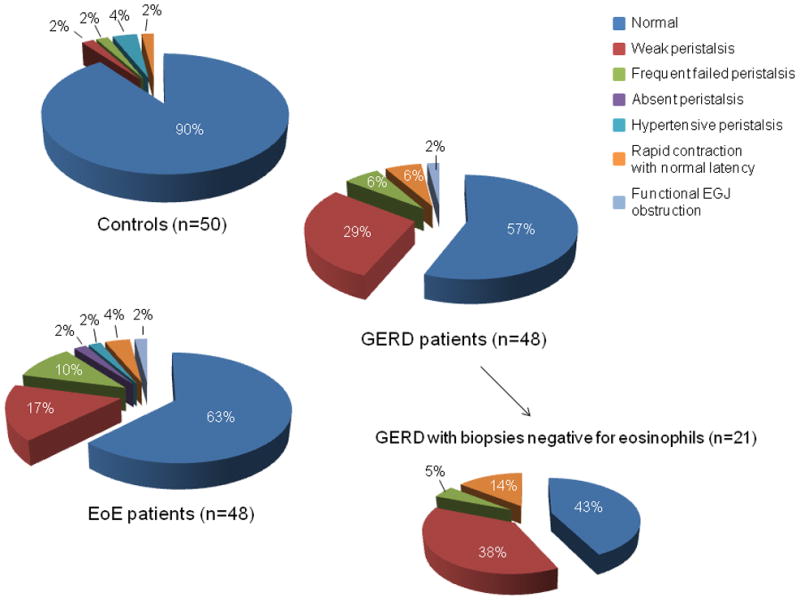
Distribution of manometric diagnosis in controls, EoE, and GERD patients. Weak peristalsis, frequent failed peristalsis, hypertensive peristalsis and rapid contraction with normal latency are present in the 3 groups of subjects in different proportions. The motility disorders were more frequent in EoE and GERD patients (p=0.02) but no significant difference was observed between EoE and GERD patients (p=0.61) The distribution of manometric diagnosis is also shown in the subgroup of GERD patients with negative esophageal biopsies and was not significantly different from that of the EoE patients (p=0.25).
Esophageal pressurization
The distribution of pressurization patterns among subject groups is shown in Table 3. There was a significant difference in the prevalence of abnormal pressurization (p<0.01) among the 3 groups. It is important to note that abnormal pressurization was not observed in any control subject. However, abnormal pressurization patterns were found in 36% of EoE patients (pan-esophageal pressurization 17%; compartmentalized distal pressurization 19%) and 12% of GERD patients (pan-esophageal pressurization 2%; compartmentalized distal pressurization 10%). The pan-esophageal pressurization occurred within 2-s of the esophageal contraction (early pan-esophageal pressurization) in all but one EoE patient. When pressurization was present the median (range) number of affected swallows per subject was 1(1–9) for pan-esophageal pressurization and 2 (1–9) for compartmentalized distal pressurization in EoE patients. In the group of GERD patients, the only subject with pan-esophageal pressurization had only 1 swallow affected and the median (range) number of swallows with compartmentalized pressurization was similar to what was observed in EoE patients (2 (1–8), p=0.45, Mann Whitney test). Figure 3 illustrates the distribution of maximal IBP among the 3 subject groups. There was no difference between controls and EoE patients (p=0.1) whereas GERD patients had a lower IBP than controls (p=0.02) or EoE patients (p<0.01).
Figure 3.
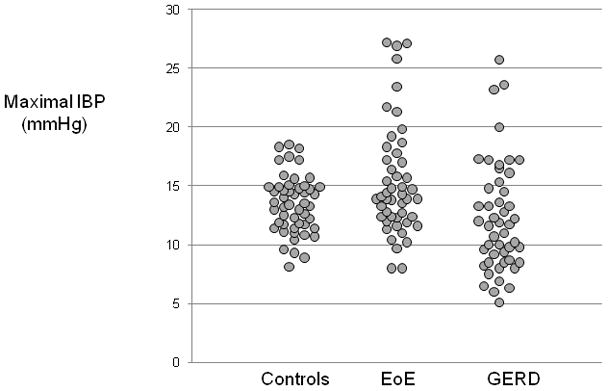
Distribution of the maximal IBP among the controls, EoE and GERD patients. The distribution was significantly different between controls and GERD (p=0.02) and between EoE and GERD (p=0.01), but not between controls and EoE (p=0.1).
The majority of EoE patients with abnormal pressurization had normal EGJ findings (76%) with only three patients exhibiting an elevated IRP and one subject exhibiting type III EGJ morphology. Of the 17 patients with abnormal pressurization; 2 had a normal endoscopy while the other patients had endoscopic features consistent with EoE. Seven of the EoE patients with abnormal pressurization had obstructive findings on endoscopy (Schatzki ring, stricture, esophageal narrowing, or dominant stricture) while 10 did not (p=1.00). All but one of the GERD patients with abnormal pressurization had esophagitis; one subject had a normal endoscopy with an elevated IRP. Only 2 of the 21 GERD patients with biopsies negative for eosinophils had an instance of esophageal pressurization (1 pan-esophageal pressurization and 1 compartmentalized distal pressurization).
Pressurization for 5-, 10- and 20-ml swallows
Ten-ml test swallows were done in 32 controls, 38 EoE and 23 GERD patients; 20-ml swallows in 32 controls, 37 EoE and 12 GERD patients. Comparing only for the final 5- and 10-ml swallow to the 20 ml swallows, the bolus challenge increased the number of swallows with pressurization in the control subjects and EoE patients. Thirty-two percent of EoE patients had early pan-esophageal pressurization for the 20-ml swallow versus 3% of controls and 8% of GERD patients (p=0.04) (Figure 4).
Figure 4.
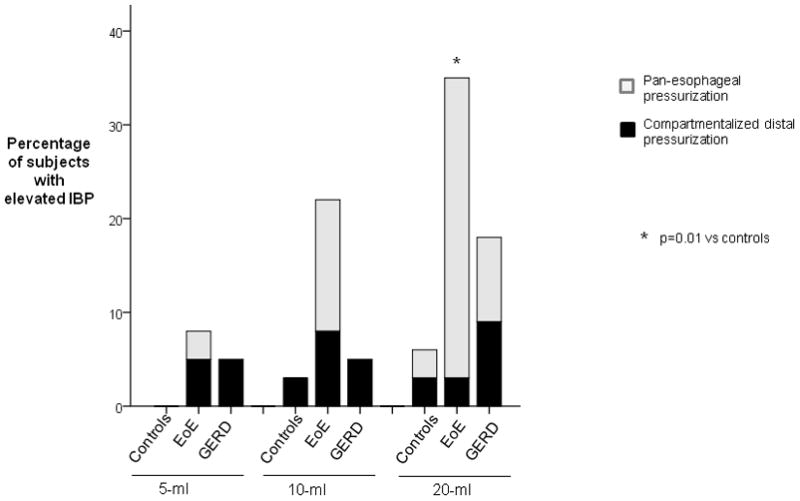
Percentage of subjects with pressurization at 25-mmHg isobaric contour for 5, 10, and 20-ml swallows. The number of subjects with pressurization was significantly different among the 3 groups (p=0.04).
Discussion
This study represents the first systematic investigation of EPT findings in EoE patients, comparing them to normal control subjects and GERD patients. Abnormal EPT was encountered in 37% of EoE patients with the most frequent diagnoses being weak peristalsis and frequent failed peristalsis. While these findings distinguish the EoE group from the control subjects, the same spectrum of diagnoses were encountered in GERD patients. However, EoE patients were much more likely to have abnormal bolus pressurization in the esophagus compared to GERD patients or control subjects and it appeared that early pan-esophageal pressurization after swallowing was a specific finding in EoE.
Various esophageal motility disorders have been described in previous smaller series of adult and pediatric EoE patients using conventional manometry (10, 21–25). These studies report a wide range of prevalence rates for esophageal motor abnormalities (22 to 90%) with the most frequent observed disorders being weak and failed peristalsis. However, no control populations were included in these studies and the motility patterns encountered do not seem to be specific for EoE. In fact, our data suggest that although the frequency of weak and failed peristalsis is increased in EoE, this finding is similar to what one observes in a GERD population. This finding is intriguing given the suggested clinical overlap between GERD and EoE.
A novel finding in this study was that increased bolus pressurization occurred in significantly more EoE patients than GERD patients or control subjects. A unique feature of EPT is the ability to assess pressurization patterns in the esophagus that occur independent of lumen-obliterating contractions. More than one third of the EoE patients had abnormalities in pressurization noted on EPT, compared to only 12% of GERD patients and none of the control subjects. Although these findings suggest some overlap with GERD, most of the overlap was associated with the pattern of distal compartmentalized pressurization in the context of abnormal EGJ structure or inflammation. Maximal IBP appears to be less useful than the panesophageal pressurization pattern to discriminate EoE patients from controls. This may be partly a methodological consideration as we measured the maximal IBP sustained for a period of 3 seconds and panesopahgeal pressurization is generally sustained for only shorter periods (Figure 1). The pattern of early pan-esophageal pressurization was unique to EoE patients during 5 ml swallows and this pattern of pressurization was more easily elicited during large volume (20 ml) swallows. We hypothesize that this pressurization pattern is a manifestation of reduced compliance in the esophagus. Recent work utilizing the functional luminal imaging probe (FLIP), a modification of impedance planimetry, to assess the luminal cross-sectional area during pressure distention, reported reduced esophageal wall distensibility in EoE compared to healthy control subjects (26). An alternative explanation for the pattern of early pan-esophageal pressurization would be exaggerated longitudinal muscle contraction and esophageal compression. That explanation, however, is unlikely as there is no evidence of augmented esophageal shortening in this patient population. Recent work utilizing intraluminal ultrasound suggests that longitudinal muscle contraction is reduced in EoE and further blunted after pharmacologic stimulation (27).
Our study has some limitations attributable to its retrospective design. Our control subjects were younger than both the EoE and the GERD patient groups and, thus, we cannot rule out an age effect on the occurrence of weak and failed peristalsis. However the distribution of age, although statistically different, was similar among EoE and GERD patients (42 versus 48) and the pattern of early pan-esophageal pressurization was much more frequent in EoE patients than in GERD (and completely absent in control subjects). We also have no data regarding esophageal acid exposure in our EoE patients and it is possible that some of the overlap in EPT findings between GERD and EoE could be secondary to reflux. However, this would not alter the observation that EoE is not associated with unique EPT diagnoses as the prevalence of weak and frequent failed peristalsis would be reduced if one excluded the potential effect of abnormal acid exposure and the associated peristaltic abnormalities that coexist with GERD. Vice-versa, there is also a possibility that some of the GERD patients could have overlap with EoE based on the fact that we only had histopathologic data on 21 GERD patients. However, our sub-analysis utilizing only GERD patients with a negative biopsy did not show a significant difference in the prevalence of contractile patterns between EoE and GERD patients with negative histopathology.
In conclusion, this study suggests that the most common EPT abnormalities found in EoE, weak persitalsis and frequent failed peristalsis, occur with similar frequency in EoE and GERD. Hence, neither is specific for EoE. However, pan-esophageal pressurization after swallowing in conjunction with normal EGJ relaxation was an EPT finding almost exclusive to EoE. This pattern of pressurization can be accentuated with larger volume swallows and is likely related to decreased esophageal compliance in EoE. Whether this finding is associated with more advanced disease or whether it can be utilized to monitor disease activity will require pre- and post-intervention studies with validated symptom end-points.
Acknowledgments
Acknowledgements and disclosures
This work was supported by R01 DK056033 (PJK) and R01 DK079902 (JEP) from the Public Health Service
Abbreviations
- CFV
Contractile front velocity
- DCI
Distal contractile integral
- EGJ
Esophagogastric junction
- EoE
Eosinophilic esophagitis
- EPT
Esophageal pressure topography
- GERD
Gastro-esophageal reflux disease
- HRM
High resolution manometry
- IBP
Intra bolus pressure
- IRP
Integrative relaxation pressure
- LES
Lower esophageal sphincter
- PPI
proton pump inhibitors
Footnotes
Competing Interest: the authors have no competing interests
Author’s contributions
Sabine Roman: Analysis and interpretation of data, drafting of the manuscript
Ikuo Hirano: Study concept and design, Analysis and interpretation of data
Monika Kwiatek: Analysis and interpretation of data
Nirmala Gonsalves: Analysis and interpretation of data
Joan Chen: Analysis and interpretation of data
Peter J. Kahrilas: Study concept and design, analysis and interpretation of data, drafting of the manuscript
John E. Pandolfino: Study concept and design, analysis and interpretation of data, drafting of the manuscript
References
- 1.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Fox VL. Eosinophilic esophagitis: endoscopic findings. Gastrointest Endosc Clin N Am. 2008;18:45–57. viii. doi: 10.1016/j.giec.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Nurko S, Rosen R. Esophageal dysmotility in patients who have eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:73–89. ix. doi: 10.1016/j.giec.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zagai U, Skold CM, Trulson A, Venge P, Lundahl J. The effect of eosinophils on collagen gel contraction and implications for tissue remodelling. Clin Exp Immunol. 2004;135:427–433. doi: 10.1111/j.1365-2249.2004.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevoff C, Rao S, Parsons W, Kahrilas PJ, Hirano I. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54:373–377. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 6.Hogan SP, Mishra A, Brandt EB, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 7.Levi-Schaffer F, Garbuzenko E, Rubin A, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci U S A. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahrilas PJ. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed? Am J Gastroenterol. 2010;105:981–987. doi: 10.1038/ajg.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 10.Hejazi RA, Reddymasu SC, Sostarich S, McCallum RW. Disturbances of Esophageal Motility in Eosinophilic Esophagitis: A Case Series. Dysphagia. 2009 doi: 10.1007/s00455-009-9248-6. [DOI] [PubMed] [Google Scholar]
- 11.Gonsalves N, Kahrilas PJ. Eosinophilic oesophagitis in adults. Neurogastroenterol Motil. 2009;21:1017–1026. doi: 10.1111/j.1365-2982.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668–685. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 14.Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–1043. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil. 2010;22:395–400. doi: 10.1111/j.1365-2982.2009.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh SK, Kahrilas PJ, Lodhia N, Pandolfino JE. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1023–1028. doi: 10.1152/ajpgi.00384.2007. [DOI] [PubMed] [Google Scholar]
- 19.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–2225. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman S, Lin Z, Kwiatek M, Pandolfino J, Kahrilas PJ. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.384. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett J, Maydonovitch C, Perry J, Sobin L, Osgard E, Wong R. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Dis Esophagus. 2009;22:543–548. doi: 10.1111/j.1442-2050.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 22.Lucendo AJ, Castillo P, Martin-Chavarri S, et al. Manometric findings in adult eosinophilic oesophagitis: a study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19:417–424. doi: 10.1097/MEG.0b013e328010bd69. [DOI] [PubMed] [Google Scholar]
- 23.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–771. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 24.Nurko S, Rosen R, Furuta GT. Esophageal dysmotility in children with eosinophilic esophagitis: a study using prolonged esophageal manometry. Am J Gastroenterol. 2009;104:3050–3057. doi: 10.1038/ajg.2009.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical Properties of the Esophagus in Eosinophilic Esophagitis. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.09.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korsapati H, Babaei A, Bhargava V, Dohil R, Quin A, Mittal RK. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58:1056–1062. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]


