Abstract
The mood-stabilizing and anticonvulsant drug valproic acid (VPA) inhibits histone deacetylases (HDACs). The aim of the present study was to determine the effect of HDAC inhibition on overall and target gene promoter-associated histone methylation in rat cortical neurons and astrocytes. We found that VPA and other HDAC inhibitors, including sodium butyrate (SB), trichostatin A (TSA), and the Class I HDAC inhibitors MS-275 and apicidin all increased levels of histone 3 lysine 4 dimethylation and trimethylation (H3K4Me2 and H3K4Me3); these processes are linked to transcriptional activation in rat cortical neurons and astrocytes. VPA, SB, TSA, MS-275, and apicidin also upregulated levels of the neuroprotective heat shock protein 70 (HSP70) in rat astrocytes. Moreover, Class I HDAC inhibition by VPA and MS-275 increased H3K4Me2 levels at the HSP70 promoter in astrocytes and neurons. We also found that VPA treatment facilitated the recruitment of acetyltransferase p300 to the HSP70 promoter and that p300 interacted with the transcription factor NF-Y in astrocytes. Taken together, the results suggest that Class I HDAC inhibition is key to upregulating overall and gene-specific H3K4 methylation in primary neuronal and astrocyte cultures. In addition, VPA-induced activation of the HSP70 promoter in astrocytes appears to involve an increase in H3K4Me2 levels and recruitment of p300.
Keywords: valproic acid, histone methylation, heat shock protein 70, MS-275, NF-Y, p300
1. Introduction
Epigenetic regulation alters the conformation state of chromatin and the accessibility of specific gene promoters to transcriptional machinery (reviewed in Beiger, 2007). In the brain, epigenetic changes in chromatin remodeling have been observed in association with a number of neuropsychiatric and neurodegenerative disorders. Notable changes include altered levels of histone acetylation, histone methylation, and DNA methylation in both animal models of these diseases and human samples (reviewed in Akbarian and Huang, 2009; Chuang et al., 2009; Costa et al., 2009; Tsankova et al., 2007). In most cases, histone acetylation has been associated with gene transcriptional activation and DNA methylation with repressed gene activity. However, histone methylation can have the opposite effect on transcription, depending on factors such as the lysine residue methylated and the number of methyl groups incorporated (reviewed in Renthal and Nestler, 2009). In general, increases in histone H3 lysine 4 trimethylation (H3K4Me3) and histone H3 lysine 4 dimethylation (H3K4Me2) correlate with activation of gene transcription, while histone H3 lysine 9 dimethylation (H3K9Me2) is associated with transcriptional silencing (Akbarian and Huang, 2009; Bernstein et al., 2002; Liang et al., 2004).
Valproic acid (2-n-propylpentanoic acid, VPA) is a histone deacetylase (HDAC) inhibitor (Göttlicher et al., 2001; Phiel et al., 2001) widely used as a mood stabilizing and anticonvulsant drug. Diverse cellular models of toxicity as well as animal models of neurodegenerative diseases have shown that VPA exerts its neurotrophic and neuroprotective effects via inhibition of HDACs to modulate histone acetylation levels and transcriptional activity (reviewed in Chuang et al., 2009). Other pan-HDAC inhibitors include the structurally similar sodium butyrate and structurally dissimilar trichostatin A (TSA) (reviewed in Chuang et al., 2009). MS-275 and apicidin are Class I-specific HDAC inhibitors (Khan et al., 2008; Simonini et al., 2006). HDAC inhibitors can elicit neuroprotection in a wide range of neurodegenerative conditions through their neurotrophic, neuroprotective, and anti-inflammatory properties (Chuang et al., 2009). In addition, several preclinical studies have indicated that the HDAC inhibitors sodium butyrate (SB) and MS-275 exert antidepressant-like effects in mice, further suggesting a role for HDAC inhibition in some mood-stabilizing effects of VPA (Covington et al., 2009; Schroeder et al., 2007). In cultured brain cells, HDAC inhibition by VPA, SB, or other inhibitors has been shown to induce prominent neurotrophic and neuroprotective molecules such as brain-derived neurotrophic factor (BDNF) (Yasuda et al., 2009), glial cell line-derived neurotrophic factor (GDNF) (Chen et al., 2006; Wu et al., 2008), and α-synuclein (Leng and Chuang, 2006).
Heat shock protein 70 (HSP70) is a member of the heat shock protein family, and a major cytoprotective protein that acts as a molecular chaperone to facilitate proper protein folding. HSP70 also has anti-apoptotic and anti-inflammatory effects. It upregulates the anti-apoptotic protein, B-cell lymphoma 2 (Bcl-2), binds to block apoptosis-inducing factor, and interferes with apoptosis protease-activating factor-1 (Yenari et al, 2005). In an experimental cerebral ischemic model of stroke, HSP70 exhibited anti-inflammatory actions by blocking NF-κB activation in the brain (Zheng et al., 2008). Overexpression of HSP70 showed beneficial effects in rodent models of cerebral ischemia, amyotrophic lateral sclerosis, and Huntington’s disease, among others (reviewed in Brown, 2007). Changes in HSP70 mRNA levels have also been observed in the brains of individuals with schizophrenia or autism (Arion et al., 2007; Garbett et al., 2008). A recent study from our laboratory found that VPA treatment increased HSP70 levels in rat cortical neurons via HDAC inhibition and subsequent transcriptional activation, and this event contributed to VPA neuroprotection against glutamate-induced N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity (Marinova et al., 2009).
It has recently been suggested that histone methylation-induced chromatin remodeling has important neurophysiological effects in the central nervous system (CNS) (Tsankova et al., 2007; Gupta et al., 2010; Akbarian and Huang, 2009; Kim et al., 2007b). However, its role in the expression of specific neuroprotective proteins in response to HDAC inhibition is unclear. In this study, we sought to determine the effects of VPA and other HDAC inhibitors on H3K4 dimethylation and trimethylation in rat cortical neurons and astrocytes. We further sought to correlate these effects with changes occurring at the promoter of a target gene HSP70 after treating these cells with HDAC inhibitors.
2. Materials and Methods
2.1. Rat astrocyte and cortical neuronal cultures
For rat astrocyte cultures, mixed glia cultures were initially prepared from the brains of one-day-old Sprague–Dawley rat pups, as described previously (Liu and Hong, 2003). Briefly, brain cells were mechanically dissociated and seeded in DMEM/F12 growth medium supplemented with 10% fetal bovine serum (FBS) on poly-L-lysine precoated 150 cm2 culture flasks. Upon reaching confluence, microglia were detached from astrocytes by shaking the flasks for five hours at 180 rpm. Astrocytes were then passaged several times and seeded onto poly-L-lysine-precoated six-well plates for experiments. Rat cortical neuronal cultures were prepared from the cerebral cortices of 18-day-old Sprague–Dawley rat embryos as described previously (Marinova et al., 2009). Briefly, cortices were dissected and cells were dissociated by trypsinization, trituration, and DNase treatment. Dissociated cells were resuspended in serum-free B27/neurobasal medium and plated on poly-D-lysine-precoated six-well plates.
2.2. Immunoblotting analysis and immunoprecipitation
Rat astrocytes or cortical neurons were washed with phosphate-buffered saline (PBS) and harvested in cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA), supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN, USA). The homogenates were sonicated, centrifuged at 20,000 g for 10 minutes at 4°C, and the supernatants were used for immunoblotting. The bicinchoninic acid protein assay reagent kit was used to determine protein concentration (Pierce, Rockford, IL, USA). Aliquots of extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 4–12% NuPage Bis–Tris gel under reducing conditions. Proteins were transferred onto a nitrocellulose membrane, blocked, and probed with primary antibody against HSP70 (provided as whole rabbit antiserum) (Assay Designs, Ann Arbor, MI, USA), acetylated histone H3 against both Lys9 and Lys14 acetylation (Millipore, Billerica, MA, USA), dimethylated Lys4 histone H3, dimethylated Lys9 histone H3, (Cell Signaling Technology, Beverly, MA, USA), trimethylated Lys4 histone H3, monomethylated Lys4 histone H3 (Abcam, Cambridge, MA, USA), acetylated tubulin (Sigma-Aldrich, Saint Louis, MO, USA), p300, Sp1 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were then probed with secondary goat anti-mouse or anti-rabbit antibodies conjugated with IRdye 680 or 800CW (LI-COR, Lincoln, Nebraska, USA). Blotted proteins were detected and quantified using the Odyssey infrared imaging system (LI-COR, Lincoln, Nebraska, USA). For immunoprecipitation, 200 µl cell lysates from rat astrocytes containing 200 µg protein were precleared with 25 µl protein G PLUS-agarose slurry (Santa Cruz Biotechnology) for 15 minutes, incubated with 2 µg NF-Y antibody at 4°C overnight and then incubated at 4°C with 30 µl protein G PLUS-agarose slurry for four hours. The samples were then washed three times with lysis buffer, mixed with loading buffer containing 5% β-mercaptoethanol, and boiled for four minutes.
2.3. Chromatin immunoprecipitation assay
Samples for chromatin immunoprecipitation (ChIP) assays were processed using the ChIP-IT Express kit protocol (Active Motif, Carlsbad, CA, USA). Briefly, rat astrocytes from two 6 cm culture dishes were treated with 1.5 mM VPA, 5 µM MS-275, or vehicle for 48 hours; alternately, 1.2 × 107 cortical neurons at DIV-8 were treated with 5 µM MS-275 or vehicle for 72 hours. After cross-linking with 1% formaldehyde at 37°C for 10 minutes, cells were washed with PBS and lysed, followed by centrifugation at 2,400 g. Nuclear pellets were resuspended in shearing buffer and sonicated (10 pulses of 15 seconds each for astrocytes and 12 pulses of 15 seconds each for neurons) using a sonicator (Sonic and Materials, Newtown, CT, USA) to shear DNA into 500–1000 bp fragments. Ten percent of the mixture of protein/DNA complex was taken for 'input DNA' analysis. An equal amount of the protein/DNA complex was then incubated with magnetic beads and an antibody against dimethylated H3K4, p300 or without an antibody at 4°C overnight. DNA was immunoprecipitated and eluted from the magnetic beads and the cross-linking was reversed. Input and ChIP DNA were analyzed using polymerase chain reaction (PCR). The HSP70-2 (HSPA1B) promoter region was PCR amplified using forward primer 5'-CCCAGCCCCTAAAGTTTGTT-3' and reverse primer 5'-GGGGATAGGGCTGATTAAGATT-3'. ChIP and input DNA were mixed with ReactionReady Hotstart 'Sweet' PCR mix (SA Biosciences, Frederick, MD, USA) and the amplification was carried out for 33 cycles. The PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized by UV illumination.
2.4. Statistical analysis
GraphPad Prism (GraphPad, San Diego, CA, USA) was used to analyze results for statistical significance, using two-sample t-tests or one-way analysis of variance. Data shown are representative from three independent experiments.
3. Results
3.1. HDAC inhibition increased H3K4 dimethylation, trimethylation and monomethylation levels in rat cortical neurons
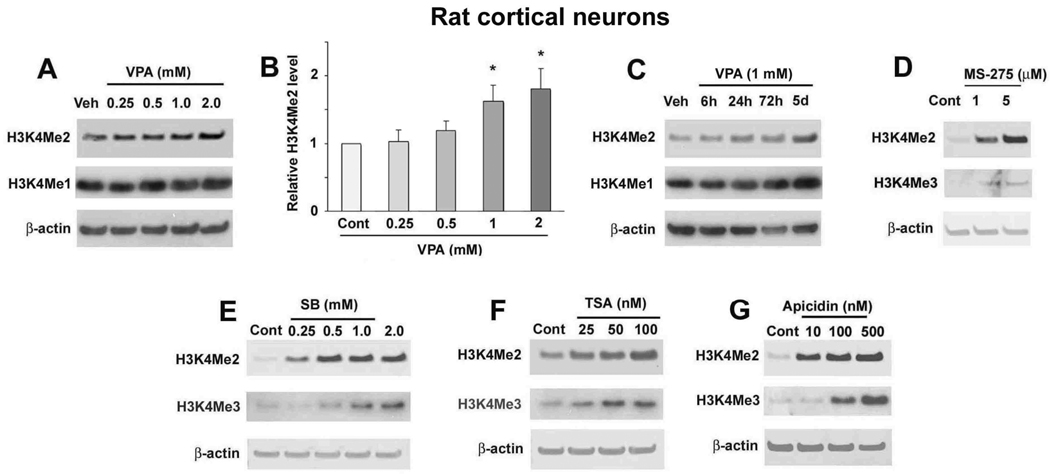
A recent study from our laboratory found increased H3K4Me3 in rat cortical neurons treated with VPA (Marinova et al., 2009). Building on his work, we sought to determine the effect of VPA on H3K4Me2 and H3K4Me1 levels, which are also linked to transcriptionally active chromatin. We found that treating rat cortical neurons with 0.25–2 mM VPA for 72 hours increased levels of H3K4Me2 in a dose-dependent manner; statistical significance was noted at 1 and 2 mM (Fig. 1A, B). VPA treatment under these conditions also apparently increased H3K4Me1 levels. At a dose of 1.0 mM VPA, the increase in H3K4Me2 and H3K4Me1 was observed at 24 and 72 hours post-treatment and persisted for at least five days (Fig. 1C). To confirm the role of HDAC inhibition in VPA-induced effects on histone methylation, we treated cortical neurons with several other HDAC inhibitors: MS-275, SB, TSA, and apicidin. Treatment with 1–5 µM MS-275, 0.25–2 mM SB, 25–100 nM TSA, or 10–500 nM apicidin for 72 hours increased H3K4Me2 and H3K4Me3 levels in cortical neurons (Fig. 1D–G).
Figure 1.
VPA and other general and class I-specific HDAC inhibitors increased histone methylation levels in rat cortical neurons. (A) Cortical neurons were treated with the indicated concentrations of VPA for 72 hours, starting from DIV-8. Samples were collected and immunoblotted for dimethylated H3K4 (H3K4Me2) and monomethylated H3K4 (H3K4Me1). Results for H3K4Me2 were quantified and shown in (B). Data are presented as mean±SEM from three independent experiments. *p<0.05 compared with control. (C) Cortical neurons were treated with 1 mM VPA for the indicated times ending on DIV-11 and samples were immunoblotted for H3K4Me2 and H3K4Me1. Cortical neurons were treated with the indicated concentrations of MS-275 (D), SB (E), TSA (F), or apicidin (G) for 72 hours, starting from DIV-8. Samples were collected and analyzed for H3K4Me2 and trimethylated H3K4 (H3K4Me3). In all cases β-actin levels were used as the loading control and for normalization of H3K4Me2 levels.
3.2. HDAC inhibition increased H3K4Me2 and H3K4Me3 levels in rat astrocytes
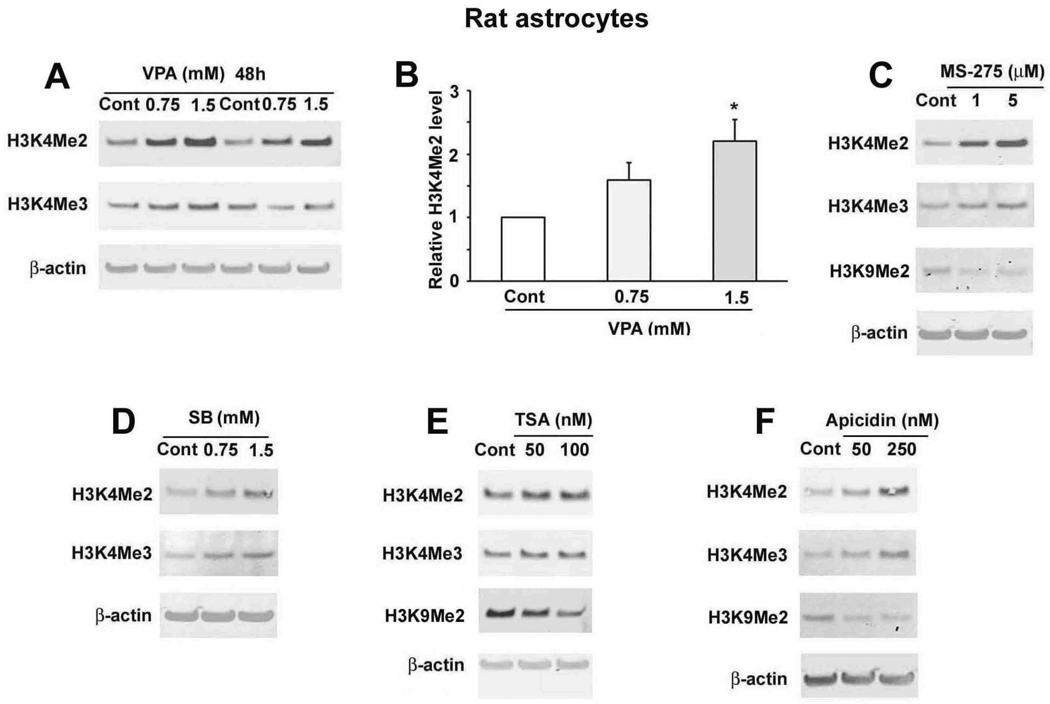
We then determined the effects of VPA and other HDAC inhibitors on H3K4Me2 and H3K4Me3 in a primary culture of rat astrocytes. Treatment of astrocytes with 0.75 or 1.5 mM VPA for 48 hours increased levels of H3K4Me2 and H3K4Me3 (Fig. 2A, B). Furthermore, treatment with 1 or 5 µM MS-275, 0.75 or 1.5 mM SB, 50 or 100 nM TSA, or 50 or 250 nM apicidin for 48 hours also elevated H3K4Me2 and H3K4Me3 levels in astrocytes (Fig. 2C–F). Taken together, these data suggest that general and Class I-specific HDAC inhibition increased H3K4Me2 and H3K4Me3 in both primary neuronal and astrocytic rat cultures. Interestingly, levels of H3K9Me2, which is linked to transcriptional silencing, were decreased in astrocytes treated with MS-275 (Fig. 2C), TSA (Fig. 2E) or apicidin (Fig. 2F).
Figure 2.
VPA and other general and class I-specific HDAC inhibitors increased H3K4Me2 and H3K4Me3 levels in rat astrocytes. (A) Astrocytes were treated with the indicated concentrations of VPA for 48 hours, and the H3K4Me2 and H3K4Me3 levels were analyzed. H3K4Me2 levels were quantified in (B). Data are presented as mean±SEM from three independent experiments. *p<0.05 compared with control. Astrocytes were treated with the indicated concentrations of MS-275 (C), SB (D), TSA (E), or apicidin (F) for 48 hours. Samples were immunoblotted for H3K4Me2, H3K4Me3, H3K9Me2 and β-actin.
3.3. VPA, MS-275, and other HDAC inhibitors increased HSP70 levels in astrocytes
Next, we sought to determine whether treatment with HDAC inhibitors is associated with changes in levels of the transcriptional activator H3K4Me2 at a specific target gene. Since previous work from our laboratory demonstrated that Class I HDAC inhibition increased HSP70 levels in rat cortical neurons (Marinova et al, 2009), we examined the effects of HDAC inhibitors on HSP70 levels in astrocytes.
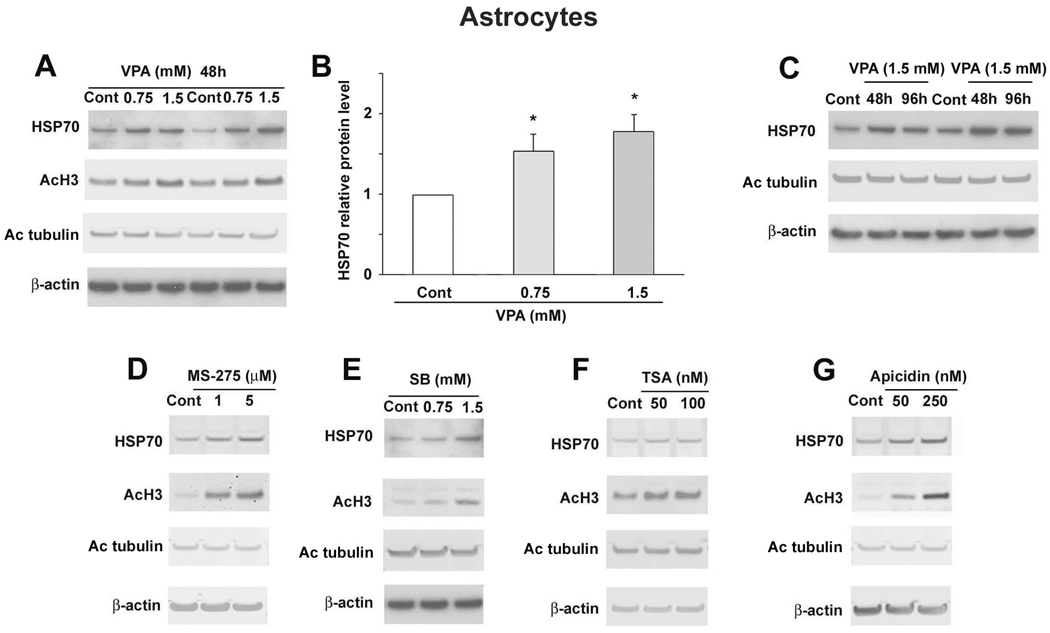
Treatment with VPA for 48 hours at 0.75 or 1.5 mM increased astrocytic HSP70 levels; statistical significance was noted at both doses (Fig. 3A, B). The increase was observed after 48 or 96 hours treatment with 1.5 mM VPA (Fig. 3C). Treatment with MS-275 (1 or 5 µM), SB (0.75 or 1.5 mM), TSA (50 or 100 nM), and apicidin (50 or 250 nM) also increased HSP70 levels in astrocytes after 48 hours (Fig. 3D–G). As expected, all HDAC inhibitors increased levels of H3 acetylation. Only TSA at 100 nM marginally increased levels of acetylated tubulin, a substrate of Class IIb HDAC 6 isoform (Fig. 3).
Figure 3.
VPA and other HDAC inhibitors increased HSP70 levels in rat astrocytes. (A) Astrocytes were treated with the indicated concentrations of VPA for 48 hours. Samples were collected and immunoblotted for HSP70, acetylated H3 (AcH3), acetylated tubulin (Ac tubulin) and β-actin. The blots for HSP70 were quantified and are shown in (B). Data are presented as mean±SEM from three independent experiments. *p<0.05 compared with control. (C) Astrocytes were treated with 1.5 mM VPA for the indicated times. Astrocytes were treated for 48 hours with the indicated concentrations of MS-275 (D), SB (E), TSA (F), or apicidin (G). Samples were subjected to immunoblotting for HSP70, AcH3, Ac tubulin, and β-actin.
3.4. VPA and MS-275 increased H3K4 dimethylation on the HSP70 promoter
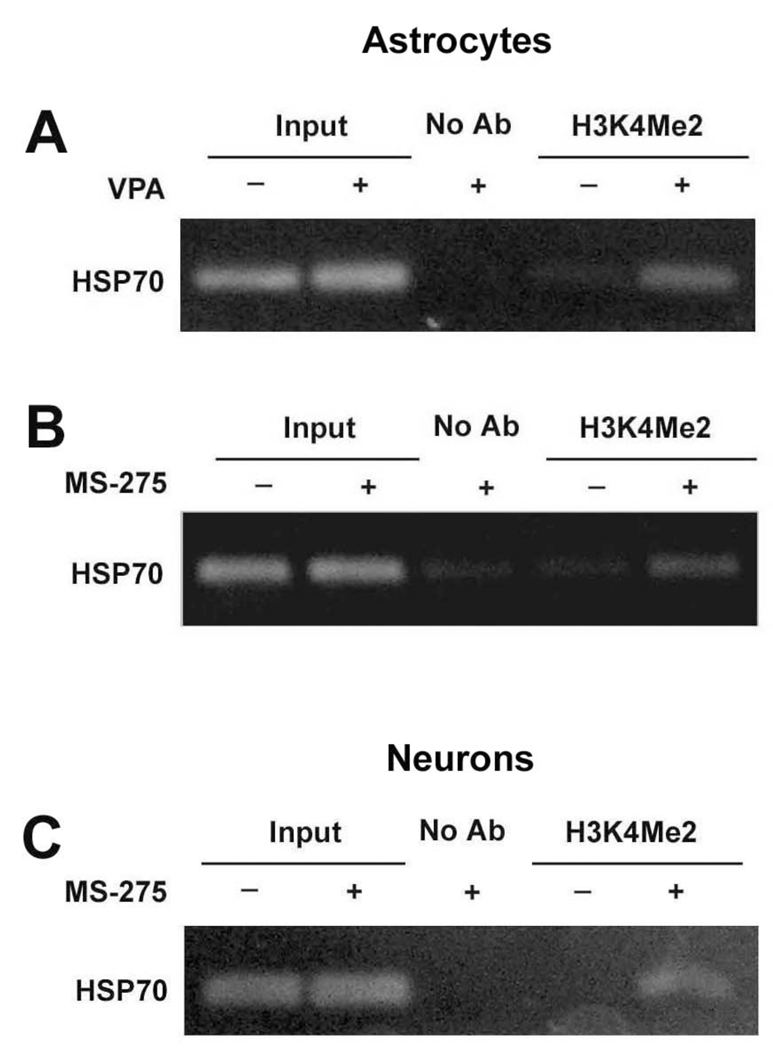
Next, we used a ChIP assay to determine the levels of H3K4Me2 bound to the HSP70 promoter region in astrocytes and neurons after treatment with VPA or MS-275. Both untreated and treated cells were cross-linked with formaldehyde and lysed to prepare the nuclear pellet, which was then sonicated to shear DNA. The protein/DNA complex was incubated with anti-H3K4Me2 antibody and the immunoprecipitated DNA was purified and PCR-amplified using primers specific for HSP70 promoter. Treatment of astrocytes with 1.5 mM VPA for 48 hours markedly enhanced HSP70 promoter-associated H3K4Me2 levels, compared with vehicle-treated control (Fig. 4A). A similar increase in H3K4Me2 levels at the HSP70 promoter was found in astrocytes treated with 5 µM MS-275 for 48 hours (Fig. 4B). Further, in cortical neurons treated with 5 µM MS-275, H3K4Me2 levels at the HSP70 promoter were also markedly enhanced compared with vehicle-treated neurons (Fig. 4C).
Figure 4.
VPA and MS-275 increased H3K4Me2 at the HSP70 promoter. Astrocytes were treated with 1.5 mM VPA (A) or 5 µM MS-275 (B) for 48 hours. (C) Cortical neurons were treated with 5 µM MS-275 for 72 hours starting from DIV-8. After chromatin sonication, the protein/DNA complex was incubated with antibody against dimethylated H3K4 or without antibody (No Ab) and analyzed by ChIP assay. PCR products were run on a 2% agarose gel and stained with ethidium bromide. Representative results from gels for the HSP70-2 gene promoter are shown.
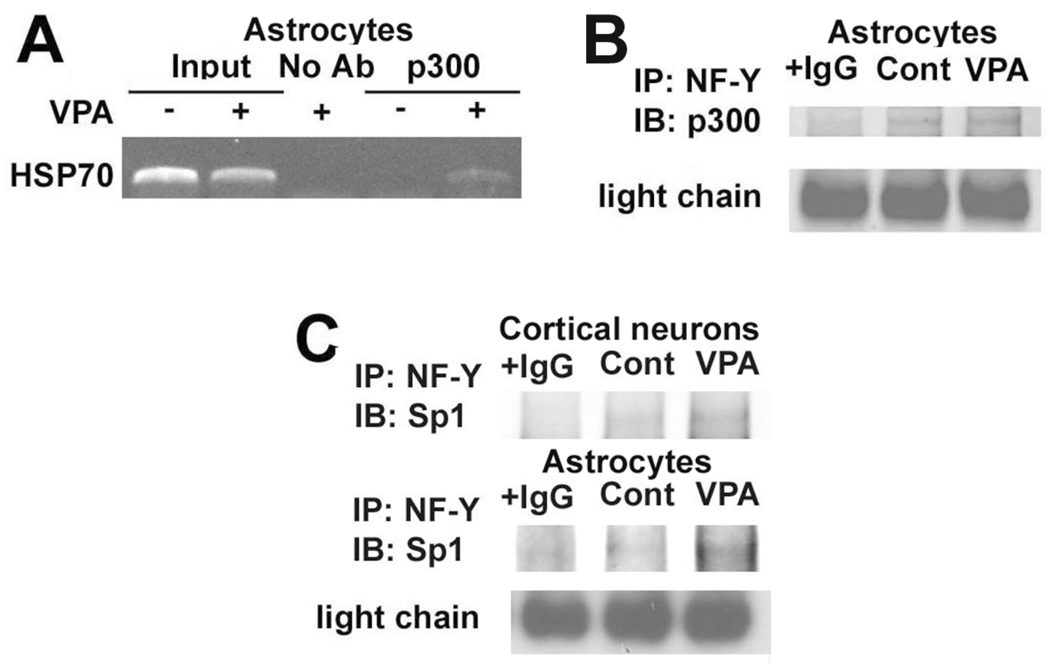
3.5. p300 and NF-Y are involved in the induction of HSP70 by VPA in rat astrocytes
We previously observed an increase in p300 levels at the HSP70 promoter in rat cortical neurons treated with VPA (Marinova et al., 2009). Therefore, we tested whether VPA affects the recruitment of p300 to the HSP70 promoter in rat astrocytes. Treatment with 1.5 mM VPA for 48 hours led to an increase in p300 occupancy of the HSP70 promoter detected by ChIP assay (Fig. 5A). Because NF-Y can interact with p300 and is an important regulator of the acetylation responsiveness in the Xenopus HSP70 promoter (Huang et al., 2005; Li et al., 1998), we used immunoprecipitation with anti-NF-Y antibody to determine whether NF-Y and p300 interact in astrocytes. Results showed that p300 and NF-Y were both present in a complex in astrocytes treated with vehicle or 1.5 mM VPA for 48 hours, and that this interaction was enhanced by VPA (Fig. 5B). Our previous study suggested a role for the transcription factor Sp1 binding to the HSP70 promoter in the induction of HSP70 in cortical neurons (Marinova et al., 2009). We therefore also used anti-NF-Y antibody to examine whether NF-Y and Sp1 can be co-immunoprecipitated. Data showed that Sp1 was indeed bound to NF-Y and such interaction was enhanced by VPA treatment in both cortical neurons and astrocytes (Fig. 5C).
Figure 5.
p300 and NF-Y were involved in HSP70 induction by VPA in rat astrocytes. (A) Astrocytes were treated with 1.5 mM VPA for 48 hours. After chromatin sonication the protein/DNA complex was incubated with an antibody against p300 or without antibody (No Ab) and analyzed by ChIP assay. The PCR products were run on a 2% agarose gel and stained with ethidium bromide. Representative results from gels for the HSP70-2 gene promoter are shown. (B) Astrocytes were treated with 1.5 mM VPA for 48 hours and cell lysates were collected and immunoprecipited with an antibody against NF-Y or normal rabbit IgG. The samples were then immunoblotted with an antibody against p300. Immunoglobulin light chain is shown as a loading control. (C) Astrocytes or neurons were treated with 1.5 mM VPA for 48 hours or 1.0 mM VPA for 72 hours, respectively. Samples were processed as described in (B) using, however, an antibody against Sp1.
4. Discussion
The present study and our previous data demonstrate that VPA, as well as other pan- and Class I-specific HDAC inhibitors, increase H3K4Me2 and H3K4Me3 levels in rat cortical neurons and astrocytes (Figs. 1 and 2). HDAC inhibitors are well known to activate gene transcription by increasing histone acetylation levels, thereby unraveling DNA around the histone core to recruit transcriptional machinery. However, HDAC inhibitors may exhibit additional chromatin-modifying properties. For example, VPA and MS-275 have been found to facilitate DNA demethylation of promoters of reelin and glutamic acid decarboxylase 67 in cell lines and animal models (Dong et al., 2007; Kundakovic et al., 2009). HDAC inhibitors also affect histone methylation, thus implying a cross-talk among histone acetylation, DNA methylation, and histone methylation (Gavin et al., 2009; Nightingale et al., 2007; Perisic et al., 2010).
Also consistent with this notion is the observation that the hyperacetylated state of H3K9 is correlated with hypermethylated H3K4 in vitro, as determined by mass spectrometric western blotting (Zhang et al., 2004). Moreover, physical and functional interactions between histone deacetylases and histone demethylases have been demonstrated in cell-free systems and intact cells (Lee et al., 2006a). Emerging evidence suggests that histone H3 methylation may play a crucial regulatory role in the neurophysiology of the CNS. For example, abnormal histone covalent modifications, including histone methylation, have been implicated in the pathophysiology of schizophrenia and other mental disorders (Akbarian and Huang, 2009). It is also noteworthy that treatment of rat neural precursor cells with VPA or phenylbutyrate enhanced FGF withdrawal induced upregulation of glutamate decarboxylase 1 and neuropeptide Y mRNA, as well as H3K4Me3 at their promoters (Huang et al., 2007). Contextual fear conditioning causes both trimethylation of H3K4 and dimethylation of H3K9 in the hippocampus of rodents, suggesting that histone methylation-mediated transcriptional activation and silencing occur during memory formation (Gupta et al., 2010). The same study found that HDAC inhibition by SB treatment upregulated H3K4Me2 but downregulated H3K9Me2 after contextual fear conditioning, further supporting a link between histone acetylation and histone methylation. The current study showed that treatment of astrocytes with MS-275, TSA and apicidin all decreased the levels of H3K9Me2 (Fig. 2). Our preliminary results also showed that HDAC inhibition induced a decrease in H3K9Me2 in cortical neurons (data not shown). Further studies in this area are warranted.
VPA, SB, and TSA are non-selective HDAC inhibitors, while MS-275 and apicidin selectively inhibit Class I HDACs. The fact that H3K4Me2 and H3K4Me3 levels were increased in neurons and astrocytes after treatment with MS-275 or apicidin suggests that inhibition of Class I HDACs is intricately coupled to enhanced histone H3K4 dimethylation and trimethylation (Figs. 1 and 2). Similarly, Class I HDAC inhibition is likely the target for HSP70 induction in astrocytes (Fig. 3) and cortical neurons (Marinova et al., 2009). The HDAC isoform(s) affected by HDAC inhibitors to elicit H3K4 methylation upregulation and HSP70 induction remains to be identified. Given that MS-275 and apicidin have the highest affinity for HDAC1 and 2, and HDAC2 and 3, respectively, one may speculate that that HDAC1, 2 and 3 isoforms are possible candidates for HDAC inhibitors to induce changes in the histone H3 methylation state. In this context, a multiple protein complex containing HDAC1/2 and the histone demethylase BHC110 (LSD1) has been detected (Lee et al., 2006a). HDAC2 has recently been shown to have negative effects on dendritic spine density, synapse formation, synaptic plasticity, and memory formation (Guan et al., 2009). Further, BDNF can be induced in cortical neurons by siRNA-mediated silencing of HDAC1 (Yasuda et al., 2009) or by contextual fear conditioning in rodent hippocampus with increased H3K4Me3 at the BDNF promoter (Gupta et al., 2010). Regardless of the HDAC isoform(s) involved, inhibition of Class I HDACs has been reported to have important neurophysiological consequences; these include reversal of contextual memory deficits in a mouse model of Alzheimer’s disease (Kilgore et al., 2010), and antidepressant-like properties in rodent models of depression (Covington et al., 2009).
Here, we detected increased H3K4Me2 levels at the HSP70 promoter after treatment with VPA and MS-275 (Fig. 4). Because H3K4Me2 levels are associated with active gene transcription, these effects may contribute to the upregulation of HSP70 by VPA and MS-275. Changes in H3K4Me2 and H3K4Me3 may also occur in response to treatment with psychoactive drugs. For example, tranylcypromine, a nonselective monoamine oxidase inhibitor (MAOI) antidepressant, inhibits H3K4Me2 demethylation by BHC110/LSD1 (Lee et al., 2006b). Furthermore, levels of H3K4Me3 in cortical interneurons depend on the activity of histone methyltransferase mixed-lineage leukemia 1 (Mll1), and H3K4Me3 levels at the GABAergic gene promoters are increased by treatment with the atypical antipsychotic clozapine (Huang et al., 2007).
In the present study we observed that the acetyltransferase p300 was recruited to the HSP70 promoter in rat astrocytes after VPA treatment, and that p300 formed a complex with the transcription factor NF-Y (Fig. 5A, B). NF-Y also appears to interact with the transcription factor Sp1 in astrocytes and neurons (Fig. 5C). NF-Y has been identified as important for acetylation responsiveness of the HSP70 promoter in Xenopus (Li et al., 1998). Our data imply that the recruited p300 interacts with NF-Y. The importance of the formation of a multiprotein complex between PCAF/p300 and Sp1/NF-Y for TSA induction of transforming growth factor beta II promoter activity in pancreatic cell lines has been demonstrated (Huang et al., 2005). A study from our laboratory also observed that Sp1 plays a role in HSP70 induction by VPA in cortical neurons (Marinova et al., 2009). Taken together, these results suggest that the formation of a complex between NF-Y and Sp1 and the recruitment of p300 may be necessary for the induction of HSP70 by VPA.
The heat shock response is a conserved, ubiquitous cytoprotective mechanism and plays a critical role in cellular defense against diverse insults. Our previous study found that HSP70 is an important target for VPA and other HDAC inhibitors, and is part of the mechanism through which they exert neuroprotective effects against glutamate-induced excitotoxicity in primary neuronal cultures (Marinova et al., 2009; Leng et al., 2010), as well as in a rat model of cerebral ischemia (Ren et al., 2004; Kim et al., 2007a). HSP70 upregulation has been shown to stabilize the NF-κB-IκB complex, thereby preventing the nuclear translocation of the activated NF-κB subunits (Rel A and p50) to elicit inflammatory responses (Zheng et al., 2008). Therefore, induction of HSP70 by VPA or other HDAC inhibitors likely contributes to the anti-inflammatory effects of these drugs in vitro and in vivo (Chen et al., 2006; Kim et al., 2007a). Growing evidence suggests that astrocytes help control the amount of glutamate acting on neurons and provide them with trophic support, and that abnormal astrocyte function may be key to a variety of brain disorders (Miller, 2005). VPA has been shown to induce BDNF from both neurons and astrocytes in culture (Wu et al., 2008; Yasuda et al., 2009) and GDNF from cultured astrocytes (Chen et al., 2006; Wu et al., 2008), suggesting that both neurons and glia are direct targets of VPA. Our current findings that HSP70 is induced by VPA through HDAC inhibition in both neurons and glia suggest that these actions on HSP70 expression in both types of brain cells may have a considerable impact on use of this drug in neurodegenerative and neuropsychiatric diseases.
In conclusion, the present study demonstrated for the first time that Class I HDAC inhibition by VPA and other compounds increased levels of dimethylation and trimethylation of histone H3K4 in rat cortical neurons and astrocytes, suggesting the interplay between histone acetylation and histone methylation. HSP70 protein levels were markedly increased under these experimental conditions and H3K4Me2 levels associated with the HSP70 promoter in astrocytes were robustly increased after treatment with VPA or MS-275, a Class I HDAC inhibitor. The induction of HSP70 by VPA in astrocytes involved recruitment of the acetyltransferase p300 and its interaction with the transcription factor NF-Y. These findings have profound implications for the use of HDAC inhibitors to induce neuroprotective proteins in neurodegenerative conditions.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), and a gift fund from the HSU Family Foundation. We thank Ioline Henter, Emily Fessler and Kelly Shan of NIMH for editorial assistance and discussions.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- GDNF
glial cell line-derived neurotrophic factor
- H3K4Me2
histone 3 lysine 4 dimethylation
- H3K4Me3
histone 3 lysine 4 trimethylation
- H3K9Me2
histone H3 lysine 9 dimethylation
- HDAC
histone deacetylase
- HSP70
heat shock protein 70
- SB
sodium butyrate
- TSA
trichostatin A
- VPA
valproic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Huang HS. Epigenetic regulation in human brain-focus on histone lysine methylation. Biol. Psychiatry. 2009;65:198–203. doi: 10.1016/j.biopsych.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol. Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins and protection of the nervous system. Ann. N Y Acad. Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW, Chuang DM, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychi. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Chen Y, Dong E, Grayson DR, Kundakovic M, Maloku E, Ruzicka W, Satta R, Veldic M, Zhubi A, Guidotti A. GABAergic promoter hypermethylation as a model to study the neurochemistry of schizophrenia vulnerability. Expert Rev. Neurother. 2009;9:87–98. doi: 10.1586/14737175.9.1.87. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J. Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. U S A. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol. Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin DP, Rosen C, Chase K, Grayson DR, Tun N, Sharma RP. Dimethylated lysine 9 of histone 3 is elevated in schizophrenia and exhibits a divergent response to histone deacetylase inhibitors in lymphocyte cultures. J. Psychiatry Neurosci. 2009;34:232–237. [PMC free article] [PubMed] [Google Scholar]
- Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJF, Zhou Y, Wang X, Mazitschek R, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J. Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1.NF-Y complex. J. Biol. Chem. 2005;280:10047–10054. doi: 10.1074/jbc.M408680200. [DOI] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J. Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem. J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharm. Exper. Therapeut. 2007a;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kim SY, Levenson JM, Korsmeyer S, Sweatt JD, Schumacher A. Developmental regulation of Eed complex composition governs a switch in global histone modification in brain. J. Biol. Chem. 2007b;282:9962–9972. doi: 10.1074/jbc.M608722200. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol. Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Bochar DA, Hakimi MA, Cooch N, Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol. Cell. Bio. 2006a;26:6395–6402. doi: 10.1128/MCB.00723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biology. 2006b;13:563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. Endogenous α-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J. Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Marinova Z, Reis-Fernandes MA, Nau H, Chuang DM. Potent neuroprotective effects of novel structural derivatives of valproic acid: potential roles of HDAC inhibition and HSP70 induction. Neurosci. Lett. 2010;476:127–132. doi: 10.1016/j.neulet.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Herrler M, Landsberger N, Kaludov N, Ogryzko VV, Nakatani Y, Wolffe AP. Xenopus NF-Y pre-sets chromatin to potentiate p300 and acetylation-responsive transcription from the Xenopus hsp70 promoter in vivo. EMBO J. 1998;17:6300–6315. doi: 10.1093/emboj/17.21.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Lin JC, Wei V, Yoo C, Cheng JC, Nguyen CT, Weisenberger DJ, Egger G, Takai D, Gonzales FA, Jones PA. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc. Natl. Acad. Sci. USA. 2004;101:7357–7362. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hong JS. Primary rat mesencephalic neuron-glia, neuron-enriched, microglia-enriched, and astroglia-enriched cultures. Methods Mol. Med. 2003;79:387–395. doi: 10.1385/1-59259-358-5:387. [DOI] [PubMed] [Google Scholar]
- Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang DM. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J. Neurochem. 2009;111:976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. The dark side of glia. Science. 2005;308:778–781. doi: 10.1126/science.308.5723.778. [DOI] [PubMed] [Google Scholar]
- Nightingale KP, Gendreizig S, White DA, Bradbury C, Hollfelder F, Turner BM. Cross-talk between histone modifications in response to histone deacetylase inhibitors: MLL4 links histone H3 acetylation and histone H3K4 methylation. J. Biol. Chem. 2007;282:4408–4416. doi: 10.1074/jbc.M606773200. [DOI] [PubMed] [Google Scholar]
- Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, Holsboer F, Rein T, Zschocke J. Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology. 2010;35:792–805. doi: 10.1038/npp.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin. Neurosci. 2009;11:257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Simonini MV, Camargo LM, Dong E, Maloku E, Veldic M, Costa E, Guidotti A. The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proc. Natl. Acad. Sci. U S A. 2006;103:1587–1592. doi: 10.1073/pnas.0510341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Xhang W, Lu RB, Hong JS. Histone deacetylase inhibitors up-regulate astrocytes GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 2008;11:1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol. Psychi. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. N. Y. Acad. Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Zhang K, Siino JS, Jones PR, Yau PM, Bradbury EM. A mass spectrometric “Western blot” to evaluate the correlations between histone methylation and histone acetylation. Proteomics. 2004;4:3765–3775. doi: 10.1002/pmic.200400819. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J. Cereb. Blood flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]