Abstract
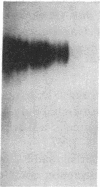
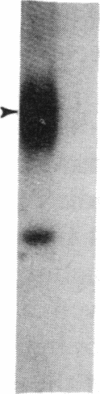
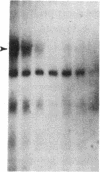
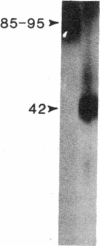
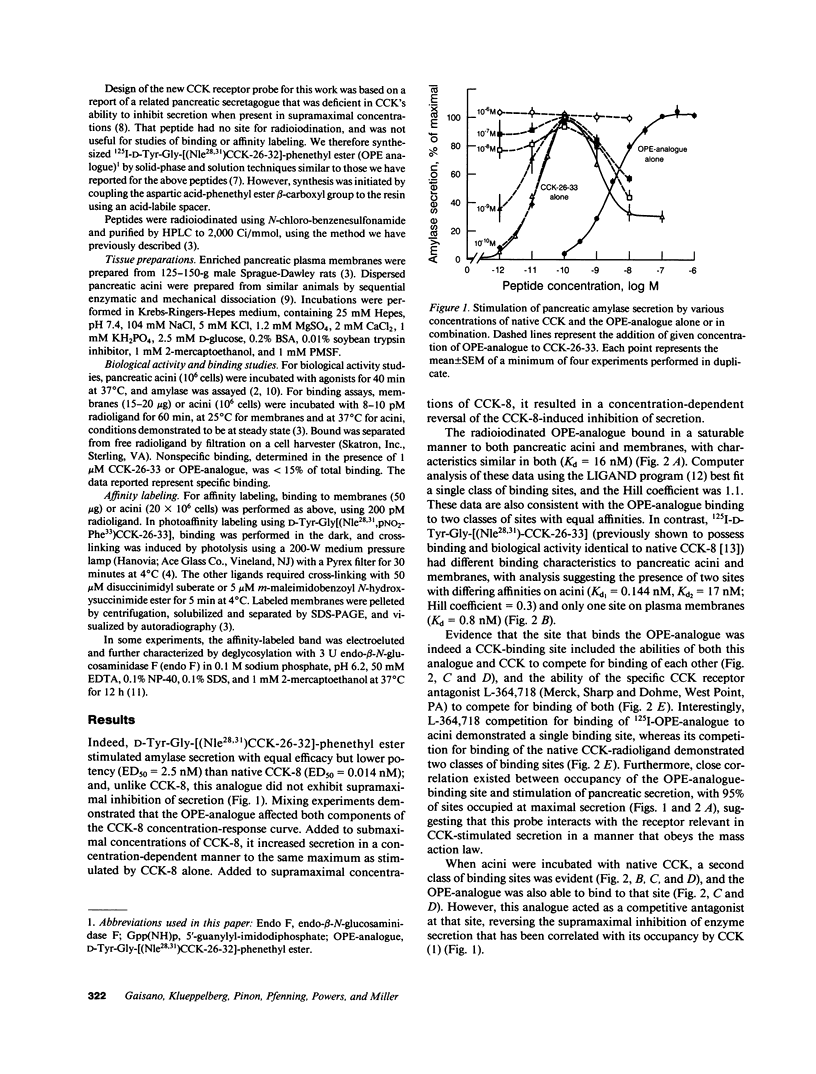
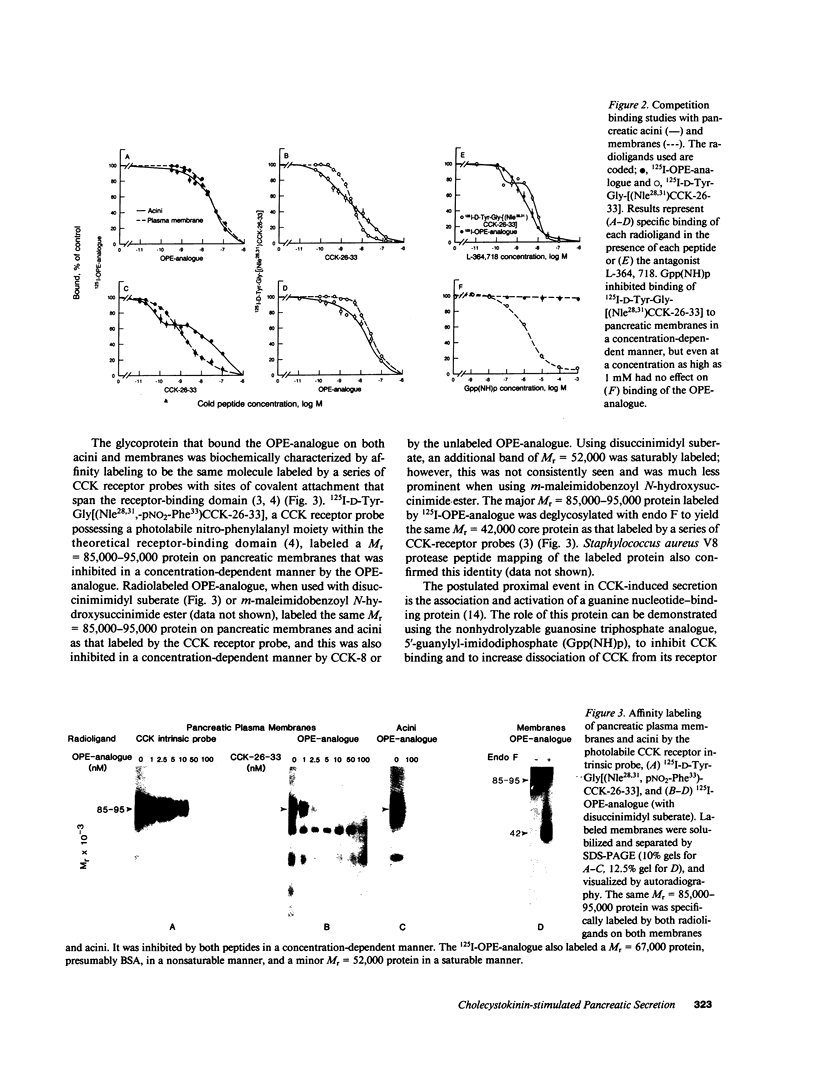
The molecular events that mediate cholecystokinin (CCK)-stimulated pancreatic secretion are not well defined because of the complex receptor-binding and concentration-response characteristics of this hormone. Functional models of receptor occupancy initiating the cascade leading to secretion have been complicated by the inhibition of secretion effected by supramaximal concentrations of CCK. Recent report of a CCK analogue that does not exhibit supramaximal inhibition led us to synthesize a similar analogue that could also be radiolabeled for studies of receptor binding and affinity labeling, and for studies of second messenger activity. This probe, D-Tyr-Gly-[(Nle28,31)CCK-26-32]-phenethyl ester, was a fully efficacious secretagogue with no supramaximal inhibition, and, unlike native hormone, bound to a single class of sites present on both acini and membranes. Occupation of this site correlated well with stimulation of secretion. Evidence that this was indeed a CCK-binding site were the abilities of CCK and the antagonist L-364, 718 to inhibit binding of this analogue. Affinity labeling confirmed the identity of the site mediating secretory stimulation as a Mr = 85,000-95,000 protein. Whereas the nonhydrolyzable guanosine triphosphate analogue, 5'-guanylyl-imidodiphosphate, was a potent inhibitor of CCK binding, it had no effect on binding of this secretagogue, suggesting that a novel cascade not involving a guanine nucleotide-binding protein mediates CCK stimulation of pancreatic secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNFELD P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982 May 25;257(10):5554–5559. [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Rosenzweig S. A., Jamieson J. D. Preparation and characterization of a probe for the cholecystokinin octapeptide receptor, N alpha (125I-desaminotyrosyl)CCK-8, and its interactions with pancreatic acini. J Biol Chem. 1981 Dec 10;256(23):12417–12423. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pearson R. K., Miller L. J. Affinity labeling of a novel cholecystokinin-binding protein in rat pancreatic plasmalemma using new short probes for the receptor. J Biol Chem. 1987 Jan 15;262(2):869–876. [PubMed] [Google Scholar]
- Pearson R. K., Miller L. J., Hadac E. M., Powers S. P. Analysis of the carbohydrate composition of the pancreatic plasmalemmal glycoprotein affinity labeled by short probes for the cholecystokinin receptor. J Biol Chem. 1987 Oct 5;262(28):13850–13856. [PubMed] [Google Scholar]
- Pearson R. K., Powers S. P., Hadac E. M., Gaisano H., Miller L. J. Establishment of a new short, protease-resistant, affinity labeling reagent for the cholecystokinin receptor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):346–353. doi: 10.1016/s0006-291x(87)80128-6. [DOI] [PubMed] [Google Scholar]
- Powers S. P., Fourmy D., Gaisano H., Miller L. J. Intrinsic photoaffinity labeling probes for cholecystokinin (CCK)-gastrin family receptors. D-Tyr-Gly-[Nle28,31,pNO2-Phe33)CCK-26-33). J Biol Chem. 1988 Apr 15;263(11):5295–5300. [PubMed] [Google Scholar]
- Powers S. P., Pinon D. I., Miller L. J. Use of N,O-bis-Fmoc-D-Tyr-ONSu for introduction of an oxidative iodination site into cholecystokinin family peptides. Int J Pept Protein Res. 1988 May;31(5):429–434. doi: 10.1111/j.1399-3011.1988.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S. A., Miller L. J., Jamieson J. D. Identification and localization of cholecystokinin-binding sites on rat pancreatic plasma membranes and acinar cells: a biochemical and autoradiographic study. J Cell Biol. 1983 May;96(5):1288–1297. doi: 10.1083/jcb.96.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., Sarras M. P., Jr, Gunther G. R., Hull B. E., Alicea H. A., Gorelick F. S., Jamieson J. D. Guinea pig pancreatic acini prepared with purified collagenase. Exp Cell Res. 1980 Nov;130(1):49–62. doi: 10.1016/0014-4827(80)90041-5. [DOI] [PubMed] [Google Scholar]
- Wank S. A., Jensen R. T., Gardner J. D. Kinetics of binding of cholecystokinin to pancreatic acini. Am J Physiol. 1988 Jul;255(1 Pt 1):G106–G112. doi: 10.1152/ajpgi.1988.255.1.G106. [DOI] [PubMed] [Google Scholar]
- Williams J. A., McChesney D. J. Cholecystokinin induces the interaction of its receptor with a guanine nucleotide binding protein. Regul Pept. 1987 Aug 3;18(2):109–117. doi: 10.1016/0167-0115(87)90041-3. [DOI] [PubMed] [Google Scholar]