Abstract
Delay discounting (DD) refers to the preference for smaller immediate rewards over larger but delayed rewards, and is considered to be a distinct component of a broader “impulsivity” construct. Although greater propensity for discounting the value of delayed gratification has been associated with a range of problem behaviors and substance abuse, particularly in adolescents, the origins of individual differences in DD remain unclear. We examined genetic and environmental influences on a real-life behavioral measure of DD using a longitudinal twin design. Adolescent participants were asked to choose between a smaller ($7) reward available immediately and a larger ($10) reward to be received in 7 days. Biometrical genetic analysis using linear structural equation modeling showed significant heritability of DD at ages 12 and 14 (30 and 51%, respectively) and suggested that the same genetic factors influenced the trait at both ages. DD was significantly associated with symptoms of conduct disorder, attention deficit hyperactivity disorder, substance use, and with higher novelty seeking and poor self-regulation. This study provides the first evidence for heritability of DD in humans and suggests that DD can be a promising endophenotype for genetic studies of addiction and externalizing disorders.
Keywords: Delay discounting, Impulsivity, Decision making, Adolescents, Heritability
Introduction
Delay discounting (DD) typically refers to the preference for smaller immediate rewards over larger but delayed rewards and to the well established fact that the subjective value of reward decreases with increasing delay to its receipt. In more general terms, when the consequences of one’s actions are delayed, they decrease in value and are less effective in controlling ongoing behavior (Reynolds 2006). Individuals with a high rate of DD tend to discount longer-term consequences of their decisions and actions, and their behavior is largely driven by the prospects of immediate gratification rather than the pursuit of longer term goals. Experimental procedures for measuring DD were originally developed in animal studies of operant behavior. In these procedures, animals chose between smaller immediate rewards such as one pellet of food and larger rewards such as two pellets of food delivered after a certain delay (Ainslie 1975; Monterosso and Ainslie 1999). In humans, different measures of DD involving hypothetical or real monetary rewards have been used as a model for impulsive choice, i.e. the tendency to select small immediate gains rather than larger but delayed rewards (reviewed in Reynolds 2006).
Neurobiological bases of inter-temporal choice including DD have been relatively well investigated in animal models. These studies (reviewed in Dalley et al. 2008) strongly implicate the orbitofrontal cortex and the core of the nucleus accumbens as key neural substrates underlying the choice between immediate and delayed reward and suggest that brain regions underlying DD are distinct from those underlying response inhibition, another aspect of impulsivity. More recently, human studies using neuroimaging techniques have shown that the subjective value of a monetary reward is represented by activity in the ventral striatum, the medial prefrontal cortex, and the posterior cingulate cortex (reviewed in Cardinal 2006), although the extent to which activity in this functional network differentiates between immediate and delayed reward is a matter of ongoing debate. In one neuroimaging study, choices of an immediate reward were associated with greater activity in these brain regions, whereas choices of delayed reward were associated with greater activity in the lateral prefrontal and posterior parietal cortical regions (McClure et al. 2004). These findings provide support for a “competing brain systems” hypothesis, according to which the choice of immediate versus delayed reward depends on the competition between two distinct neural systems, the mainly limbic and paralimbic “impulsive” system and the cortical fronto-parietal “reflective” system (Bechara 2005; Bickel et al. 2007). In contrast, another neuroimaging study provided evidence that both immediate and delayed rewards are represented within a single reward valuation network that tracks the subjective value of possible rewards during choice (Kable and Glimcher 2007). Importantly, a neurometric discount function reflecting tradeoffs between amount and delay showed a good match with the psychometric discount function captured by behavioral tradeoffs between these variables (Kable and Glimcher 2007).
Increasing evidence suggests that individual differences in DD are associated with liability to addiction and comorbid impulse control disorders. Human studies using various DD procedures have demonstrated that addicted individuals show a strong tendency to discount delayed rewards (Perry and Carroll 2008; de Wit 2009), and animal studies have shown an association between DD and the propensity to substance dependence such as alcohol preference and rates of cocaine self-administration (Perry and Carroll 2008; Verdejo-Garcia et al. 2008). It should be noted, however, that although the association between DD and different aspects of addictive behaviors is well established, the direction of causation remains unclear: this association can arise both due to pre-existing cognitive deficits leading to addiction and due to chronic drug exposure causing cognitive deficits (Audrain-McGovern et al. 2004; Reynolds 2006).
The role of impulsive choice in vulnerability to addictive disorders is particularly important during adolescence. First, adolescence is a period of increased risk for onset of substance use and the development of dependence. Second, adolescence is characterized by continuing development of the brain, particularly its prefrontal cortical areas, as evidenced by morphological (Yakovlev and Lecours 1967), electrophysiological (Hudspeth and Pribram 1992; Anokhin et al. 1996; Segalowitz and Davies 2004), and imaging (Ernst and Mueller 2008; Giedd 2008) data. Importantly, due to uneven neurodevelopmental trajectrories of different brain regions and systems, adolescence is characterized by a relative immaturity of the frontally mediated cognitive control system and relative maturity of the mostly sub-cortical systems underlying motivation and reward processing (Chambers et al. 2003). Consistent with this notion, adolescents show greater delay aversion compared to young adults (Scheres et al. 2006; Olson et al. 2007). Thus, in line with the competing neural systems model (Bickel et al. 2007), adolescents can be more susceptible to addictions because their “impulsive” system prevails over the “reflective” executive control system. Indeed, studies using various DD paradigms showed that a greater tendency to discount delayed gratification is associated with substance use in adolescents and young adults (Wulfert et al. 2002; Kollins 2003; Reynolds et al. 2003; Audrain-McGovern et al. 2004; Reynolds 2006; Bickel et al. 2007; Perry and Carroll 2008; Verdejo-Garcia et al. 2008), as well as with psychopathology associated with increased risk for substance use and dependence such as externalizing (aggressive and delinquent) disorders (Krueger et al. 1996) and ADHD (Scheres et al. 2006). Taken together, converging evidence from animal and human studies suggests that addiction liability can in part be mediated by individual differences in the neural mechanisms affecting decision making, such as propensity for impulsive choices.
Due to its strong association with addiction, availability of well established animal models, and relatively well elucidated neural substrates, DD can potentially serve as an intermediate biobehavioral phenotype that could help bridge the gap between genetic risk factors and complex psychiatric phenotypes such as substance use or externalizing disorders in which impulsivity is implicated as a core underlying dysfunction. An important requirement of such an intermediate phenotype is its heritability. However, little is currently known about the relative contribution of genetic and environmental factors to individual differences in DD in humans. Several previous studies have provided converging evidence for about 45% heritability for self-reported measures of impulsivity (Pedersen et al. 1988; Hur and Bouchard 1997; Seroczynski et al. 1999). However, these studies provide little information about genetic influences on DD because the self-report instruments used in these studies did not incorporate temporal discounting as a distinct component. Furthermore, correlations between DD measures and self-reported impulsivity have been fairly modest (Beck and Triplett 2009; Richards et al. 1999) or non-significant (Reynolds et al. 2006), suggesting that DD may represent a construct that is distinct from other aspects of a broader “impulsivity” construct. Studies have demonstrated good test–retest reliability of DD measures (Ohmura et al. 2006; Beck and Triplett 2009), but the origins of these stable individual differences remain unclear. Recently, studies using animal models have provided some evidence for genetic influences on DD: significant strain differences in DD were found in rats (Wilhelm and Mitchell 2009) and increased DD has been reported in mice selectively bred for alcohol preference (Mitchell et al. 2006; Oberlin and Grahame 2009). However, we are not aware of published studies estimating heritability of DD in humans.
Accordingly, the main goal of the present study was to estimate the heritability of DD, i.e. the proportion of inter-individual variability accounted for by genetic factors, using a classical twin design. Another goal was to assess age-related changes and developmental stability of individual differences in DD during early adolescence using a longitudinal approach.
An additional goal was to provide further evidence for the validity of the DD measure by investigating DD’s association with relevant personality variables and behavioral outcomes such as substance use and other problem behaviors. Consistent with previous studies implicating DD in a broader impulsivity construct, we hypothesized that participants choosing immediate but smaller reward would be characterized by a personality and behavior profile indicating elevated level of eternalizing symptoms. Specifically, we expected that participants making an “impulsive” choice would score higher on the Novelty Seeking (NS) and lower on the Self-Directedness (SD) scales of the Temperament and Character Inventory (TCI) (Cloninger and Svrakic 1997; Constantino et al. 2002), would show more symptoms of conduct disorder and ADHD (particularly its impulsivity component), and would be more likely to report substance use. Finally, we examined possible influence of family socioeconomic status (SES) on the participants’ decision in the reward choice task.
Materials and methods
Sample
Subjects were 744 adolescent twins (48.0% female; age at first assessment: M ± SD = 12.5 ± 0.21 years) participating in a longitudinal twin study of the genetics of brain function and behavior. The sample ascertained at age 12 included 169 MZ pairs (82 male, 87 female) and 203 DZ pairs (71 male, 49 female, and 83 opposite sex). Twins were recruited from the general population through a twin registry and represented three consecutive birth-year cohorts. The study was approved by Washington University Institutional Review Board, and written informed assent and consent were obtained from adolescent participants and their parents, respectively, after complete description of the study to the subjects and their parents. The subjects were retested at age 14 (M ± SD = 14.6 ± 0.24 years; n = 606, or 81.5% of the original sample). It is important to note that because the collection of follow-up data was still ongoing at the time of the analysis, the smaller number of subjects at age 14 reflects not only dropouts, but also incomplete follow-up.
Subjects with a history of serious head trauma or health conditions precluding a laboratory visit or the ability to perform the experimental tasks (e.g. severe visual impairment or mental retardation) were excluded. In addition, data from African American participants were not included in the present analyses because preliminary analyses showed substantial ethnic heterogeneity with respect to the studied measure, but due to the small size of the African American group (13.9% of the sample) we did not have sufficient statistical power to model group differences or to conduct separate analyses within this group. Zygosity was determined based on a standard interview administered to the twins’ parents, on the research assistants’ ratings of the twins’ physical similarity, and on a set of seven DNA markers genotyped in 94% of the participants. The reliability of zygosity diagnosis by questionnaire has been demonstrated in previous studies (Kasriel and Eaves 1976), and the agreement between interview and genotyping data was 95.1%.
Reward choice procedure
At the end of the laboratory session, which included a series of psychophysiological and behavioral tests, the participants were told that they had earned an additional bonus for their cooperation and good performance. They were asked to choose between two options: to receive $7 in cash immediately (the bills were placed on the desktop in front of them) or to receive $10 in cash by mail in 7 days. The test was administered individually to each twin, such that the twins were unaware of their co-twin’s choice. If the subjects chose the immediate reward, they were handed the $7 bonus, and if they chose a delayed reward, a $10 bill was mailed to them in a regular envelope on the 4th day following the lab visit (the local mail delivery takes 2–3 days, with larger delays being extremely rare). During a routine follow-up call, the receipt of the reward was confirmed and the subject was queried about their experience. No complaints about failed delivery or delays were received.
We used a delay of gratification procedure that has been shown previously to predict problem behaviors in high school children (Wulfert et al. 2002). This test is quick, places a minimum burden upon the subjects, is ecologically valid, and models real-world choice situations. It also has the advantage of being a naturalistic reward choice test whereby the respondents directly experience the consequences of their choices (i.e. obtain a real rather than a hypothetical reward and experience a real rather than a hypothetical delay), which ensures active motivational engagement by the participant. Although previous studies have established a good correspondence between the discounting of real and hypothetical rewards (Johnson and Bickel 2002; Madden et al. 2003), other studies found systematic differences in the rate of discounting depending on the reward type (Hinvest and Anderson 2010). Furthermore, the use of real rewards is more suitable for research with children because they require minimum abstraction on the part of the child participant (Reynolds 2006).
Other assessments
Participants were administered the TCI (Cloninger and Svrakic 1997; Constantino et al. 2002) to assess the personality dimensions of NS and SD. In addition, a proxy measure of SES was derived from parental occupation data. Parental occupations were classified into nine categories according to Hollingshead (1975) and the score categories were averaged for both parents. If only one parent provided occupational data, the family was assigned a score based on the available data from one parent. Occupational score has been shown to correlate highly with education, income, and “prestige” rating (Hollingshead 1975) and thus can serve as a good proxy indicator of SES of the family. Participants were administered Missouri Assessment of Genetics Interview for Children (Todd et al. 2003), a semi-structured, diagnostic interview for children and adolescents that has been developed and extensively used in epidemiological family genetic studies of psychiatric disorders in adolescents. In addition, participants completed a paper-and-pencil substance use screen.
Statistical analyses
The relationship between DD and personality, social status, psychiatric symptoms, and substance use variables was examined using ANOVA with DD as a categorical (grouping) variable or χ2 tests, depending on the variable type.
To estimate heritability, i.e. the relative contribution of genetic and environmental sources to individual differences in choice behavior, we performed a biometrical genetic analysis using linear structural equation model (SEM) fitting, a standard approach in twin genetic research (Neale and Cardon 1992; Rijsdijk and Sham 2002). SEM models were fitted using the Mx package, which was specifically developed to model genetically informative data (Neale et al. 2002). These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), non-additive genetic influences (D), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). It was not possible to test a model including both D and C with our data, thus we tested ADE and ACE models separately. It is important to note that A, D, and C increase intrapair twin similarity whereas E decreases it.
We fit a bivariate Cholesky model (see Neale and Cardon 1992) with measurements taken at ages 12 and 14 entered as separate variables. In a longitudinal analysis, the bivariate model permits the estimation of both the genetic and environmental influences at each age and the correlations between genetic and environmental factors across ages, which is important for examining whether same or different genetic and environmental factors influence the trait at different ages. Since our sample included both male and female twins and preliminary analyses suggested some differences between male and female twin correlations, we investigated potential sex differences in genetic and environmental effects on the DD phenotype by allowing genetic and environmental parameters to differ between males and females.
Path coefficients corresponding to these genetic and environmental factors were estimated using maximum likelihood, and the goodness of model fit was indicated by −2LL (log likelihood). Submodels were tested by dropping individual paths from the full model. The significance of individual paths was tested by comparing the fit of the submodel to the fit of the saturated model using a χ2 test of the difference between the log likelihoods for the two models with degrees of freedom corresponding to the difference in the degrees of freedom between two models. Model fit was assessed using the Root Mean Square Error of Approximation (RMSEA; equation per Chen et al. 2008; Kenny and McCoach 2003; Rigdon 1996) and Akaike’s Information Criterion (AIC). If dropping a path reduced the goodness of fit (i.e. the RMSEA and AIC increased), the path was retained in the model, otherwise the more parsimonious model was chosen. Heritability was estimated as the percentage of the total variance of the trait attributed to genetic factors; in addition, 95% confidence intervals of the estimates were computed. A detailed description of the model fitting approach and assessment of heritability can be found elsewhere (Neale and Cardon 1992; Rijsdijk and Sham 2002).
For all quantitative genetic analyses, the occupational status measure was further collapsed into a five-level measure with approximately equal proportions of respondents in each category. These five levels were used to create four dummy variables, with the highest occupational status group as the comparison group. These dummy variables were included in all quantitative genetic analyses, to control for any effects of occupational status on DD.
Results
Developmental stability and change
There was a significant decrease in the prevalence of impulsive choices with age: 35.0 and 27.5% of the sample preferred the smaller but immediate reward at ages 12 and 14, respectively (McNemar’s test: (χ2 = 8.45, df = 1, P = .004). There were no significant sex differences in the preference of immediate versus delayed rewards at either age (χ2 = .60 and .46, P = .44 and P = .50 at ages 12 and 14, respectively). There was a highly significant within-subject association between choices made at ages 12 and 14 (χ2 = 33.9, df = 1, P < .0001; tetrachoric r = .44) indicating significant developmental stability of temporal discounting preferences despite significant age-related changes.
Association with personality, behavior, and SES
At both ages, DD was significantly associated with the Novelty Seeking (NS) and Self-Directedness (SD) scales of the TCI in the predicted direction: participants choosing the immediate but smaller reward scored higher on NS (F1,646 = 11.7, P = .001 and F1,511 = 3.94, P = .048 at ages 12 and 14, respectively) and lower on SD (F1,646 = 6.39, P = .012 and F1,511 = 5.08, P = .025 at ages 12 and 14, respectively).
Preference for immediate reward was associated with larger number of Conduct Disorder symptoms (F1,741 = 10.27, P = .001 at age 12 and F1,490 = 6.80, P = .009 at age 14) and ADHD symptoms from all three domains at age 12 (Inattentive: F1,638 = 9.85, P = .002; Hyperactive: F1,638 = 10.73, P = .001; Impulsive: F1,638 = 5.35, P = .021); however, at age 14 only the association with Impulsive symptoms was significant (inattentive: F1,638 = 3.87, P = .050; hyperactive: F1,638 = 3.79, P = .052; impulsive: F1,638 = 6.14, P = .014).
Although preference for immediate reward was associated with substance use over the past year (any substance use, χ2 = 7.46, P = .006; tobacco, χ2 = 8.76, P = .003; alcohol, χ2 = 8.68, P = .003; marijuana, χ2 = 7.16, P = .007; df = 1 for all tests) at age 14, none of these associations reached significance at age 12.
Finally, DD was significantly associated with family SES derived from parental occupation data: F1,644 = 10.47, P = 0.001 at age 12 and F1,531 = 11.86, P = 0.001 at age 14, although effect sizes were modest (d = .27 and .35, respectively). Specifically, at both ages participants from families with lower SES tended to prefer smaller but immediate rewards. SES was included as a covariate (definition variable) in the genetic analysis through the use of four dummy variables (with the highest income bracket as the comparison group). Percentages of respondents in the lowest through highest socioeconomic brackets were 16, 26, 25, 17, and 15% respectively (1% of respondents had not socioeconomic data and were set to missing for these variables).
Twin correlations and heritability
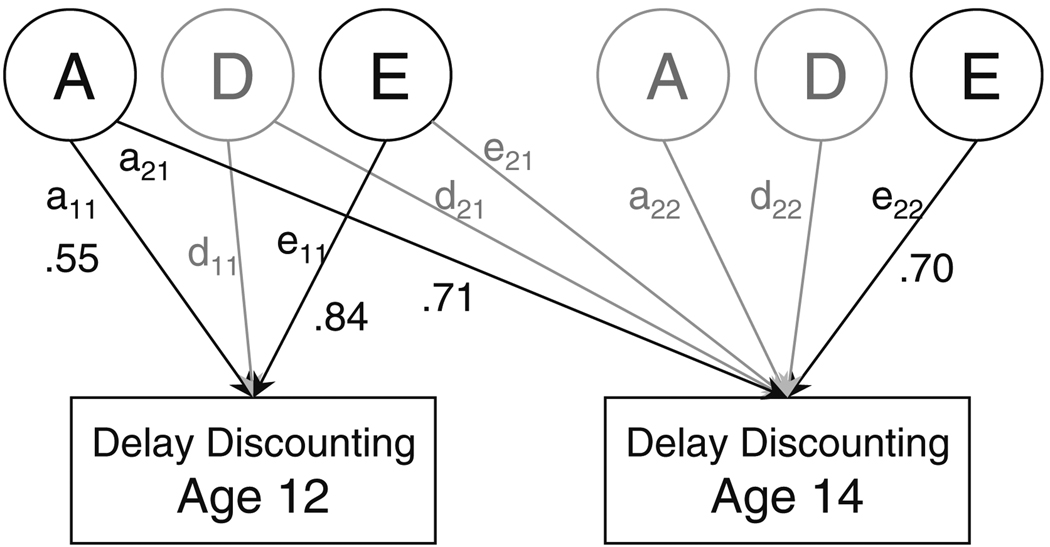
Tetrachoric correlations computed within the twin pairs (Table 1) were significant for MZ twins at both ages, whereas DZ twin correlations were generally lower and mostly non-significant, suggesting the presence of genetic influences. The results of model fitting are presented in Table 2. First, we fit a saturated model, which estimated a variance/covariance matrix for each group (10 parameters for each of 5 twin groups; 50 parameters total), thresholds for each twin for each variable (5 groups, 2 twins per group, 2 variables per twin; 20 parameters total), and regression coefficients for each definition variable (5 groups, 2 twins per group, 4 definition variables per twin, 2 variables per twin; 80 parameters total). More restrictive genetic models were then tested against the saturated model to determine whether they provided an adequate fit with a more parsimonious model. Our first genetic model included additive genetic, non-additive genetic, and unique environmental influences (ADE) because in most cases the MZ correlations were more than twice the DZ correlations, suggesting the contribution of non-additive genetic factors. The initial ADE model (Model 1) allowed for the differences in parameter estimates between males and females, equated thresholds for members of same-sex twin pairs (males and females estimated separately), and equated the impact of SES on same-sex twin pairs (males and females estimated separately). Thresholds and regression parameters for members of DZO pairs were estimated separately from each other and from the same-sex pairs. Under this model, broad-sense heritability (including both additive and non-additive genetic influences) was significant at both ages (age 12: 37 and 38% for males and females, respectively; age 14: 45 and 80% for males and females, respectively). However, equating male and female parameters did not lead to a significant deterioration of fit (Model 2). Because there was some evidence of shared environmental influences (C) at age 12, we also tested ACE models. As with the ADE models, ACE models incorporating shared environmental influences (C) indicated that male and female parameters could be equated (Model 4 vs. Model 3). Because the full ADE model fit the data slightly better than the full ACE model (according to both the RMSEA and the AIC), the ADE model was selected for further analyses. Additional analyses indicated that non-additive genetic influences (D) could be dropped without a significant deterioration of fit (Model 5) and that additive genetic influences specific to age 14 could be dropped from the AE model without a significant decrement in fit (Model 7), but that dropping additive genetic influences common to both ages led to a highly significant deterioration of fit (Model 6). It was also possible to drop the path linking unique environmental influences across the two ages (Model 8), indicating that the unique environmental influences (which include actual individual-specific effects as well as error variance) were not overlapping across the two assessments. Thus, the best-fitting model (Model 9) included additive genetic influences that were common to ages 12 and 14 and age-specific non-shared (individual) environmental influences. Standardized parameter estimates for this model are shown in Fig. 1, and heritability estimates under this model can be found in Table 1. At both ages, heritability was significant, with a slight increase at age 14. Importantly, because age-specific genetic influences at age 14 could be dropped completely, genetic correlation between DD at different ages was 1.0.
Table 1.
Twin correlations and heritability estimates under the best-fitting model for the delay discounting measure in adolescent twins as a function of age and sex
| Longitudinal assessment wave |
rMZF, n = 77/66 |
rDZF, n = 41/31 |
rMZM, n = 77/63 |
rDZM, n = 57/50 |
rDZOS, n = 68/56 |
Variance components |
|
|---|---|---|---|---|---|---|---|
| a2 (95% CI) | e2 (95% CI) | ||||||
| Age 12 | .38 (.06, .71) | .29 (−.20, .78) | .45 (.14, .75) | .02 (−.40, .44) | .34 (.02, .73) | .30 (.15, .49) | .70 (.51, .85) |
| Age 14 | .46 (.08, .73) | −.14 (−.71, .53) | .82 (.52, .95) | .14 (−.33,.56) | .17 (−.29, .57) | .51 (.28, .71) | .49 (.29, .72) |
Tetrachoric twin correlations: rMZF and rDZF are intrapair correlations for female MZ and DZ twins, and rMZM and rDZM are intrapair correlations for male MZ and DZ twins, respectively; rDZOS are intrapair correlations for opposite-sex twin pairs. The lower and upper bounds of the 95% confidence interval for the tetrachoric correlation coefficients are shown in brackets. Significant correlations are shown in bold font. The number of twin pairs in each of the zygosity groups is shown for both ages (age 12/age 14). Variance component estimates for the best-fitting model (#7 in Table 1): a2 is the proportion of total phenotypic variance explained by genetic factors (heritability), e2 is the proportion of variance explained by non-shared environmental factors including measurement error (95% confidence intervals of the maximum likelihood estimates are shown in brackets)
Table 2.
Goodness-of-fit statistics for different submodels
| Model | Model −2*log-likelihood | Model df | Δχ2 | Δdf | RMSEA | AIC |
|---|---|---|---|---|---|---|
| Saturated model | 1244.170 | 1040 | - | - | - | - |
| 1. ADE, sex differences | 1335.811 | 1116 | 91.641 | 76 | 0.0254 | −60.359 |
| 2. ADE, genetic and environmental parameters equated for males and females |
1343.502 | 1125 | 99.332 | 85 | 0.0230 | −70.668 |
| 3. ACE, sex differences | 1337.058 | 1116 | 92.888 | 76 | 0.0264 | −59.112 |
| 4. ACE, genetic and environmental parameters equated for males and females |
1344.066 | 1125 | 99.896 | 85 | 0.0234 | −70.104 |
| 5. AE, drop D (all d paths on Fig. 1) | 1344.103 | 1128 | 99.933 | 88 | 0.0206 | −76.067 |
| 6. AE, drop genetic influences common to both ages (a21 path) | 1365.608 | 1129 | 121.438 | 89 | 0.0337 | −56.562 |
| 7. AE, drop genetic influences specific to age 14 (a22 path) | 1344.568 | 1129 | 100.398 | 89 | 0.0200 | −77.602 |
| 8. AE, drop individual environmental influences common to both ages (e21 path) |
1344.114 | 1129 | 99.944 | 89 | 0.0196 | −78.056 |
| 9. AE, drop age-specific A at age 14 and E common to both ages (a22 and e21)a |
1344.301 | 1130 | 100.131 | 90 | 0.0186 | −79.869 |
Notes: A, C, D, and E stand for additive genetic, shared environmental, non-additive genetic, and non-shared (individual) environmental influences; Δχ2 chi-square difference between the submodel and the saturated model; Δdf difference in the degrees of freedom between the submodel and the saturated model; RMSEA √[(χ2 − df)/(N − 1)*df]; AIC Akaike’s Information Criterion (χ2 − 2df)
Final model
Fig. 1.
Path diagram for the best-fitting structural equation model. The paths retained in the model along with the estimated path coefficients are shown in black, and the paths that could be dropped without significant deterioration of fit are shown in grey. Rectangles represent the observed phenotype (choice of immediate versus delayed reward) measured at ages 12 and 14; circles represent the corresponding latent additive genetic, non-additive genetic, and non-shared environmental factors (A, D, and E, respectively). Standardized parameter estimates are shown for each path retained in the final model
Discussion
This study provides the first estimate of the heritability of DD in humans. Using a real-money choice paradigm, this study demonstrated that individual differences in the discounting of delayed gratification among adolescents are moderately to strongly influenced by genetic factors at ages 12 and 14 respectively. The results also suggest an increasing role of genetic factors with age. Given that DD is considered to be one of the key components of a broader impulsivity trait (Perry and Carroll 2008; de Wit 2009), the demonstration of a significant genetic component in DD has important implications for genetic studies of psychopathological conditions characterized by increased impulsivity, particularly those involving impulsive decision-making, such as addictions, pathological gambling, externalizing disorders, and ADHD.
The present study also adds to the literature on DD and SES. We found that adolescents from lower-income families were more likely to choose immediate but smaller rewards, which is consistent with a previous study involving choices between hypothetical amounts of money conducted on a population of older adults (Green et al. 1996). Taken together, these findings suggest that SES is inversely associated with DD across age groups (adolescents and elderly) and reward types (real and hypothetical rewards). One possible explanation for this finding is that there is a causal relationship between propensity to discount delayed rewards and SES, and that this relationship is genetically mediated. There is evidence for genetic influences on SES indicators such as occupational status and educational attainment (Heath et al. 1985; Tambs et al. 1989), and these genetic influences could be mediated by individual differences in DD. For example, individuals with a genetically influenced “impulsive” decision making style may have a propensity to choose smaller but immediate gratification (such as going to parties or watching television) at the cost of larger but delayed rewards (such as acquisition of a professional degree) and thereby be less likely to attain higher SES. Further research would be needed to test such a possibility.
Taken together, the present findings and previous literature suggest that DD may represent a core neurocognitive dysfunction contributing to a range of problem behaviors characterized by reduced sensitivity to the delayed consequences of one’s decisions and actions. The preference for immediate but smaller rewards may be a behavioral marker of underlying genetic liability to impaired decision making and could serve as a useful endophenotype for genetic studies of the etiology of substance use disorders. The finding of genetic influences on this behavioral phenotype raises the question about genetic influences on specific neural substrates underlying intertemporal choices. These issues need to be addressed in future studies using cognitive brain electrophysiology and/or functional neuroimaging methods in genetically informative designs.
Another important issue concerns specific genes accounting for the heritability of DD. A recent study using hypothetical money choices reported an association between the rate of discounting and two dopamine-related genes (Eisenberg et al. 2007), although these associations accounted for less than 5% of total variance. Given that the present findings indicate that genetic factors account for about 50% of the total phenotypic variance (at least for the real-money choice paradigm used) it is likely that many more genes are involved DD. Future studies should aid in the identification of individual genes influencing variability in decision making.
The present findings also have implications for the understanding of normal-range individual differences in personality and behaviors involving economic decisions. A particularly intriguing possibility is that heritable individual differences in inter-temporal decision style may be associated with real-world economic behavior such as saving, investing, purchasing decisions, etc. It is also important to determine whether hypothetical rewards have comparable heritability to real rewards.
The results of this longitudinal study also indicate significant age-related changes in DD, suggesting a decrease in the propensity for impulsive choice during early adolescence. This finding is consistent with the notion that adolescence is a period of significant changes in cognitive and emotional systems and their underlying neural substrates (Ernst and Mueller 2008). Despite the relatively narrow age range in the present study, the findings suggest a significant trend towards the increasing role of the “reflective” system and decreasing role of the “impulsive” system as adolescents grow older (Bickel et al. 2007).
Some important limitations of the present study need to be acknowledged. First, data from the ongoing longitudinal study were available for only a limited age range of 12–14 years and it remains to be seen how the propensity for DD and genetic influences on this measure will change during mid- and late adolescence. Second, this study used a simple categorical measure of DD which did not allow us to model the DD function and estimate its quantitative characteristics. Finally, the results of model fitting must be interpreted with caution because our relatively modest sample size resulted in limited power to identify possible differences in male and female heritability.
In summary, this study provides the first estimate of the relative contribution of genetic and environmental factors to an experimental behavioral measure of DD, a distinct facet of the impulsivity construct, and suggests that DD can be a promising endophenotype in psychiatric genetic research of addiction and frequently comorbid disorders such as conduct disorder and ADHD.
Acknowledgment
Supported by NIH grants DA027096 and DA018899 from the National Institute on Drug Abuse and the Midwest Alcoholism Research Center (P60 AA011998).
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Birbaumer N, Lutzenberger W, Nikolaev A, Vogel F. Age increases brain complexity. Electroencephalogr Clin Neurophysiol. 1996;99:63–68. doi: 10.1016/0921-884x(96)95573-3. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychol Addict Behav. 2004;18:64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck RC, Triplett MF. Test–retest reliability of a group-administered paper–pencil measure of delay discounting. Exp Clin Psychopharmacol. 2009;17:345–355. doi: 10.1037/a0017078. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90 Suppl 1:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Curran PJ, Bollen KA, Kirby J, Paxton P. An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Sociol Methods Res. 2008;36:462–494. doi: 10.1177/0049124108314720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM. Integrative psychobiological approach to psychiatric assessment and treatment. Psychiatry. 1997;60:120–141. doi: 10.1080/00332747.1997.11024793. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Cloninger CR, Clarke AR, Hashemi B, Przybeck T. Application of the seven-factor model of personality to early childhood. Psychiatry Res. 2002;109:229–243. doi: 10.1016/s0165-1781(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychol Aging. 1996;11:79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Heath AC, Berg K, Eaves LJ, Solaas MH, Corey LA, Sundet J, Magnus P, Nance WE. Education policy and the heritability of educational attainment. Nature. 1985;314:734–736. doi: 10.1038/314734a0. [DOI] [PubMed] [Google Scholar]
- Hinvest NS, Anderson IM. The effects of real versus hypothetical reward on delay and probability discounting. Q J Exp Psychol (Colchester) 2010;63(6):1072–1084. doi: 10.1080/17470210903276350. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Hollingshead four factor index of social status. New Haven: Department of Sociology, Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hudspeth WJ, Pribram KH. Psychophysiological indices of cerebral maturation. Int J Psychophysiol. 1992;12:19–29. doi: 10.1016/0167-8760(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Hur YM, Bouchard TJ., Jr The genetic correlation between impulsivity and sensation seeking traits. Behav Genet. 1997;27:455–463. doi: 10.1023/a:1025674417078. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasriel J, Eaves L. The zygosity of twins: further evidence on the agreement between diagnosis by blood groups and written questionnaires. J Biosoc Sci. 1976;8:263–266. doi: 10.1017/s0021932000010737. [DOI] [PubMed] [Google Scholar]
- Kenny DA, McCoach DB. Effect of the number of variables on measures of fit in structural equation modeling. Struct Equ Modeling. 2003;10:333–351. [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, White J, Stouthamer-Loeber M. Delay of gratification, psychopathology, and personality: is low self-control specific to externalizing problems? J Pers. 1996;64:107–129. doi: 10.1111/j.1467-6494.1996.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl) 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. vol 67. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: statistical modeling. 6th edn. Richmond: Department of Psychiatry; 2002. [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33(7):1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol. 2006;14:318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Individ Dif. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, McClearn GE, Friberg L. Neuroticism, extraversion, and related traits in adult twins reared apart and reared together. J Pers Soc Psychol. 1988;55:950–957. doi: 10.1037//0022-3514.55.6.950. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Karraker K, Horn K, Richards JB. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behav Processes. 2003;64:333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon EE. CFI versus RMSEA: A comparison of two fit indexes for structural equation modeling. Struct Equ Modeling. 1996;3:369–379. [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman CS, Coccaro EF. Etiology of the impulsivity/aggression relationship: genes or environment? Psychiatry Res. 1999;86:41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Tambs K, Sundet JM, Magnus P, Berg K. Genetic and environmental contributions to the covariance between occupational status, educational attainment, and IQ: a study of twins. Behav Genet. 1989;19:209–222. doi: 10.1007/BF01065905. [DOI] [PubMed] [Google Scholar]
- Todd RD, Joyner CA, Heath AC, Neuman RJ, Reich W. Reliability and stability of a semistructured DSM-IV interview designed for family studies. J Am Acad Child Adolesc Psychiatry. 2003;42:1460–1468. doi: 10.1097/00004583-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8(4):426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M. Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J Pers. 2002;70:533–552. doi: 10.1111/1467-6494.05013. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minowski A, editor. Regional development of the brain in early life. Blackwell, Oxford; 1967. pp. 3–70. [Google Scholar]