Abstract
Since 1992 we have prospectively included all head and neck cancer patients in our health region in a departmental based register. Our hospital takes care of all head and neck cancer patients in our health region consisting of approximately 1 million people. In 1997, we evaluated the results of the treatment of oropharyngeal cancer in the 1992–1997 period. On the basis of this evaluation, we changed our treatment policy for tonsillar and base of tongue carcinoma. We first changed the treatment for the lesions with worst prognosis, i.e., those with T3–T4 carcinomas, from radiotherapy only, to radical surgery and postoperative radiotherapy. We have since that time increasingly also operated the smaller oropharyngeal carcinomas. The 2 years’ overall survival and disease-specific survival for all patients diagnosed in the 1992–1997 period was 56 and 63%, respectively. The results from a similar group of patients in the 6 years’ period from 2000 to 2005, after the change in treatment, have increased to 83 and 88%. When we looked at the subgroup of patients in the 2000–2005 period treated with surgery and postoperative radiotherapy, 45 out of 69 patients (65%) presenting with an oropharyngeal cancer were fit for operation. With radical surgery and postoperative radiation therapy, the 2 years overall survival is now 91%. The 2-year disease-specific survival is 96% and the locoregional control is 98%. This is a marked improvement as compared to radiotherapy alone and definitely competitive with modern radiochemotherapy.
Keywords: Oropharyngeal carcinoma, Tonsillar carcinoma, Tongue base carcinoma, Squamous cell carcinoma, Survival
Introduction
The treatment of oropharyngeal cancer varies widely between different institutions. The choice of treatment is based on local and to some extent regional traditions and availability of resources. The dominating treatments have been radiotherapy as a single treatment or a combination of surgery and radiotherapy. Lately, chemotherapy concomitant to radiotherapy or as adjuvant therapy has increased in use [1, 2].
When looking into the overall results of the treatment of oropharyngeal cancer, few studies show the overall, population based patient material. Most studies show only the results of the treated groups and give little or no information of the size and character of the non-included patient group. This is a bias in most studies and makes the overall survival (OS) for the whole oropharyngeal patient group impossible to validate and comparison of studies inaccurate.
One of the largest studies of overall results is the study of tongue base cancer by Zhen et al. [3] of more than 16,000 patients from the National Cancer Data Bank. They found a 2 years’ OS of 48.7% and a 5 years’ OS of 27.8%. Other results from studies including population-based patient material are: The Dutch multi-institutional study of tonsillar cancer by Mak-Kregar et al. [4] with a 5 years’ OS of 32%, the Danish study of oropharyngeal cancers by Johansen et al. [5] with a 5 years’ OS of 31%, the Finnish study on oropharyngeal cancer by Makitie et al. [6] with a 3 years’ OS of 58% and a 5 years OS of 45%, and the study from Oslo by Hannisdal et al. [7] of tonsillar cancer with a 5 years’ OS of 34% (Table 1). We have not found any population-based study based on modern chemo-radiation treatment.
Table 1.
Studies based on overall survival in squamous cell carcinoma of the oropharynx
Many studies have been designed to compare the success of radiotherapy to that of combination therapy with surgery and radiotherapy. The large multicenter study of Parson et al. [8] collecting data from a large number of studies on tonsillar and tongue base cancers from USA and Canada, with about 6,400 patients, did not show any significant difference in outcome between the two treatment modalities. Mendenhall et al. [9] came to the same conclusion in their article based on 400 patients treated with radiotherapy only, or in combination with neck dissection. Other studies [4, 10] have found combination treatment better than radiotherapy as a single treatment.
A cancer register including all patients in a well-defined region gives us the chance to follow and evaluate the longitudinal flow of patients. The patient material in any time period in such a longitudinal follow up will not change to any large extent. By introducing a change in treatment, defined in time, we can monitor any change in outcome caused by such a change. This is the basis of this study.
Patients and methods
Since the first of January 1992 all patients seen with a new diagnosis of cancer in the Department of Otolaryngology/Head & Neck Surgery have been registered in a hospital-based cancer register (HBCR). The basis of the HBCR is the Western Health Care Region of Norway (WHCR). The region today includes approximately one million inhabitants. All patients evaluated and treated in our hospital are seen in a joint oncology meeting with staff from the departments of Otolaryngology/Head & Neck Surgery and Oncology, and when required, also from other departments such as Plastic Surgery and Oral Surgery. This meeting also includes all patients with incurable cancer and all patients where no treatment is provided. Therefore, the HBCR includes very close to 100% of all new head and neck cancer patients within our well-defined geographical area. All patients from outside the WHCR treated in our hospital are omitted from our study. The patient referral base is therefore identical in the two periods.
The HBCR is based on a Filemaker Pro data file. The data base is registered and approved by the Norwegian Social Science Data Services.
The patients are registered with name, date of birth, date of diagnosis, ICD classification, TNM classification, and treatment. All new cancer events such as recurrences, new treatments, new cancers, and deaths are continuously recorded.
The present study is based on the data of oropharyngeal cancer patients in two defined periods. The first period includes all patients from 1992 up to the end of 1997 and the second period includes all patients from 2000 up to the end of 2005.
The first evaluation based on the data from the HBCR was done in 1997–1998 with focus on tongue base and tonsillar cancers. This treatment group therefore includes all patients registered in the 6-year period 1992–1997.
To improve the outcome for patients with advanced oropharyngeal cancers, we decided to change our treatment policy from radiation only, to radical surgery and postoperative radiation therapy. We always start radical surgery with a tracheotomy. This removes the tubing from the operating field, and secures the airway in the postoperative period. It also helps in the protection of the airway when the patient starts oral feeding. A neck dissection on the same side as the primary tumor is standard and a bilateral neck dissection when the primary tumor goes to the midline or to the contralateral side. We perform an open mandibular split in the area of teeth 3–4 with simultaneous splitting of the lip. The splitting and subsequent lateral displacement of the mandible give us a very good control over the area between the primary tumor and the neck area and with macroscopical surgical margins when resecting the primary tumor. We find this approach also of great benefit in the reconstruction phase of the surgery. The majority of patients have been reconstructed with a free radial forearm flap. All surgeries were performed by the two senior head and neck surgeons at the department and a microvascular team.
The second phase of the study is based on the changes done after the evaluation of the first phase (1992–1997). This part of the study includes all patients registered in the period 2000–2005. We have omitted the patients in the 1998–1999 period since we did not have the new treatment policy fully implemented in this intermediate period. Since 2000, all patients diagnosed with a tonsillar or tongue base cancer have been recommended and offered surgery and postoperative radiation treatment if the tumor and the patient have been found suitable for such treatment.
All data are shown as crude 2 years’ overall survival and 2 years’ disease-specific survival (DSS), calculated from the time of diagnosis. The analysis of results as early as 2 years after treatment is valid due to the fact that almost all recurrences occur within this short time interval. In a total of 142 patients from 1992 to 2005, we have had only two recurrences occurring after the 2-year post-treatment period. One was a regional recurrence on the contralateral side at 26 months (treated and cured) and one was a possible recurrence at 44 months (could have been a new primary cancer not far from the location of the first cancer). Hinerman et al. [11] in their study of 134 patients also found only one patient with a cancer recurrence occurring later than 2 years after treatment (77 months after treatment!). Harrison et al. [12] in their study of 68 patients had no local failures after 16 months and one regional failure after 2 years. Based on these figures, the chance of a locoregional recurrence for a patient surviving 2 years without such a recurrence, is <2%.
Radiotherapy has been given as external beam radiation with a linear accelerator in one course treatment. We used one dose of 2 Gy/day, 5 doses/week as standard treatment. Radiotherapy dose was 70 Gy to all macroscopic tumors and to postoperative areas with marginal histological borders. No major change in radiotherapy treatment policy has been made in the study period except for the two younger inoperable patients who in recent years have been treated with concomitant chemo-radiation treatment. Intensity-modulated radiotherapy (IMRT) has been used on a few selected patients in the later years.
Two and five years OS were calculated by using computerized software package (SPSS).
Patient characteristics are shown in Table 2. We diagnosed 45 patients in the 1992–1997 period and 69 patients in the 2000–2005 period. Median age was 59 years; 7.5 patients per year were included in the first period and 11.5 patients per year in the second period. At the time of diagnosis the T stages were as follows: T1: n = 22 (19%); T2: n = 36 (32%); T3: n = 27 (24%); T4: n = 29 (25%).
Table 2.
Patient characteristics
| Period 1995–1997 | Period 2000–2005 | |
|---|---|---|
| Patients included | 45 | 69 |
| Average age (years) | 57.6 | 60.6 |
| Proportion females (%) | 24 | 26 |
| Patients per year | 7.5 | 11.5 |
| Proportion tongue base cancers (%) | 18 | 30 |
| Node positive patients (pretreatment) (%) | 76 | 81 |
| T3 + T4 (%) | 40 | 54 |
| Stage III + IV (%) | 84 | 91 |
Stage distribution at the time of diagnosis were: I: n = 4 (3.5%); II: n = 9 (8%); III: n = 18 (16%); IV: n = 83 (73%). Tongue base was dominated by stage IV cancers (83%).
Neck metastases at the time of diagnosis were detected in 79% of the patients (76% of the patients in the 1992–1997 period and 81% in the 2000–2005 period). The patients with T3 + T4 tumors made up 49% of the patient material (40% in the first period and 54% in the second period). Stage III + Stage IV made up 89% of the patient material (84% in the first period and 91% in the second period).
Eight (7%) patients were not treated or did not survive the treatment period (three patients started but did not finish radiotherapy treatment). All other patients received treatment with curative intent (i.e. no treatment group received palliative treatment).
Results
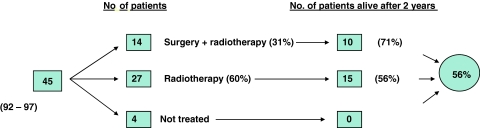
A total of 114 patients with squamous cell carcinoma of the lateral pharyngeal wall and tongue base have been diagnosed in our region in these two periods; 85 patients had tonsillar cancer and 29 tongue base cancer; 85 were men and 29 were women with a mean age of 59 years. In the 1992–1997 period, 45 patients fitting the inclusion criteria were diagnosed; 4 patients were not treated; 27 (60%) patients received radiotherapy as single modality treatment; 14 (31%) patients were operated and given postoperative radiotherapy. Almost all the operations in this period consisted of tonsillectomy and neck dissection (four patients were operated with more radical surgery). Two years from the time of diagnosis no patients in the non-treated group were alive; 15 (56%) in the radiotherapy only group; 10 (71%) in the group treated with operation and postoperative radiotherapy (Fig. 1). Two years’ OS and DSS for the entire group were 56 and 63%, respectively. Two years’ OS and DSS for the treated patients were 60 and 66%. Five years’ OS and DSS were 49 and 59% (Table 3). The difference between the 2 and 5 years’ OS was due to one cancer death and two non-cancer deaths.
Fig. 1.
Two years overall survival for the whole patient material in the 1992–1997 period
Table 3.
Two years overall and disease spesific survival
| Overall survival (%) | Disease spesific survival (%) | |
|---|---|---|
| 1992–1997 All patients | 56 | 63 |
| 1992–1997 Treated patients | 60 | 66 |
| 2000–2005 All patients | 83 | 88 |
| 2000–2005 Treated patients | 88 | 93 |
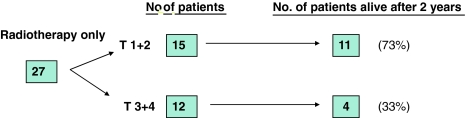
In the further analysis of the radiotherapy only group, we divided the group into two treatment groups: T1 + T2 and T3 + T4. The T1 + T2 group consisted of 15 patients, 11 (73%) of whom survived 2 years. The T3 + T4 group consisted of 12 patients, 4 (33%) of whom survived 2 years (Fig. 2).
Fig. 2.
Results from the group of patients treated with radiotherapy only in the 1992–1997 period
On the basis of the poor outcome in the T3 + T4 group, we decided to operate and give postoperative radiotherapy to patients with T3 + T4 cancer of the lateral oropharyngeal wall and base of tongue. We have lately also operated on T1 and T2 tumors.
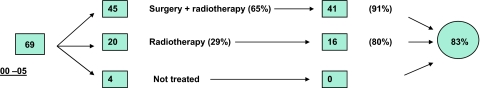
In the 2000–2005 period, 69 patients fitting the inclusion criteria were diagnosed. Four patients were not treated; 20 (29%) patients received radiotherapy treatment as single modality treatment; 45 (65%) patients were operated and given postoperative radiotherapy. Two years from the time of diagnosis no patients in the non-treated group were alive; 16 (80%) patients in the radiotherapy group and 41 (91%) patients in the group treated with operation and postoperative radiotherapy were alive (Fig. 3). Two years’ OS and DSS for the entire group including the non-treated patients, were 83 and 88%. Two years’ OS and DSS for the treated patients were 88 and 93% (Table 3). In the later study period, we have operated a total of 45 patients (31 with radical operations) (Fig. 4). The stages of the radically operated patients were as follows: II: 2; III: 6; IV: 23.
Fig. 3.
Two years overall survival for the whole patient material in the 2000–2005 period
Fig. 4.
Overall survival for all patients diagnosed with tongue base and lateral wall oropharyngeal cancer in the 1992–1995 and 2000–2005 period
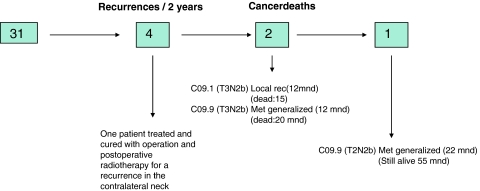
We have surgically removed and given postoperative radiotherapy to the local tumor in 40 patients (31 as part of radical surgery and in 9 patients with T1–T2 cancers as a transoral excision in patients with tonsillar cancers). Only one of these patients experienced a local recurrence. This patient had a tonsillar tumor with extensive growth into the retro-molar area (C09.1/T3N2b).
Forty-five patients were treated with neck dissection and postoperative radiotherapy (31 as part of radical surgery, in 9 patients with T1–T2 cancers as a transoral excision in patients with tonsillar cancers and in 5 patients with T1–T2 cancers were only biopsy were done locally). No recurrences have occurred in the treated necks. One patient experienced a recurrence in the contralateral untreated neck. This patient was treated with surgery and postoperative radiotherapy and is cured. This gives us a 2 years’ DSS and locoregional control in the surgically treated group of 96 and 98%, respectively.
Two of the 45 operated patients have had a distal recurrence. Both these patients had N2b disease, with four and five positive glands respectively, and both had extranodal tumor growth. This gives us a 4 years’ disease-specific survival in the combined treatment group of 96%. (One patient with bone metastasis is still alive more than 55 months from date of diagnosis. No histological confirmation has confirmed the nature of these lesions).
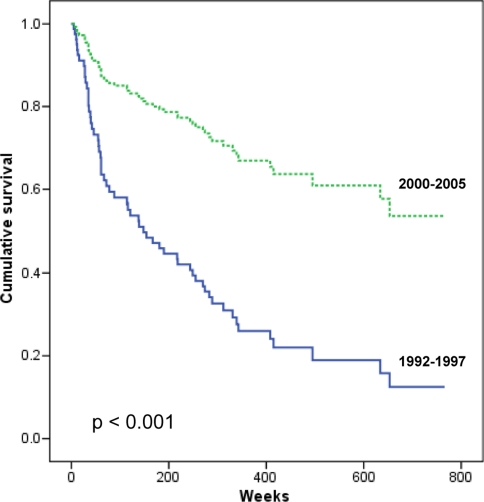
Kaplan–Meier calculation of 5 years survival for the entire patient population in this period is 67% (Fig. 5). Log Rank (Mantel–Cox) overall comparison for the two study periods is p < 0.001.
Fig. 5.
Results of the radically operated patients with postoperative radiotherapy
Discussion
In 1997, we evaluated the results of the treatment of oropharyngeal cancer in the 1992–1997 period. On the basis of this evaluation, we changed our treatment policy for tonsillar and base of tongue carcinoma. We first changed the treatment for the lesions with worst prognosis, i.e., those with T3–T4 carcinomas, from radiotherapy only, to radical surgery and postoperative radiotherapy. We have since that time increasingly also operated the smaller oropharyngeal carcinomas. The 2 years’ overall survival and disease-specific survival for all patients diagnosed in the 1992–1997 period were 56 and 63%. The result from a similar group of patients in the 6 years’ period from 2000 to 2005, after the change in treatment, is increased to 83 and 88%.
When we look at the subgroup of patients in the 2000–2005 period treated with surgery and postoperative radiotherapy, we found that two out of three patients presenting with an oropharyngeal cancer were fit for operation. With radical surgery and postoperative radiotherapy, the 2 years’ overall survival is now 91%. The 2-year disease-specific survival is 96% and the locoregional control is 98%.
The rise in number of patients with oropharyngeal cancer per year, here shown in our population, is in accordance with the reports by others [5, 13, 14]. Hammarstedt et al. [13] found a parallell rise in HPV positive tumors with time, based on re-evaluation of the histopathology, indicating a probable cause for the sharp rise in incidence.
The group of non-treated patients in our study is 7%. All patients in this group were dead within 6 months except one patient who lived 13 months after date of diagnosis. There are very few studies in the literature with control over the non-treated group in the patient material. In the few studies where this has been documented, we find a surprisingly large difference in the size of this group, from the Danish study [5] where only 6% of the patients did not receive curative intended treatment, to the Dutch study [4] where 22% of the patients did not receive curative intended treatment. By increasing the non-treatment group, the danger, from a clinical point of view, is that the overall survival will decrease (and the result in the treated group will theoretically go up, which is of no clinical importance).
Our results in the 1992–1997 period compare favorably with the results from other studies [3–6, 8, 13]. We found, never the less, T3–T4 cancers of the lateral wall of the oropharynx and tongue base had an unacceptable mortality rate. Up to this point, almost all operations in our hospital had been a combination of neck dissection and a tonsillectomy. The decision to operate the T3–T4 oropharyngeal cancers implied that many patients would be treated with more extensive radical surgery.
Since 2000 we have recommended operative treatment, if possible to all patients with cancer of the base of tongue or lateral pharyngeal wall. Patients with T3–T4 tumors and larger T2 tumors have been recommended radical surgery. T1 and smaller T2 tumors have been treated with transoral extirpation of tumors with free margins and a neck dissection. All patients were informed about the different possibilities of treatment (operation and postoperative radiotherapy, radiotherapy only or chemo-radiotherapy) and the advantages and disadvantages of the different alternatives. We always try to involve the patients to actively take part in the selection of treatment. The clinical contraindications we found to surgical treatment were almost always based upon poor general health. The only technical contraindication we found to surgery was cancer surrounding the internal carotid artery in two patients. In general, patients found unfit for surgery due to poor general health were also found unfit for chemo-radiotherapy.
In the 2000–2005 study period, 65% of the patients receiving treatment were treated with surgery and postoperative radiotherapy, and most of these surgical procedures were major radical surgery (31/45). The results from the later period showed an increase in 2 years overall survival for the surgical group compared with the first period from 71 to 91%. This increase, combined with the fact that we moved patients from the worst prognostic group (T3–T4 treated with radiotherapy only) to the surgery plus radiotherapy group, gave the rise in 2 years OS for the entire group from 56 to 83%, and for the treated patients from 60 to 88% (Table 3). The increase in survival in the group treated with radiotherapy only is most likely caused by the fact that the most advanced tumors have been transferred to the group treated with operation and postoperative radiotherapy.
When we compare the patient material from the first and second study periods, we find that the TNM classification has become more advanced. We believe this is mainly the result of increased use of MRI of the oropharynx and ultrasound of the neck in the second study period. The importance of this increased diagnostic capability is that it gives us a better chance to tailor the treatment to the individual patient’s disease both in terms of surgical resections and radiotherapy planning.
Functional results in the radically operated group of patients have not been systematically evaluated. However, only one patient ended up with a permanent feeding tube, all other patients are orally fed. Speech is satisfactory in all patients, but some nasal sound is persistent in the patients where large parts of the soft palate have been removed. The most common complaint from the patients is dryness of the mouth and pharynx. Data on functional results and quality of life are presently investigated and will be reported.
Similar results are reported by transoral laser microsurgery followed by radiotherapy or chemo-radiotherapy with 94 and 88% overall survival at 2 and 5 years, respectively [15].
So called organ preservation techniques using induction plus concurrent chemo-radiotherapy (carboplatin and paclitaxel) for advanced resectable oropharyngeal carcinomas yielded a 3 year actuarial locoregional control of 82% [16]. Finnegan et al. [17] using neoadjuvant chemotherapy followed by concurrent hyperfractionated radiation therapy and sensitizing chemotherapy for locally advanced T3 or T4 oropharyngeal carcinomas in 23 patients reported an absolute 2 and 5 years survival rate of 71 and 55%, respectively.
The use of intensity-modulated radiation treatment (IMRT) may improve the control rate and minimize toxicity and even better results may be achieved by using IMRT together with chemotherapeutic agents [18].
Huang et al. [19] using intensity-modulated chemo-radiotherapy for treatment of stages III and IV oropharyngeal carcinoma yielded a 3 year overall survival of 83%. Similar results are reported with concurrent chemotherapy and intensity-modulated radiation therapy, but including a mixed oropharyngeal and laryngeal material and yielding a 3 year overall survival rate of 81.2% [20].
There are also other good results reported by primary surgery followed by radiotherapy. Röösli et al. [21] reported a 5 year overall survival of 57.9% for their entire cohort of 427 consecutive patients with oropharyngeal squamous cell carcinoma treated from 1990 to 2006; 102 patients were treated by surgery alone and 159 with adjuvant chemo-radiotherapy. The 5 year overall survival for surgery alone was 70.3% and for surgery with radiation 66.6%. Their conclusion was that surgery alone for early oropharyngeal carcinomas and surgery followed by radiation for advanced oropharyngeal carcinomas remain a valuable treatment option but they consider primary chemo-radiotherapy as a strong alternative for those patients who are not candidates for functional preserving surgery.
Cohan et al. [22] state that “Advanced SCCs of the oropharynx (T3–T4, nodally aggressive, or both) require multimodal approaches consisting of either surgery along with adjuvant radiotherapy or concurrent chemo-radiotherapy along with salvage surgery (as necessary)”.
Jones et al. [23] state that for locally advanced carcinomas of the oropharynx primary surgery with immediate microvascular reconstruction followed by radical radiotherapy would be the treatment of choice. They seem, however, not especially optimistic as they at the same time state that the treatment with curative intent for advanced disease is not any better than palliation alone in terms of survival at 5 years. They also find that surgery and radiotherapy are equivalent in controlling the disease.
Low survival rates have been reported in a fairly large series of 361 patients treated in 1990–2001 in Brazil yielding a 5 year overall survival rate of only 17.6% [24]. These patients were treated by primary radiotherapy alone or with chemotherapy. They found that age, Zubrod scale, weight loss, involvement of the adjacent soft tissue area and bone, lymph node mobility, clinical stage, and radiotherapy dose were all prognostic factors.
Haigentz et al. [25] reviewing the current trends in the initial management of oropharyngeal cancer state that the true role of chemotherapy in oropharyngeal cancer requires further research. Anyhow most authors today recommend chemo-radiotherapy for advanced oropharyngeal carcinomas due to better quality of life despite that prospective randomized studies are missing [26].
Concluding remarks
The treatment of oropharyngeal cancer in our institution has become more individualized. We favor a combination of surgery with postoperative radiotherapy as the treatment of choice for most oropharyngeal patients, if the patients are motivated and medically fit for such treatment. It must be stressed that our improved results are based on a combined treatment. Improvements in both surgery, radiotherapy and diagnostic work-up are all important factors for the improvements in outcome that we have seen. For patients not treated with operation, we have introduced chemo-radiotherapy treatment in the later years. All younger patients, found technically inoperable, are offered such combined treatment, but this is a very small group of patients. Only two patients were found inoperable for technical reasons (both had tumors surrounding the internal carotid artery). For all other non-operated patients, the reasons were inoperability based on poor general health, and this is a patient population generally not fit for chemo-radiotherapy treatment either.
We have shown survival figures that are comparable or even better than modern chemo-radiotherapy. The functional results are good with most persons returning to work and only one of the patients treated with surgery plus radiotherapy has a permanent feeding tube (PEG). The functional results will be further elaborated on in a subsequent review.
Acknowledgments
This work was supported by The Norwegian Cancer Society.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Denis F, Garaud P, et al. Final results of the 94–01 French head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitany radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Shirazi HA, Sivaandan R, et al. Advanced-staged tonsillar squamous carcinoma: organ preservation versus surgical management of the primary site. Head Neck. 2006;28:587–594. doi: 10.1002/hed.20372. [DOI] [PubMed] [Google Scholar]
- 3.Zhen W, Karnell LH, et al. The national cancer data base report on squamous cell carcinoma of the base of tongue. Head Neck. 2004;26:660–674. doi: 10.1002/hed.20064. [DOI] [PubMed] [Google Scholar]
- 4.Mak-Kregar S, Hilgers FJM, et al. Disease-specific survival and locoregional control in tonsillar carcinoma. Clin Otolaryngol Allied Sci. 1996;21:550–556. doi: 10.1111/j.1365-2273.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 5.Johansen LV, Grau C, et al. Squamous cell carcinoma of the oropharynx-an analysis of the treatment results in 289 consecutive patients. Acta Oncol. 2000;39:985–994. doi: 10.1080/02841860050215981. [DOI] [PubMed] [Google Scholar]
- 6.Makitie AA, Pukkila M, et al. Oropharyngeal carcinoma and its treatment in Finland between 1995 and 1999: a nationwide study. Eur Arch Otorhinolaryngol. 2006;263:139–143. doi: 10.1007/s00405-005-0975-5. [DOI] [PubMed] [Google Scholar]
- 7.Hannisdal K, Boysen M, et al. Different prognostic indices in 310 patients with tonsillar carcinomas. Head Neck. 2003;25:123–131. doi: 10.1002/hed.10175. [DOI] [PubMed] [Google Scholar]
- 8.Parsons JT, Mendenhall WM, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–2980. doi: 10.1002/cncr.10567. [DOI] [PubMed] [Google Scholar]
- 9.Mendenhall WM, Amdur RJ, et al. Radiation therapy for squamous cell carcinoma of the tonsillar region: a preferred alternative to surgery? J Clin Oncol. 2000;18:2219–2225. doi: 10.1200/JCO.2000.18.11.2219. [DOI] [PubMed] [Google Scholar]
- 10.Pericot J, Escribà JM, et al. Survival evaluation of treatment modality in squamous cell carcinoma of the oral cavity and oropharynx. J Craniomaxillofac Surg. 2000;28:49–55. doi: 10.1054/jcms.1999.0091. [DOI] [PubMed] [Google Scholar]
- 11.Hinerman RW, Parsons JT, et al. External beam irradiation alone or combined with neck dissection for base of tongue carcinoma: an alternative to primary surgery. Laryngoscope. 1994;104:1466–1470. doi: 10.1288/00005537-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Harrison LB, Lee HJ, et al. Long term results of primary radiotherapy with/without neck dissection for squamous cell cancer of the base of tongue. Head Neck. 1998;20:668–673. doi: 10.1002/(SICI)1097-0347(199812)20:8<668::AID-HED2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Hammarstedt L, Lindquist D, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 14.Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing? Oral Oncol. 2003;39:31–36. doi: 10.1016/S1368-8375(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 15.Rich JT, Milov S, et al. Transoral laser microsurgery (TLM) ± adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machtay M, Rosenthal I, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a university of Pennsylvania phase II trial. J Clin Oncol. 2002;20:3964–3971. doi: 10.1200/JCO.2002.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Finnegan V, Parsons JT, et al. Neoadjuvant chemotherapy followed by concurrent hyperfractionated radiation therapy and sensitizing chemotherapy for locally advanced (T3–T4) oropharyngeal squamous cell carcinoma. Head Neck. 2009;31:167–174. doi: 10.1002/hed.20947. [DOI] [PubMed] [Google Scholar]
- 18.Narayan S. The use of intensity-modulated radiation therapy in the treatment of oropharyngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2005;13:226–232. doi: 10.1097/01.moo.0000170528.97134.20. [DOI] [PubMed] [Google Scholar]
- 19.Huang K, Xia P, et al. Intensity-modulated chemoradiation for treatment of stage III and IV oropharyngeal carcinoma. Cancer. 2008;113:497–507. doi: 10.1002/cncr.23578. [DOI] [PubMed] [Google Scholar]
- 20.Saba NF, Edelman S, et al. Concurrent chemotherapy with intensity-modulated radiation therapy for locally advanced squamous cell carcinoma of the larynx and oropharynx: a retrospective single-institution analysis. Head Neck. 2009;31:1447–1455. doi: 10.1002/hed.21120. [DOI] [PubMed] [Google Scholar]
- 21.Röösli C, Tschudi DC, et al. Outcome of patients after treatment for a squamous cell carcinoma of the oropharynx. Laryngoscope. 2009;119:534–540. doi: 10.1002/lary.20033. [DOI] [PubMed] [Google Scholar]
- 22.Cohan DM, Popat S, et al. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg. 2009;17:88–94. doi: 10.1097/MOO.0b013e32832984c0. [DOI] [PubMed] [Google Scholar]
- 23.Jones AS, Rafferty M, et al. Treatment of squamous cell carcinoma of the tongue base: irradiation, surgery or palliation? Ann Otol Rhinol Laryngol. 2007;116:92–99. doi: 10.1177/000348940711600203. [DOI] [PubMed] [Google Scholar]
- 24.Pedruzzi PAG, Kowalski LP, et al. Analysis of prognostic factors in patients with oropharyngeal squamous cell carcinoma treated with radiotherapy alone or in combination with systemic chemotherapy. Arch Otolaryngol Head Neck Surg. 2008;134:1196–1204. doi: 10.1001/archotol.134.11.1196. [DOI] [PubMed] [Google Scholar]
- 25.Haigentz M, Jr, Silver CE, et al. Current trends in initial management of oropharyngeal cancer: the declining use of open surgery. Eur Arch Otorhinolaryngol. 2009;266:1845–1855. doi: 10.1007/s00405-009-1109-2. [DOI] [PubMed] [Google Scholar]
- 26.Boscolo-Rizzo P, Stellin M, et al. Long-term quality of life after treatment for locally advanced oropharyngeal carcinoma: surgery and postoperative radiotherapy versus concurrent chemoradiation. Oral Oncol. 2009;45:953–957. doi: 10.1016/j.oraloncology.2009.06.005. [DOI] [PubMed] [Google Scholar]