Abstract
Our aim was to assess the ability of tocilizumab monotherapy to reduce progressive structural joint damage in rheumatoid arthritis patients at high risk of progression. This study was a subanalysis from a prospective 1-year, multicenter, X-ray-reader-blinded, randomized controlled trial of tocilizumab [Study of Active Controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 Inhibitor (SAMURAI) trial]. All patients were categorized into two or three groups according to four independent predictive markers for progressive joint damage [urinary C-terminal crosslinking telopeptide (uCTX-II), urinary pyridinoline/deoxypyridinoline (uPYD/DPD) ratio, body mass index (BMI), and joint-space narrowing (JSN) score at baseline]. One-year progression of joint destruction was assessed in high-risk versus low-risk groups receiving tocilizumab monotherapy and compared with patients receiving conventional disease-modifying antirheumatic drugs (DMARDs) (n = 157 and 145, respectively). In patients at high risk of progression of erosion as estimated by high uCTX-II, uPYD/DPD, or low BMI, and at high risk of progression of JSN as estimated by low BMI or high JSN score, the 52-week changes in radiological erosion and JSN, respectively, were significantly less in patients treated with tocilizumab monotherapy compared with those receiving DMARDs for each type of risk factor. In patients at low risk, those receiving tocilizumab also progressed less than those on DMARDs, although the difference did not reach statistical significance. Tocilizumab monotherapy is more effective in reducing radiological progression in patients presenting with risk factors for rapid progression than in low-risk patients. Patients at high risk for progression may benefit more from tocilizumab treatment.
Keywords: CTX-II, PYD/DPD, Rheumatoid arthritis, Interleukin-6, Tocilizumab, Joint destruction
Introduction
Biological agents targeting inflammatory cytokines have proven more effective than conventional disease-modifying antirheumatic drugs (DMARDs) for suppressing disease activity and progressive joint damage in rheumatoid arthritis (RA) [1–11]. Patients at high risk of progressive joint damage who are difficult to treat with conventional DMARDs may be a particularly important target population for treatment with biologics. Our recent studies showed that increased urinary levels of C-terminal crosslinked telopeptide of type II collagen (uCTX-II), total pyridinoline/total deoxypyridinoline ratio (uPYD/DPD ratio), joint-space narrowing (JSN) score, and low body mass index (BMI) at baseline were all independent predictive markers for radiographically evident joint damage in patients with RA of <5 years and treated with conventional DMARDs. Although targeting patients with rapid progression, as assessed by these predictive factors, for treatment with biologics may be beneficial, to date there is no evidence that these agents are equally effective in such high-risk patients. Therefore, this study aimed to investigate the ability of tocilizumab monotherapy to reduce progressive structural joint damage in high-risk patients.
Methods
Patients with RA of <5 year duration participating in a prospective 1-year randomized controlled trial of tocilizumab [Study of Active Controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 Inhibitor (SAMURAI) trial] [10] receiving anti-interleukin (IL)-6-receptor antibody monotherapy (8 mg/kg intravenously every 4 weeks, n = 154), were categorized into two or three groups according to their uCTX-II values, uPYD/DPD ratio, BMI, and JSN scores at baseline (cutoff values: 500 ng/mmol creatinine for uCTX-II, median for uPYD/DPD, 0 units for JSN, 18.5 and 25 for BMI). These four factors were shown to be independent predictive markers for radiographically evident joint damage progression in 148 patients with RA treated with conventional DMARDs in the control arm of the SAMURAI trial [12]. Briefly, this trial established that high baseline uCTX-II, uPYD/DPD ratio and JSN score and a low BMI were independent risk factors for progression of bone erosion as evaluated with the van der Heijde modified Sharp method in patients with RA receiving conventional DMARDs. In addition to these three variables, the JSN score at baseline was also significantly associated with an increased risk of progression of JSN score and total Sharp score (TSS). In this study, we compared the 1-year progression of joint destruction in these high- and low-risk groups in the two cohorts of 148 and 154 patients with RA receiving conventional DMARDs or tocilizumab monotherapy, respectively.
SAMURAI trial
In the SAMURAI trial [10], patients were >20 years and fulfilled the American College of Rheumatology (ACR; formerly the American Rheumatism Association) 1987 revised criteria for the classification of RA, with a disease duration of ≥6 months and <5 years. In addition, they had ≥6 tender joints (of 49 evaluated), ≥6 swollen joints (of 46 evaluated), an erythrocyte sedimentation rate (ESR) of ≥30 mm/h, and C-reactive protein (CRP) of ≥20 mg/l. All patients had an inadequate response to at least one DMARD or immunosuppressant. Use of anti-tumor necrosis factor (anti-TNF) agents and leflunomide were not allowed within the 3 months prior to the first dose. Change in dose and type of DMARDs and/or immunosuppressants, plasma exchange therapies, and surgical treatments were not allowed within the previous 4 weeks. Oral corticosteroids (prednisolone up to 10 mg/day) were allowed if the dosage had not been changed during the prior 2 weeks. Patients were randomly assigned to receive either tocilizumab monotherapy at 8 mg/kg intravenously every 4 weeks or conventional DMARD therapy for 52 weeks. For the tocilizumab group, DMARDs and/or immunosuppressants were discontinued from the start of the study. Oral corticosteroids (up to 10 mg prednisolone per day) were allowed, but the dosage could not be increased during the study. Use of one nonsteroidal anti-inflammatory drug (NSAID), including switching to another NSAID, was allowed. For the conventional DMARD group, the dose, type, and combination of DMARDs and/or immunosuppressants, except for anti-TNF agents and leflunomide, could be varied according to disease activity at the discretion of the treating physician. Variations of NSAIDs and/or corticosteroids including intra-articular corticosteroid injections were also allowed.
Assessment of risk of radiographic progression
Posteroanterior radiographs of the hands and anteroposterior radiographs of the feet were acquired at baseline, week 28, and week 52 (or at the last visit for patients who withdrew from the study prior to week 52). Radiographs were scored using the van der Heijde modified Sharp method [13] for bone erosion, JSN, and TSS independently by two readers who were well trained and competent to score radiographs in accordance with the method. The readers were blinded to the treatment group and chronologic order of the images. We measured urinary total deoxypyridinoline (uDPD) and total pyridinoline (uPYD) by high-performance liquid chromatography (HPLC) and uCTX-II by enzyme-linked immunosorbent assay (ELISA) (CTX-II CartiLaps® ELISA, NORDIC Biosciences, Herlev, Denmark).
Statistical analysis
All statistical analyses were two-sided, and p values <0.05 were considered significant. All statistical analyses were carried out using SAS (SAS Institute, Cary, NC, USA version 8.2, TS2M0).
Results
Groups were comparable at baseline for the risk factors previously identified for predicting radiological progression in the DMARDs group (Table 1). As shown in Figs. 1 and 2, differences in the 1-year changes in radiological erosion and JSN scores between patients in the DMARDs and tocilizumab monotherapy arms varied between subgroups divided according to the baseline levels of each predictive marker for radiographically evident joint damage (uCTX-II, uPYD/DPD, JSN, and BMI). The 1-year changes in radiological erosion scores in patients with high uCTX-II, high uPYD/DPD, or low BMI at baseline, indicating a high risk of progressive joint erosion, were significantly lower in tocilizumab-treated than in DMARD-treated patients (Fig. 1). Those changes in radiological JSN scores in patients with high uCTX-II, high uPYD/DPD, high JSN, or low BMI at baseline, indicating a high risk of progressive JSN, were also lower in tocilizumab-treated than in DMARD-treated patients (Fig. 2a–d), and there were proven to be significant differences in cases with high JSN and low BMI (Fig. 2c, d). In contrast, low-risk patients receiving tocilizumab monotherapy progressed less than patients on DMARDs, although the differences were very small and did not reach statistical significance (Figs. 1 and 2).
Table 1.
Baseline values in the different patient groups
| Variables | Cutoff value | DMARDs N = 145 |
Tocilizumab N = 157 |
||
|---|---|---|---|---|---|
| N | Mean of baseline value ± SD | N | Mean of baseline value ± SD | ||
| JSN | 0 | 30 | 0 | 32 | 0 |
| >0 | 115 | 21.1 ± 22.5 | 125 | 18.2 ± 21.8 | |
| uCTX-II (ng/mmol/creatinine) | <500 | 53 | 327.2 ± 104.6 | 66 | 337.0 ± 102.9 |
| ≥500 | 88 | 1249.0 ± 1014.9 | 83 | 1156.0 ± 585.8 | |
| uPYD/DPD | <6.8 | 72 | 5.8 ± 0.7 | 76 | 5.5 ± 0.8 |
| ≥6.8 | 73 | 8.6 ± 1.4 | 81 | 8.5 ± 1.3 | |
| BMI (kg/m2) | <18.5 | 20 | 17.5 ± 1.2 | 26 | 17.5 ± 0.6 |
| ≥18.5 and <25 | 102 | 21.5 ± 1.6 | 102 | 21.5 ± 1.8 | |
| ≥25 | 21 | 27.1 ± 1.7 | 29 | 28.2 ± 2.4 | |
Criteria were according to criteria of the World Health Organization [16] employees
JSN joint space narrowing, uPYD/DPD urinary pyridinoline/deoxypyridinoline ratio, uCTX-II urinary C-terminal telopeptide of type II collagen, BMI body mass index, SD standard deviation
BMI <18.5, underweight; ≥18.5 and <25, normal weight; 25≤, overweight or obese
Fig. 1.
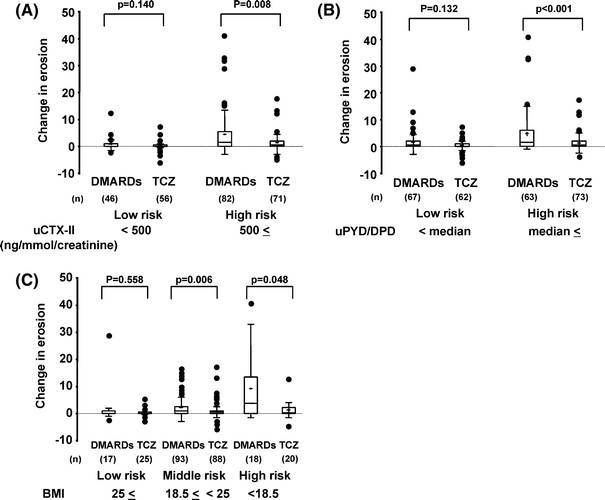
One-year change of erosion scores in tocilizumab-treated rheumatoid arthritis (RA) patients at high risk for developing erosions. One-year change in erosion scores were analyzed for individual risk factors (a uCTX, b uPYD/DPD, c BMI) at baseline. Results are expressed as median (line across boxes), 25–75% interquartile range (boxes), mean (cross symbol in boxes), standard deviation (SD) (vertical line across the top of the box), and standard error (vertical line across bottom of the box). The filled circles represent individual values over SD values. Differences in changes in erosion scores were compared by the Wilcoxon rank sum test. uPYD/DPD urinary pyridinoline/deoxypyridinoline ratio, uCTX-II urinary C-terminal telopeptide of type II collagen, BMI body mass index, TCZ tocilizumab
Fig. 2.
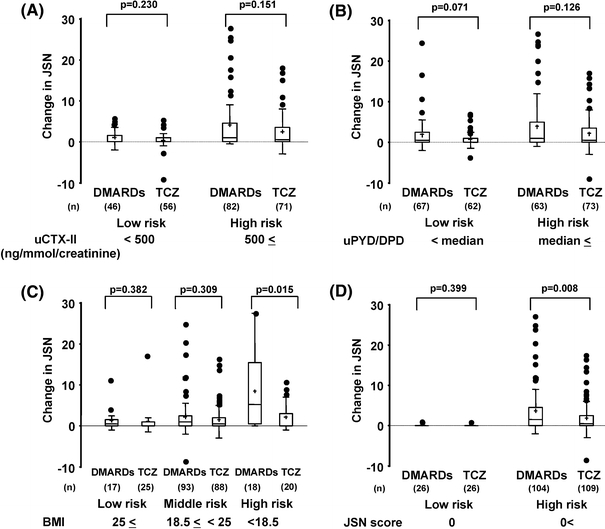
One-year change of joint space narrowing (JSN) scores in tocilizumab-treated rheumatoid arthritis (RA) patients with risk factors for developing JSN. Score changes were analyzed for individual risk factors (a uCTX, b uPYD/DPD, c BMI, d JSN) at baseline. Results are expressed as median (line across boxes), 25–75% interquartile range (boxes), mean (cross symbol in boxes), standard deviation (SD) (vertical line across top of the box), and standard error (vertical line across bottom of the box). The filled circles represent individual values over SD values. Differences in change in JSN scores were compared by the Wilcoxon rank sum test. uPYD/DPD urinary pyridinoline/deoxypyridinoline ratio, uCTX-II urinary C-terminal telopeptide of type II collagen, BMI body mass index, TCZ tocilizumab
Discussion
Biological agents targeting inflammatory cytokines are more effective at suppressing progression of joint damage than are conventional DMARDs. However, previously reported studies on the efficacies of these agents for slowing radiological damage showed that there was still significant progression in many patients [1–11]. These data allowed us to recognize that many patients on nonbiologic DMARDs show no radiographic progression, and some even show radiographic improvement. Specifically, our previous data showed that 39% of patients treated with conventional DMARDs showed no radiographic progression in 1-year follow-up [10]. However, biological agents may have side effects. With such knowledge gained from growing experience in the use of biologics, patients would benefit from personalized therapies based on accurate prognostic tools rather than standard therapies that do not evaluate risk factor to guide treatment selection. A better understanding of the prognostic factors for progressive joint damage under nonbiological treatment and appropriate targeting of biologics to patients with RA at high risk of progressive joint damage and disability could enhance risk–benefit balance of RA therapeutic strategies. Thus, many investigators have sought such prognostic factors. Young-Min et al. reported the possible usefulness of matrix metalloproteinase-3 (MMP-3) and uCTX-II in predicting radiographic outcome in RA patients treated with DMARDs [14], and Charni et al. reported the possible usefulness of urinary type II collagen helical peptide (HELIX-II) [15]. We found that high baseline levels of uCTX-II, uPYD/DPD, JSN score, and low BMI were independently significantly associated with 1-year progression of joint destruction under conventional DMARDs treatment [12]. Most of these newly developed or recognized markers are still not widely used in clinical practice; however, further understanding the significance of these markers would facilitate better therapies. Having established these risk factors, the efficacy of biologics for reducing joint destruction in high-risk groups needed to be confirmed to effectively target treatment. Therefore, in this study, subanalysis from a prospective 1-year randomized controlled trial of tocilizumab (SAMURAI trial) was performed to investigate the ability of this agent to reduce progression of structural joint damage in high-risk patients. There was no significant difference in 1-year changes of JSN scores between DMARD- and tocilizumab-treated patients in high-risk groups, as estimated by high uCTX-II, uPYD/DPD. Our data show, however, that tocilizumab monotherapy effectively blocked progression of bone erosion in all high-risk groups as estimated by high uCTX-II, uPYD/DPD, or low BMI and also effectively blocked progression of JSN in high-risk groups as estimated by low BMI or high JSN score, The differences in 1-year changes of erosion and JSN scores between the DMARD- and tocilizumab-treated patients were greatest in the high-risk groups, whereas there was little to no difference in the low-risk group. These findings indicate that the benefit of tocilizumab monotherapy to inhibit bone erosion and JSN progression is maximized in high-risk groups as estimated by high uCTX-II, uPYD/DPD, or low BMI, and by low BMI or high JSN score, respectively. To our knowledge, this is the first comprehensive report showing the usefulness of biomarker targeting strategies for biologics in treating RA. There were smaller and nonsignificant differences in 1-year changes of erosion and JSN scores between patients in the DMARD or tocilizumab monotherapy treatment groups in the low-risk category, although progression was still lower in individuals receiving tocilizumab. The lack of statistical significance can be due in part to the very limited progression in the low-risk group reducing the power to detect differences. These findings also suggest that patients at low risk may benefit less from treatment with biologics unless they develop changes in markers that indicate an increased risk of progression during treatment with conventional DMARDs. On the other hand, the lack of statistically significant differences in 1-year changes of JSN scores between patients in the DMARD or tocilizumab monotherapy treatment groups in the high-risk category might be due to the relatively weak prognostic power of high uCTX-II and uPYD/DPD for JSN progression compared with low BMI or high JSN score [12].
Establishing means of early discrimination between tocilizumab radiological responders and nonresponders is therefore raised as the next issue to be addressed when considering targeted therapeutic strategies. In conclusion, we demonstrated that tocilizumab monotherapy is effective in reducing radiological progression in patients presenting with risk factors for rapid progression of joint damage.
Acknowledgments
This work was supported by Chugai Pharmaceutical Co., Ltd., Tokyo, Japan.
Conflict of interest statement NN has served as a consultant to and received honoraria from Chugai Pharmaceutical Co. Ltd., the manufacturer of TCZ. NN also works as a scientific advisor to F. Hoffmann-La Roche, which is developing TCZ in collaboration with Chugai Pharmaceutical Co., Ltd.
References
- 1.Lipsky PE, Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 2.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 3.Klareskog L, Heijde D, Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 4.St Clair EW, Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 5.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 6.Genovese MC, Bathon JM, Fleischmann RM, Moreland LW, Martin RW, Whitmore JB, et al. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol. 2005;32(7):1232–1242. [PubMed] [Google Scholar]
- 7.Heijde D, Landewe R, Klareskog L, Rodriguez-Valverde V, Settas L, Pedersen R, et al. Presentation and analysis of data on radiographic outcome in clinical trials: experience from the TEMPO study. Arthritis Rheum. 2005;52(1):49–60. doi: 10.1002/art.20775. [DOI] [PubMed] [Google Scholar]
- 8.Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54(4):1063–1074. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 9.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 10.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genant HK, Peterfy CG, Westhovens R, Becker JC, Aranda R, Vratsanos G, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis. 2008;67(8):1084–1089. doi: 10.1136/ard.2007.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto J, Garnero P, Heijde D, Miyasaka N, Yamamoto K, Kawai S, et al. A combination of biochemical markers of cartilage and bone turnover, radiographic damage and body mass index to predict the progression of joint destruction in patients with rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs. Mod Rheumatol. 2009;19(3):273–282. doi: 10.1007/s10165-009-0170-4. [DOI] [PubMed] [Google Scholar]
- 13.Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–263. [PubMed] [Google Scholar]
- 14.Young-Min S, Cawston T, Marshall N, Coady D, Christgau S, Saxne T, et al. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared with traditional markers. Arthritis Rheum. 2007;56(10):3236–3247. doi: 10.1002/art.22923. [DOI] [PubMed] [Google Scholar]
- 15.Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52(4):1081–1090. doi: 10.1002/art.20930. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO expert consultation, January 28–February 1, 2002. Geneva: WHO; 2003. http://www.fao.org/DOCREP/005/AC911E/AC911E00.HTM


