Abstract
Objective of this paper is to study how DNA-test result information was communicated and perceived within families. A retrospective descriptive study in 13 probands with a BRCA1/2 unclassified variant, 7 with a pathogenic mutation, 5 with an uninformative result, and in 44, 14, and 12 of their 1st and 2nd degree relatives respectively. We examined differences and correlations between: (a) information actually communicated (b) probands’ perception, (c) relatives’ perception. The perception consisted of recollections and interpretations of both their own and their relatives’ cancer-risks, and heredity-likelihood (i.e. likelihood that cancer is heritable in the family). Differences and low correlations suggested few similarities between the actually communicated information, the probands’ and the relatives’ perception. More specifically, probands recalled the communicated information differently compared with the actually communicated information (R = .40), and reinterpreted this information differently (R = .30). The relatives’ perception was best correlated with the proband’s interpretation (R = .08), but this perception differed significantly from their proband’s perception. Finally, relatives reinterpreted the information they received from their proband differently (R = .25), and this interpretation was only slightly related with the original message communicated by the genetic-counsellor (R = .15). Unclassified-variants were most frequently misinterpreted by probands and relatives, and had the largest differences between probands’ and relatives’ perceptions. Like in a children’s whisper-game, many errors occur in the transmission of DNA-test result information in families. More attention is required for how probands disseminate information to relatives. Genetic-counsellors may help by supporting the probands in communicating to relatives, e.g. by providing clear summary letters for relatives.
Keywords: BRCA1/2, Oncology, Psychology, Genetic-counselling, Familytherapy, Risk-perception
Introduction
Having multiple family members with breast and ovarian cancer may lead an individual to request for DNA-testing. Usually, a DNA-test is first performed in an individual with cancer, a proband. The detection of a pathogenic BRCA1/2 mutation provides probands with precise information about their own cancer-risks. Contralateral breast-cancer recurrence risks for affected women are 30–60%, primary breast and ovarian-cancer risks for unaffected women are respectively 60–80% and 30–60% (BRCA1)/5–20% (BRCA2). The majority of probands receives an uninformative-result, and about 10% an unclassified-variant/variant-of-uncertain-clinical-significance (UV). In these cases, cancer-risks are primarily calculated on the basis of the pedigree [49]. Subsequently, risk management options, such as surveillance and prophylactic surgery of ovaries and breasts depend on the pathogenic-result or the pedigree.
Many studies showed that probands may experience a significant influence of DNA-testing on their psychological wellbeing and medical decisions [2, 4, 29, 30]. Fewer studies have examined how probands communicate DNA-test results to untested relatives, and how a test result influences their relatives’ lives. The perception and impact of relatives has not been studied from the relatives’ own perspective [12], despite the fact that relatives are often closely involved in genetic-counselling.
First, many relatives provide medical information on the proband’s request to complete pedigree information, which is the basis for DNA-testing and risk-estimation.
Second, many probands undergo DNA-testing for the reason of receiving genetic-information for their relatives [15, 16, 48]. Detection of a pathogenic-result enables relatives to request for DNA-testing, and other DNA-results allow calculation of a priori cancer-risks for relatives on the basis of the pedigree.
Third, most relatives are informed by the proband about the DNA-test result, mostly within 4 months after testing [37]. Especially pathogenic-mutations are communicated, in particular to first-degree female relatives from cohesive families for whom DNA-test results may have medical consequences [5, 6, 11, 18, 23, 37]. The communicated DNA-test result may subsequently cause distress in relatives [12, 18, 22, 47], awaken familial conflicts and myths [3, 8, 40], and influence the relatives’ well-being, medical-decisions and intention to request DNA-testing [12, 24, 28, 32–34, 36].
Family communication timeline
We examined the relatives’ perception as a part of the family communication timeline of genetic counselling.
Family communication of genetic-counselling involves two senders of genetic-information, viz. the genetic-counsellor and the proband, and two receivers, viz. the proband and the relative. The communication of genetic-information may involve ‘noise’, either caused by genetic-counsellors and probands who disclose information inaccurately, and/or the probands and relatives who receive information inaccurately.
First, noise may occur in the receipt of information. We showed in previous studies that probands may recall the DNA-test result differently compared to what had actually been communicated [50–52, 54]. Subsequently, these probands did not interpret the risk-information result identical to how they recalled it. Hence, the receival of information—either by probands or relatives- consists of three different processes: actual communicated information, recollections and interpretations.
Second, noise may occur due to ineffective disclosure of genetic-information. In this family study, we focus on the proband, who is not only receiver, but also sender of information. It is unclear how the proband makes this role transformation, and whether she communicates what she recalls or whether she mainly communicates her own interpretation and makes a selection of the information when disclosing to relatives. We expect that the probands’ main message is their subjective interpretation because the interpretation has been reported as the most important aspect of their perception, and strongly influences well-being and decision-making [50, 52, 54].
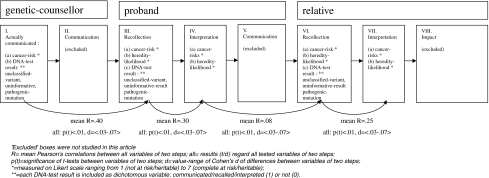
Figure 1 depicts our hypothesized family communication timeline of genetic counselling. (I) A DNA-test result and cancer-risks are obtained; (II) the genetic-counsellor communicates this to a proband. (III) The proband recalls and (IV) interprets this information. (V) The proband communicates her interpretation of the DNA-test result to the relative, which is (VI) recalled and (VII) interpreted by the relative, and (VIII) may have consequences for the relatives’ lives. Because of logistic reasons, II, V and VIII were excluded from this study.
Fig. 1.
Family communication timeline of genetic counselling, showing variables included in this article, resulting correlations an differences
Hypotheses and research questions
The difficulty of communicating information accurately can be illustrated by children’s whisper games, in which one child whispers a word to another child who subsequently whispers the word to another child. In most cases, the last child in the line of whisperers understands another word than the initial word.
We hypothesized that the family communication of a DNA-test result functions like a whisper game, in which the originally communicated information fades out more at every step in the communication timeline. More specifically, we asked:
Is there a significant difference between each step in the family communication timeline of genetic-counselling? The steps in the family communication timeline of genetic-counselling consist of the genetic-information actually communicated by the genetic-counsellor (i.e. DNA-test result category and cancer-risks), and the recollections and interpretations that probands and relatives have regarding this genetic-information (cf. Fig. 1). We expected to find significant differences between all variables of respectively steps I–III, III–IV, IV–VI, and VI–VII.
Does the initially communicated genetic-information fade out more and more at every next step in the communication model? More specifically: does the information transmitted at the first step correlate less and less with each step further away from the first step? We expected that the correlations would decrease between the following steps, i.e.: I–III > I–IV > I–VI > I–VII; III–IV > III–VI > III–VII; IV–VI > IV–VII; small correlations between VI–VII.
Are there differences in the information transfer (i.e. correlations and decrease in correlations) between unclassified-variants, pathogenic-mutations and unformative-results?
Do the following covariates influence the information transfer: sociodemographics, pedigree, familial relationship, cancer-history of proband and relative? We expected that the whispergame-effect would be stronger than the communicated DNA-test result and covariates.
Method
Procedure
Eligible participants in current study were probands from families with intermediate or high cancer-risks who had received a BRCA1/2 DNA-test result in the period 1998–2008 at the Leiden University Medical Center or the VU Medical Center Amsterdam [50–52]. Because the primary focus of our study concerns unclassified-variants, we first approached probands with an unclassified-variant, communicated as ‘a mutation/genetic-change for which the clinical meaning is not known (yet)’. In addition, we approached women with a pathogenic-mutation or uninformative-result, with matching year of result-disclosure.
We asked all 89 probands in this study for their approval to contact their 1st and 2nd degree relatives in the affected branch of the family. Subsequently, in line with the proband’s preference, we either sent our invitation letter to relatives directly, or to the proband who distributed the letters. We administered the relatives’ questionnaire both in a paper-and-pencil-version as in an Internet version. The study was approved by the medical ethical committees of the participating medical centers.
Instruments
Development and description of the questions about the probands’ and relatives’ recollections and interpretations of both cancer-risks and heredity-likelihood have been described elsewhere [50, 51] (see Fig. 1; Table 1).
Table 1.
Overview of instruments and items
| Actual communicated information by genetic-counsellor | Scaling | References | Items |
|---|---|---|---|
| Cancer-risks | Cancer-risks in %, rescaled to a 1–7 scale to match counsellees’ recollections and interpretations (derived from medical file and summary letter sent to proband) | ||
| DNA-test result | Scored as 3 dummy-items: communicated (1) or not (0) | Pathogenic-mutation, unclassified-variant, uninformative | |
| Probands’ perception | |||
| Recollection of DNA-test result | 1 item with 3 options | Options: (a) ‘no genetic change detected’, (b) ‘a genetic change was detected meaning that cancer is heritable in my family’, (c) ‘a genetic change was detected for which the meaning for breast/ovarian cancer is unknown at this moment, and therefore tells nothing about the heredity of cancer in my family’ | |
| Recollections of own cancer-risks and heredity-likelihood | 2 items (1–7 scale: not-complete at risk/heritable) | [50–54] | (1) What is your risk to develop cancer (again), according to your genetic-counsellor; (2) according to your genetic-counsellor, what does your pedigree/DNA-result mean for the likelihood that cancer is heritable in your family (pathogenic-mutation: result-based; other DNA-results: pedigree-based) |
| Interpretations of own cancer-risks and heredity-likelihood | 2 items (1–7 scale: not-complete at risk/heritable) | [50–54] |
What are your own thoughts and feelings about: (1) your risk to develop cancer (again), (2) the likelihood that cancer is heritable in your family |
| Interpretations of healthy relatives’ cancer-risks | 1items (1–7 scale: not-complete at risk) | [50–54] | What are your own thoughts and feelings about the risk for a healthy female relative in your family to develop cancer? |
| Relatives’ perception | Relative’s questionnaire: identical to proband’s perception, except ‘healthy relatives’ risks’ | ‘Genetic-counsellor’ was replaced for ‘your relative’ (i.e. proband) | |
| Covariates |
(1) 3 items derived from medical files (%); (2) 6 binary items in questionnaire (yes/no); (3) 8 items (several scales) |
(1) Percentage of affected 1st, 2nd and 3rd degree relatives; (2) gender: woman, children, married, religiously active, employed, high school and higher, or lower educated; (3) age, breast or ovarian or other cancer, metastases, year of diagnoses, mastectomy, adnexextirpation, radio/chemotherapy in the past or now |
Statistical analysis
Research question 1 was answered by performing t-tests to calculate differences: (a) between all variables of steps I and III, (b) between all variables of steps III and IV, (c) between all variables of steps IV and VI, (d) and between all variables of steps VI and VII. Figure 1 shows which variables are included in each step. To facilitate presentation of the large number of t-tests, we only present an overview of the results; details can be requested from the authors.
Research question 2 was analyzed in two phases. In phase 1, all applicable correlations between all variables of all steps were calculated (Fig. 1 shows all variables). In phase 2, mean correlations were calculated between all variables of the steps required for answering research question 2: I–III, I–IV, I–VI, I–VII; III–IV, III–VI, III–VII; IV–VI, IV–VII; VI–VII. To facilitate data presentation, we only present phase 2; data from phase 1 can be requested from the authors.
Research question 3 was answered by calculating mean correlations regarding research question 2 separately for each of the three DNA-test results. Research question 4 was explored by calculating partial correlations for research question 2, corrected for covariates.
Missing values (<2%) were imputed by multiple imputing within each step. To correct for three DNA-test-result categories, p-values smaller than .01 were regarded as significant. Effect sizes were calculated with Cohen’s d and correlations.
Results
Sample
Table 2 shows sample information. We approached 89 probands, but were unable to contact 44 of them (mainly due to deceased, too ill to participate and moved to another address). Twenty-five (56%) out of the remaining 45 probands participated, and 20 (44%) probands did not want that we asked their relatives; the main reported reasons for decline were: ‘I do not know whether my relatives would accept me providing you with their private addresses’; ‘I do not have contact with relatives’; ‘I do not want to burden them’; ‘I have not communicated the result’ and ‘I want to keep the genetic-counseling process closed and completed’. We approached 157 of their relatives, of whom 60 (38%) did not react, mainly due to organizational issues such as inaccurate address. Seventy out of the remaining 97 (72%) agreed up participation. Twenty-seven relatives (28%) declined; the most frequently reported reason was wanting to keep the genetic counseling process psychologically closed and being afraid that participation could remind them of painful memories. Statistical analysis of participation/decline rates did not reveal other significant patterns. In sum: the large non-response in probands and relatives was due to the retrospective design which caused high rates of decease and inaccurate addresses of eligible individuals; analyses of decliners showed that participation in this study was regarded as a sensitive theme, involving ethical issues and wanting to keep counseling psychologically closed.
Table 2.
Information about procedure and sample
| Name | M(sd) | N(%) |
|---|---|---|
| Probands | ||
| Total number of contacted probands | 45(100%) | |
| Probands declining | 20(44%) | |
| Probands agreeing to approach their relatives | 25(56%) | |
| Relatives | ||
| Total number of contacted relatives | 97(100%) | |
| Relatives declining | 27(28%) | |
| Participating relatives | 70(72%) | |
| Relationship of relative to proband | ||
| 1st degree | 45(64%) | |
| 2nd degree | 12(17%) | |
| 3rd degree | 12(17%) | |
| 4th degree | 1(2%) | |
| Sociodemographics of relatives | ||
| Women | 54(77%) | |
| High-school or higher | 26(37%) | |
| Employed | 50(71%) | |
| Cancer-history of relatives | ||
| Breast cancer | 15(21%) | |
| Ovarian cancer | 0 | |
| Another kind of cancer | 5(7%) | |
| Year of cancer diagnosis | 2002(4.0) | |
| Mastectomy/affected women | 6/15(40%) | |
| Mastectomy/unaffected women | 0/55 | |
| Bilateral salpingo-oophorectomy/unaffected women | 1/70(1%) | |
| Pedigree | ||
| % affected 1st degree relatives/all relatives | 37%(10%) | |
| % affected 2nd degree relatives/all relatives | 7%(7%) | |
| % affected 3rd degree relatives/all relatives | 7%(2%) | |
Included relatives were mainly first-degree (64%), especially daughters (32%) or sisters (29%). Fifty-four (77%) relatives were women, 15(21%) had had breast cancer, none ovarian cancer and 5(7%) another kind of cancer. Six of the affected and none of the unaffected women had undergone prophylactic mastectomy, and one affected woman prophylactic bilateral salpingo-oophorectomy (BSO). Perception did not differ between affected and unaffected participants.
Thirteen (52%) probands had actually received an unclassified-variant (UV), 7(28%) a pathogenic-mutation and 5(20%) an uninformative DNA-test result. Of the 70 relatives, 44(63%) belonged to a family in which an unclassified-variant was communicated, 14(20%) in a mutation-family and 12(17%) in an uninformative-family (Table 3).
Table 3.
Overview of variables
| Step | Description | Actually communicated DNA-test result (means, sd) | |||
|---|---|---|---|---|---|
| Overall | Unclassified-variant | Pathogenic-mutation | Uninformative-result | ||
| I | Actually communicated | ||||
| Communicated to proband: unclassified-variant, pathogenic-mutation, uninformative (n,%) | 13 (1.0) | 7 (1.0) | 5 (1.0) | ||
| Cancer-risks (% rescaled to 1–7 scale) | 4.9 (1.2) | 4.0 (1.0) | 6.0 (0.0) | 3.0 (0.0) | |
| III | Probands’ recollections | ||||
| Recollection of unclassified-variant, pathogenic-mutation, uninformative (n,%) | 11 (.45) | 11 (.45) | 2 (.1) | ||
| Recalled own cancer-risks | 4.7 (1.4) | 4.6 (1.5) | 5.2 (.4) | 3.5 (.6) | |
| Recalled heredity-likelihood | 4.6 (1.9) | 4.5 (.7) | 6.2 (1.2) | 2.3 (.8) | |
| IV | Probands’ interpretations | ||||
| Interpreted own cancer-risks | 6.0 (1.7) | 6.5 (1.2) | 4.1 (1.7) | 4.1 (.9) | |
| Interpreted heredity-likelihood | 6.4 (1.3) | 5.5 (.7) | 7.0 (.0) | 4.7 (2.3) | |
| Interpreted relatives’ cancer-risks | 5.5 (1.2) | 5.3 (1.4) | 6.7 (.8) | 5.3 (.8) | |
| VI | Relatives’ recollections | ||||
| Recollection of: unclassified-variant, pathogenic-mutation, uninformative (n,%) | 19 (.3) | 35 (.5) | 14 (.2) | ||
| Recalled own cancer-risks | 4.9 (1.0) | 4.9 (.9) | 5.7 (.7) | 3.9 (1.1) | |
| Recalled heredity-likelihood | 3.4 (1.4) | 3.9 (1.2) | 5.0 (.0) | 2.4 (1.2) | |
| VII | Relatives’ interpretations | ||||
| Interpreted own cancer-risks | 3.8 (1.4) | 4.3 (1.0) | 5.0 (.0) | 2.9 (1.3) | |
| Interpreted heredity-likelihood | 3.8 (1.3) | 4.0 (1.4) | 3.0 (1.2) | 4.1 (.8) | |
Question 1: differences between steps
All variables differed significantly between steps I–III, III–IV, IV–VI, and VI–VII. Al p-values were smaller than .01, and Cohen’s d’s varied between 0.3 and 0.7, which is regarded as medium effects (see Fig. 1).
Question 2: fading-out
Table 4 shows mean correlations between the steps. First, when we examined the four communicated aspects as depicted in the left columns of the geneticist, we found that the correlations decreased at every step downwards: correlations I–III > I–IV > I–VI > I–VII. Thus, the actually communicated information by the genetic-counsellor faded out more and more in respectively the proband’s recollections and interpretations and the relatives’ recollections and interpretations. Second, we found that the correlations of the proband’s recollections decreased at every step downwards in Table 4: correlations III–VI > III–VI > III–VII. Thus, the proband’s recollections faded out more and more in respectively the proband’s interpretations and the relatives’ recollections and interpretations. Third, the correlations of the probands’ interpretations with other variables decreased in each step: IV–VI > IV–VII. Thus, the proband’s interpretations faded out more and more in the relatives’ recollections and interpretations. Fourth, the relatives’ recollections VI correlated only for .25 with interpretations. Thus, the relatives’ recollections faded out in the relatives’ interpretations.
Table 4.
Mean correlations between steps: overall and specified for different DNA-test results
| DNA-test result | From this step (e.g. I → III) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. Geneticist | III. Proband: recollections | IV. Proband: interpretations | VI. Relative: recollections | ||||||||||
| Overall | uv | uninf | Path | Overall | uv | uninf | Overall | uv | uninf | Overall | uv | uninf | |
| To this step (e.g. I → III) | |||||||||||||
| III. Proband: recollections | .40 | .16 | .40 | .58 | |||||||||
| IV. Proband: interpretations | .33 | .22 | .33 | .48 | .30 | .34 | .64 | ||||||
| VI. Relative: recollections | .29 | .44 | .49 | .29 | .07 | .16 | .09 | .08 | 0 | .06 | |||
| VII. Relative: interpretations | .15 | .20 | .26 | .05 | .03 | .09 | .06 | 0 | 0 | 0 | .25 | .13 | .07 |
All correlations: p < .01; uv unclassified-variant, uninf uninformative-result, path pathogenic mutation; several cells contained too little pathogenic-mutation carriers to calculate mean correlations, therefore only correlations with step I are presented
Bold values show overall values
The mean correlations between the main steps as depicted in Fig. 1 are: .40 between the information actually communicated by the genetic-counsellor and the proband’s recollections (I–III); .30 between the proband’s recollections and interpretations (III–IV); .08 between the proband’s interpretations and the relatives’ recollections (IV–VI); and .25 between the relatives’ recollections and interpretations.
Question 3: DNA-test results
We calculated all correlations of research questions 2 and 3 separately for three different DNA-test results. The number of participants for pathogenic-mutations was too small to calculate correlations in steps III, IV and VI. Similar to overall results, the genetic-information from the first communication steps faded out in each DNA-test result group. Exceptions were the high correlations of the information actually communicated by the genetic-counsellor and the relatives’ recollections of unclassified-variants and uninformative-results (R’s = .44, .49). Unclassified-variants were recalled worse by probands compared to other results (R = .16), and the proband’s interpretations of an unclassified-variant did not correlate with the relatives’ recollections and interpretations.
Covariates
No significant effects of covariates were found, except for the proband’s mothers who interpreted higher cancer-risks, and the probands’ daughters who less often recalled having received pathogenic-mutations (R’s = .25, −.29, −24, p’s < .01).
Discussion
This study is the first to examine the relatives’ perception of genetic-counselling as part of the family communication timeline of genetic-counselling. We compared the communication of genetic-information between probands and relatives with a children’s whisper game. Our expectation was confirmed that errors would accumulate in the communication of genetic-information from step to step: from information actually communicated by the genetic-counsellor to the proband’s recollection, and from that to the proband’s interpretation, and from that to the relatives’ recollection, and from that to the relatives’ interpretation.
First, all steps differed significantly from each other, implying that noise occurred in all transfers of information between genetic-counsellor, proband and relatives. This also means that the recollections and interpretations of both probands and relatives were inaccurate, when compared with the information that was actually communicated to them.
Second, the information originally communicated by the genetic-counsellor faded out at every step in the communication timeline, like a whisper game. The final step, the relatives’ interpretation, showed a correlation of no more than .15 with the originally communicated information.
Noise
The least noise (R = .40) had arisen in the communication between genetic-counsellor and proband, and the largest noise (R = .08) between the proband’s and relatives’ perception. The correlations between recollections and interpretations were relatively low, both for probands and relatives (R’s = .30, .25), which was comparable to previous studies [50, 51].
Why did noise arise? First, probands and relatives may have difficulties understanding the meaning of DNA-test results and pedigree (cf. [51, 52]). Their inaccurate perceptions could also be caused by the time passed since communication of the DNA-test result, low education, innumeracy [1, 41, 56], and black-or-white thinking, i.e.,’either I get cancer or I do not get cancer’ [14, 19].
Second, probands and relatives may have selectively listened to the communicated information, and may have used heuristics, such as representativeness and availability biases and illusion of control [20]. They may have been stuck in specific family communication patterns [21], and have developed their own opinion about cancer-risks and heredity-likelihood on the basis of their experiences with cancer in the family [10, 38, 39, 55].
Third, probands may only have disclosed information which they perceived as most likely to be true and as most relevant for their relatives. Particularly in situations of personal threat, an individual may trust their own interpretations most. [17, 19, 27, 42].
Fourth, the largest part of the noise remained unexplained by the variables in this study. This suggests involvement of other variables.
Actually communicated information
The information communicated by the genetic-counsellor did not completely fade-out, because it correlated with the relatives’ recollections and interpretations (I–VI/VII). However, these remaining correlations were small (R’s = .29, .15). This suggests that the largest part of the relatives’ perception was not directly predicted by the actually communicated information, which confirms the whisper-game phenomenon.
Analyses yielded two results: (1) the actually communicated information predicted the relatives’ perception to some extent; (2) the relatives’ perception differed significantly from the actually communicated information. This is comparable with the results of a children’s whisper-game: (1) the first and the last communicated words may be somewhat related; (2) there may be a difference between the first and last words. Thus, the relatives’ perception was inaccurate/different compared to what was actually communicated by genetic-counsellors, but was also somewhat related. Finding significant correlations between the first and last steps suggest that the first step (slightly) predicts the last step; this suggests that the actually communicated information consistently predicted the counsellees’ inaccurate perception.
We hypothesize that the influence from the actually communicated information on the relatives’ perception is completely explained/mediated by the way how probands communicate DNA-test results to relatives [53].
DNA-test results
We found large correlations between the genetic-counsellor communication and the relatives’ recollection in families with unclassified-variants and uninformatives. The genetic-counsellor’s information predicted the relatives’ recollections even better than the proband’s recollections. Probands with these DNA-test results largely overestimated the cancer-risks and heredity-likelihood in their recollections and interpretations (cf. [51, 52, 54]), but relatives reduced the extent of this overestimation, so that the relatives’ perception was more in line with what the genetic-counsellor had actually communicated.
Possibly, relatives understood the actual meaning of the DNA-test result better. Or they deduced from nonverbal communication that their proband was exaggerating. Or the answers of the relatives showed a tendency towards the mean. Or the relatives had read the summary letter that probands had received from their genetic-counsellor; we have no information whether relatives have read this letter, but only less than 20% of the letters included explicit risk-information for relatives.
Compared to other DNA-test results, unclassified-variants were recalled and interpreted the most inaccurate, and the probands’ perception also correlated the worst with the relatives’ perception.
Implications
Large noise occured in the family communication timeline of genetic counselling. Therefore, genetic-counsellors should not only be aware of the proband in their consultation room, but also of the absent relatives to whom the proband will disclose the DNA-test result.
Genetic-counsellors should explicitly help probands in disclosing DNA-test results to their relatives [6, 9], especially regarding unclassified-variants and possible medical consequences for relatives [24]. Probands often perceive the disclosure process as difficult and stressful [6, 7, 11], especially when children are involved [45–47] or when DNA-test results are negative [43]. This could be achieved by improving the summary letters for probands, especially by including more explicit information for relatives (cf. [26]).
Direct communication between counsellor and relatives may contribute in improving family communication (cf. [31]). For instance, genetic-counsellors might send letters to relatives, summarizing the DNA-test result and providing the possibility for private consultation by phone or face-to-face. This raises ethical questions. Are genetic-counsellors obliged to inform high-risk relatives? Are they allowed to inform a non-patient population who has not requested for genetic-information? Are they allowed to violate the proband’s privacy? Is communication beneficial, when relatives do not receive risk-management options, but may feel ‘alarmed’? Guidelines should be developed for genetic-counsellors if, when and how they should communicate DNA-test results to relatives [13].
Methodological issues
This study is limited by its small sample size and retrospective design. Therefore, causal relationships remain theoretically assumed. There may have been sampling bias, because probands decided which relatives we could ask to participate, and the relatives’ participation percentage was low. The communication timeline assumes a linear feed-forward process, but feedback loops may have been present. All variables were assumed to be linear, to enable calculating mean correlations and t-tests. Non-presented analyses showed identical results with Spearman-correlations, Fisher-exact-tests and corrections for family-dynamics, second/changed DNA-test result, DNA-test-request by relatives, mastectomy and adnexextirpation. Mediation analyses including communication processes are described elsewhere [53]. Future studies should be prospective and include more variables.
Despite these limitations, this study ‘taps from the richness of family responses to create a more complete picture of the effects of genetic testing’ [25]. It underlines studies on risk-perception in probands [50–54], and suggests a broader focus on the family domain, which is both ‘critical and relatively neglected’ in the science and practice of genetic-counselling [35].
Acknowledgments
We acknowledge the Dutch Cancer Society for their financial support of this study (grant no. UL2005-3214).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Brewer NT, Tzeng JP, Lillie SE, Edwards AS, Peppercorn JM, Rimer BK. Health literacy and cancer risk perception: implications for genomic risk communication. Med Decis Making. 2009;29:157–166. doi: 10.1177/0272989X08327111. [DOI] [PubMed] [Google Scholar]
- 2.Broadstock M, Michie S, Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8(10):731–738. doi: 10.1038/sj.ejhg.5200532. [DOI] [PubMed] [Google Scholar]
- 3.Brown TC, Garber J, Muto M, Schneider KA. Case report—loyalty, legacy, and ledger: contextual therapy in a patient with a family history of ovarian cancer. J Genet Couns. 1999;8(6):359–372. doi: 10.1023/A:1022971309842. [DOI] [PubMed] [Google Scholar]
- 4.Butow PN, Lobb EA, Meiser B, Barratt A, Tucker KM. Psychological outcomes and risk perception after genetic testing and counselling in breast cancer: a systematic review. Med J Aust. 2003;178(2):77–81. doi: 10.5694/j.1326-5377.2003.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 5.Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116A(1):11–19. doi: 10.1002/ajmg.a.10868. [DOI] [PubMed] [Google Scholar]
- 6.Costalas JW, Itzen M, Malick J, Babb JS, Bove B, Godwin AK, Daly MB. Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program’s experience. A J Med Genet C Semin Med Genet. 2003;119C(1):11–18. doi: 10.1002/ajmg.c.10003. [DOI] [PubMed] [Google Scholar]
- 7.Daly MB, Barsevick A, Miller SM, Buckman R, Costalas J, Montgomery S, Bingler R. Communicating genetic test results to the family: a six-step, skills-building strategy. Fam Community Health. 2001;24(3):13–26. doi: 10.1097/00003727-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Douglas HA, Hamilton RJ, Grubs RE. The effect of BRCA gene testing on family relationships: a thematic analysis of qualitative interviews. J Genet Couns. 2009;18(5):418–435. doi: 10.1007/s10897-009-9232-1. [DOI] [PubMed] [Google Scholar]
- 9.Sermijn E, Goelen G, Teugels E, Kaufman L, Bonduelle M, Neyns B, Poppe B, De Paepe A, de Greve J. The impact of proband mediated information dissemination in families with a BRCA1/2 gene mutation. J Med Genet. 2004;41:23–26. doi: 10.1136/jmg.2003.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery J, Kumar S, Smith H. Patient understanding of genetic principles and their expectations of genetic services within the NHS: a qualitative study. Community Genet. 1998;1:78–83. doi: 10.1159/000016141. [DOI] [PubMed] [Google Scholar]
- 11.Forrest K, Simpson SA, Wilson BJ, van Teijlingen ER, Mckee L, Haites N, Matthews E. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64(4):317–326. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 12.Gaff CL, Clarke AJ, Atkinson P. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2008;16(3):402. doi: 10.1038/sj.ejhg.5201971. [DOI] [PubMed] [Google Scholar]
- 13.Godard B, Hurlimann T, Letendre M, Egalite N, Inherit BRCA. Guidelines for disclosing genetic information to family members: from development to use. Fam Cancer. 2006;5(1):103–116. doi: 10.1007/s10689-005-2581-5. [DOI] [PubMed] [Google Scholar]
- 14.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health. 2003;18(2):141–184. doi: 10.1080/088704403100081321. [DOI] [Google Scholar]
- 15.Hallowell N. Doing the right thing: genetic risk and responsibility. Sociol Health Illn. 1999;21(5):597–621. doi: 10.1111/1467-9566.00175. [DOI] [Google Scholar]
- 16.Hallowell N, Foster C, Ardern-Jones A, Eeles R, Murday V, Watson M. Genetic testing for women previously diagnosed with breast/ovarian cancer: examining the impact of BRCA1 and BRCA2 mutation searching. Genet Test. 2002;6(2):79–87. doi: 10.1089/10906570260199320. [DOI] [PubMed] [Google Scholar]
- 17.Hopwood P. Genetic risk counselling for breast cancer families. Eur J Cancer. 1998;34(10):1477–1479. doi: 10.1016/S0959-8049(98)00250-0. [DOI] [PubMed] [Google Scholar]
- 18.Hughes C, Lerman C, Schwartz M, Peshkin BN, Wenzel L, Narod S, Corio C, Tercyak KP, Hanna D, Isaacs C, Main D. All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107(2):143–150. doi: 10.1002/ajmg.10110. [DOI] [PubMed] [Google Scholar]
- 19.Kahneman D. A perspective on judgment and choice: mapping bounded rationality. Am Psychol. 2003;58(9):697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- 20.Kenen R, rdern-Jones A, Eeles R. Family stories and the use of heuristics: women from suspected hereditary breast and ovarian cancer (HBOC) families. Sociol Health Illn. 2003;25(7):838–865. doi: 10.1046/j.1467-9566.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 21.Kenen R, rdern-Jones A, Eeles R. We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC) Psycho-Oncology. 2004;13(5):335–345. doi: 10.1002/pon.745. [DOI] [PubMed] [Google Scholar]
- 22.Koehly LM, Peters JA, Kuhn N, Hoskins L, Letocha A, Kenen R, Loud J, Greene MH. Sisters in hereditary breast and ovarian cancer families: communal coping, social integration, and psychological well-being. Psycho-Oncology. 2008;17(8):812–821. doi: 10.1002/pon.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehly LM, Peterson SK, Watts BG, Kempf KKG, Vernon SW, Gritz ER. A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev. 2003;12(4):304–313. [PubMed] [Google Scholar]
- 24.Landsbergen K, Verhaak C, Kraaimaat F, Hoogerbrugge N. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4(2):115–119. doi: 10.1007/s10689-004-7991-2. [DOI] [PubMed] [Google Scholar]
- 25.Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: psychological aspects and implications. J Consult Clin Psychol. 2002;70(3):784–797. doi: 10.1037/0022-006X.70.3.784. [DOI] [PubMed] [Google Scholar]
- 26.Lewis C, Mehta P, Kent A, Skirton H, Coviello D. An assessment of written patient information provided at the genetic clinic and relating to genetic testing in seven European countries. Eur J Hum Genet. 2007;15:1012–1022. doi: 10.1038/sj.ejhg.5201874. [DOI] [PubMed] [Google Scholar]
- 27.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127(2):267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 28.McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, Morrison PJ. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psycho-Oncology. 2009;18(11):1208–1215. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- 29.Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psycho-Oncology. 2005;14(12):1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 30.Meiser B, Halliday JL. What is the impact of genetic counselling in women at increased risk of developing hereditary breast cancer? A meta-analytic review. Soc Sci Med. 2002;54(10):1463–1470. doi: 10.1016/S0277-9536(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 31.Mellon S, Berry-Bobovski L, Gold R, Levin N, Tainsky MA. Communication and decision-making about seeking inherited cancer risk information: findings from female survivor-relative focus groups. Psycho-Oncology. 2006;15(3):193–208. doi: 10.1002/pon.935. [DOI] [PubMed] [Google Scholar]
- 32.Mellon S, Gold R, Janisse J, Cichon M, Tainsky MA, Simon MS, Korczak J. Risk perception and cancer worries in families at increased risk of familial breast/ovarian cancer. Psycho-Oncology. 2008;17:756–766. doi: 10.1002/pon.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellon S, Janisse J, Gold R, Cichon M, Berry-Bobovski L, Tainsky MA, Simon MS. Predictors of decision making in families at risk for inherited breast/ovarian cancer. Health Psychol. 2009;28(1):38–47. doi: 10.1037/a0012714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellon S, Kershaw TS, Northouse LL, Freeman-Gibb L. A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psycho-Oncology. 2007;16:214–223. doi: 10.1002/pon.1074. [DOI] [PubMed] [Google Scholar]
- 35.Miller SM, McDaniel SH, Rolland JS, Feetham SL. Individuals, families, and the new era of genetics. Biopsychosocial perspectives. New York/London: W.W. Norton & Company; 2006. [Google Scholar]
- 36.Northouse LL, Mood D, Kershaw T, Schafenacker A, Mellon S, Walker S, Galvin E, Decker V. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002;20(19):4040–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 37.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 38.Peters JA, Biesecker BB. Genetic counseling and hereditary cancer. Cancer. 1997;80(3):576–586. doi: 10.1002/(SICI)1097-0142(19970801)80:3+<576::AID-CNCR7>3.0.CO;2-7. [DOI] [Google Scholar]
- 39.Ponder M, Green JM. BRCA1 testing: some issues in moving from research to service. Psycho-Oncology. 1996;5(3):223–232. doi: 10.1002/(SICI)1099-1611(199609)5:3<223::AID-PON237>3.0.CO;2-X. [DOI] [Google Scholar]
- 40.Rolland JS. Cancer and the family: an integrative model. Cancer. 2005;104(11):2584–2595. doi: 10.1002/cncr.21489. [DOI] [PubMed] [Google Scholar]
- 41.Sheridan SL, Pignone M. Numeracy and the medical student’s ability to interpret data. Eff Clin Pract. 2002;5:35–40. [PubMed] [Google Scholar]
- 42.Slovic P, Finucane ML, Peters E, MacGregor DG. Risk as analysis and risk as feelings: some thoughts about affect, reason, risk, and rationality. Risk Anal. 2004;24(2):311–322. doi: 10.1111/j.0272-4332.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith KR, West JA, Croyle RT, Botkin JR. Familial context of genetic testing for cancer susceptibility: moderating effect of siblings’ test results on psychological distress one to two weeks after BRCA1 mutation testing. Cancer Epidemiol Biomarkers Prev. 1999;8(4):385–392. [PubMed] [Google Scholar]
- 44.Tercyak KP, Hughes C, Main D, Snyder C, Lynch JF, Lynch HT, Lerman C. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42(3):213–224. doi: 10.1016/S0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 45.Tercyak KP, Peshkin BN, DeMarco TA, Brogan BM, Lerman C. Parent-child factors and their effect on communicating BRCA1/2 test results to children. Patient Educ Couns. 2002;47(2):145–153. doi: 10.1016/S0738-3991(01)00192-6. [DOI] [PubMed] [Google Scholar]
- 46.Tercyak KP, Peshkin BN, Streisand R, Lerman C. Psychological issues among children of hereditary breast cancer gene (BRCA1/2) testing participants. Psycho-Oncology. 2001;10(4):336–346. doi: 10.1002/pon.531. [DOI] [PubMed] [Google Scholar]
- 47.Tercyak KP, Streisand R, Peshkin BN, Lerman C. Psychosocial impact of predictive testing for illness on children and families: challenges for a new millennium. J Clin Psychol Med Settings. 2000;7(1):55–68. doi: 10.1023/A:1009597303743. [DOI] [Google Scholar]
- 48.van Asperen CJ, van Dijk S, Zoeteweij MW, Timmermans DRM, de Bock GH, Meijers-Heijboer EJ, Niermeijer MF, Breuning MH, Kievit J, Otten W. What do women really want to know? Motives for attending familial breast cancer clinics. J Med Genet. 2002;39(6):410–414. doi: 10.1136/jmg.39.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vink GR, van Asperen CJ, Devilee P, Breuning MH, Bakker E. Unclassified variants in disease-causing genes: nonuniformity of genetic testing and counselling, a proposal for guidelines. Eur J Hum Genet. 2004;13:525–527. doi: 10.1038/sj.ejhg.5201379. [DOI] [PubMed] [Google Scholar]
- 50.Vos J, Otten W, van Asperen C, Jansen A, Menko F, Tibben A. The counsellees’ view of an unclassified variant in BRCA1/2: recall, interpretation, and impact on life. Psycho-Oncology. 2008;17(8):822–830. doi: 10.1002/pon.1311. [DOI] [PubMed] [Google Scholar]
- 51.Vos J, Gomez-Garcia EB, Oosterwijk JC, Menko FH, Jansen AM, van Asperen CJ, Stiggelbout AM, Tibben A (2010a) Perceiving cancer-risks and heredity-likelihood in genetic-counseling: the analysis of the counselees’ recollections and interpretations of BRCA1/2-test results (submitted) [DOI] [PubMed]
- 52.Vos J, Gomez-Garcia EB, Oosterwijk JC, Menko FH, Stoel RD, van Asperen CJ, Jansen AM, Stiggelbout AM, Tibben A (2010b) Opening the psychological black box in genetic counseling. The psychological impact of DNA-testing is predicted by the counsellees’ perception, the medical impact by the pathogenic or uninformative BRCA1/2-result. Psycho-Oncology (in press) [DOI] [PubMed]
- 53.Vos J, Jansen AM, Menko FH, van Asperen CJ, Stiggelbout AM, Tibben A (2010c) Family communication matters: the impact of telling relatives about non-pathogenic DNA-test results depends on the proband’s non-verbal communication and the relatives’ subjective perception (submitted)
- 54.Vos J, Oosterwijk JC, Gomez-Garcia EB, Menko FH, van Asperen CJ, Stiggelbout AM, Tibben A (2010d) Explaining the short-term impact of DNA-testing: the counselees’ perception matters, the actual BRCA1/2-result does not (submitted) [DOI] [PubMed]
- 55.Walter FM, Emery J, Braithwaite D, Marteau TM. Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. Ann Fam Med. 2004;2(6):583–594. doi: 10.1370/afm.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woloshin S, Schwartz LM, Moncur M, Gabriel S, Tosteson ANA. Assessing values for health: numeracy matters. Med Decis Making. 2001;21:380–388. doi: 10.1177/02729890122062686. [DOI] [PubMed] [Google Scholar]