Abstract
Recent evidence demonstrating that exposure to rapamycin during viral infection increased the quantity and quality of antigen-specific T cells poses an intriguing paradox, since rapamycin is used in transplantation to dampen, rather than enhance, donor-reactive T cell responses. In this report, we compared the effects of rapamycin on the antigen-specific T cell response to a bacterial infection versus a transplant. Using a transgenic system in which the antigen and the responding T cell population were identical in both cases, we observed that treatment with rapamycin augmented the antigen-specific T cell response to a pathogen, while it failed to do so when the antigen was presented in the context of a transplant. These results suggest that the environment in which an antigen is presented alters the influence of rapamycin on antigen-specific T cell expansion, and highlights a fundamental difference between antigen presented by an infectious agent as compared to an allograft.
Keywords: CD8+ T cell, immunotherapy, transplantation, heterologous immunity
Introduction
Transplantation is a life-saving treatment option for many forms of end-stage organ disease. The advent of new immunosuppressive agents over the last thirty years has dramatically increased the graft survival of almost all types of organ and tissue transplantation. One such agent, rapamycin, was isolated in the early 1970’s from Streptomyces hygroscopicus and was found to potently inhibit cell proliferation and therefore possess immunosuppressive effects (1). Despite its current widespread use for the prevention of kidney allograft rejection (2), the precise effects of rapamycin on different cell types involved in rejection, including effector T cells, dendritic cells (DC) and regulatory T cells is an area of intense investigation (1). Rapamycin exerts its effect by targeting the mammalian target of rapamycin (mTOR) (3), a serine/threonine protein kinase which has a pervasive role in many aspects of both the innate and adaptive immune response (1). Several studies exist to suggest that blockade of mTOR by rapamycin retards dendritic cell maturation and inhibits antigen uptake and presentation by DC (4) and also attenuates T cell proliferation by inhibiting the G1→S transition. Furthermore, rapamycin has been shown by many groups to enhance the generation and function of regulatory T cells (5, 6), potentially further promoting its immunosuppressive effects during transplantation.
Recent studies in virally infected mice that had been treated with rapamycin revealed surprising and as-yet unappreciated effects on the expansion and retention of viral-specific CD8+ T cells. Specifically, Araki et al. measured the antigen-specific CD8+ T cell response following infection of mice infected with lymphocytic choriomeningitis virus (LCMV) in the presence or absence of rapamycin (7). In contrast to the expected result based on rapamycin’s known function as an immunosuppressant, this study revealed that treatment with rapamycin instead increased the quantity and quality of virus-specific memory T cells. Treatment of both mice and rhesus macaques with rapamycin resulted in an increased response to live virus or vaccination, respectively. Using RNAi knockdown of mTOR, the regulatory-associated protein of mTOR (RAPTOR), or the rapamycin-binding protein FKB12, these studies also demonstrated that inhibition of mTOR functioned in a T cell intrinsic manner to enhance the quantity and quality of antigen-specific T cells (7).
Because this drug is used in many clinical transplantation immunosuppressive regimens (2), we sought to address whether treatment with rapamycin resulted in an increase in the T cell response to additional types of pathogens, such as bacterial infection, and also to study the effect of rapamycin on the expansion and retention of donor-reactive CD8+ T cells following transplantation. In short, we sought to compare the effect of rapamycin on the CD8+ T cell response to a pathogen to its effect on the CD8+ T cell response to a transplant. In order to accomplish this, we made use of a transgenic system in which the same monoclonal TCR transgenic T cells responded to a bacterial pathogen infection or a skin graft. OT-I T cells recognized an epitope (SIINFEKL/Kb) that was expressed by both Listeria monocytogenes (LM-OVA)(8)and by donor skin under the control of the β-actin promoter (9). In comparing the CD8+ T cell responses by identical monoclonal cell populations to the same epitope in the setting of either pathogen infection or transplantation, we found that treatment with rapamycin resulted in very disparate effects on the antigen-specific cell populations. While rapamycin augmented the antigen-specific CD8+ T cell response to a bacterium, it failed to do so in response to a transplant.
Materials and Methods
Mice
Adult male 6-to 8-week old C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). TCR transgenic OT-I and OT-II mice were purchased from Taconic, Inc. and bred onto the Thy1.1+ background. Act-mOVA mice were produced by Dr. Marc Jenkins, Univ. of Minnesota (9). Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
T Cell Adoptive Transfers
OT-I and OT-II Thy1.1+ TCR transgenic T cells were harvested from spleen. The frequency of OT-I or OT-II T cells was determined prior to adoptive transfer by staining with anti-Vα2 (used by both TCRs) and anti-CD8 or anti-CD4, respectively (Pharmingen, San Diego, CA).
Listeria infection and rapamycin treatment
Forty-eight hours prior to Listeria infection, naïve B6 mice received an i.v. injection of 104 OT-I T cells. Similar results were observed if mice received both OT-I and OT-II T cells (data not shown). Mice were then infected with 104 colony-forming units (CFU) of Listeria monocytogenes expressing ovalbumin (8)gene i.p. on day 0. Where indicated, mice were treated with 1.5 ug/day rapamycin (Rapamune, Wyeth Pharmaceuticals) on days 0–10 post-infection or post-transplant as previously described (7).
Skin Grafting
Forty-eight hours prior to mOVA skin grafting, mice received an i.v. injection of 106 OT-I and OT-II T cells, to mimic the higher precursor frequencies observed in allospecific immune responses (10). Skin grafts (~1 cm2) were transplanted onto the dorsal thorax of recipient mice and secured with an adhesive bandage for 5 days. Rejection was defined as 90% loss of viable epidermal tissue.
Flow Cytometric Analyses for Frequency and Absolute Number
Splenocytes were stained with Thy1.1-PerCP, CD8-PacOrange, and CD4-PacBlue (BD Pharmingen) for flow cytometric analysis on a BD LSRII flow cytometer. Absolute numbers of antigen-specific T cells were determined by TruCount Bead Analysis (Pharmingen). Data were analyzed using FlowJo Software (Treestar, San Carlos, CA).
Intracellular Cytokine Staining
Cells were incubated for 4 hours with 10 nM OVA257–264 (SIINFEKL), and 10 μg/ml Brefeldin A (Pharmingen), and processed using an intracellular staining kit (Pharmingen).
Statistical Analyses
Groups were compared by Mann-Whitney non-parametric test (GraphPad Prism Software, La Jolla, CA).
Results
Rapamycin treatment resulted in increased antigen-specific CD8+ effectors following infection with OVA-expressingListeria monocytogenes
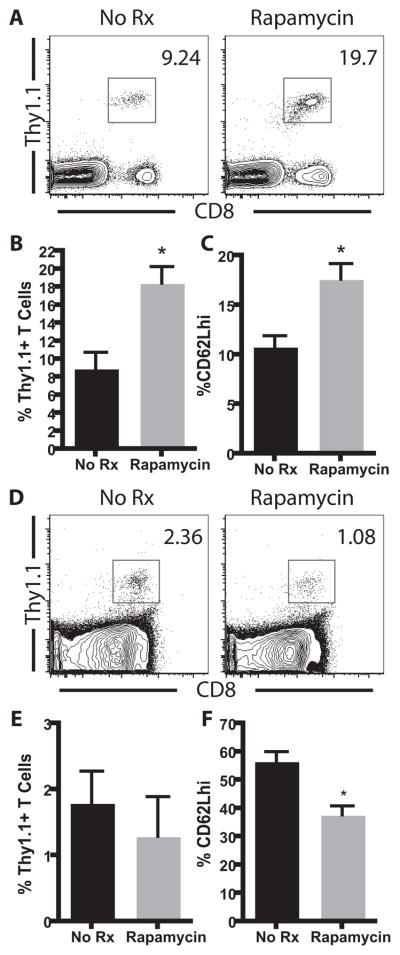
In order to assess the effects of treatment with rapamycin on the antigen-specific CD8+ T cell response following a bacterial infection, we adoptively transferred Thy1.1+ OT-I T cells specific for OVA257–264/Kb into naïve B6 recipients, which were then infected with an OVA-expressing Listeria monocytogenes (LM-OVA) in the presence or absence of rapamycin. In this model infectious bacteria are cleared from the spleen and liver by day 5 post-infection (data not shown). At the peak of the antigen-specific CD8+ T cell response (day 10 post-infection), frequency of OVA-specific OT-I T cells in the peripheral blood was quantified by flow cytometry (Figure 1A). We observed an increase in the frequency of antigen-specific T cells at day 10 post-infection in the rapamycin-treated animals as compared to untreated controls (18.4% ± 1.9% vs 8.9% ± 1.8%, respectively, p=0.001) (Figure 1B). Longitudinal analysis revealed that the enhancing effects of rapamycin were observed at all timepoints, including memory, indicating that these observations were not simply due to altered kinetics of expansion of the antigen-specific CD8+ T cell response (data not shown). Consistent with recently published results in a viral infection model(7), these data indicated that treatment with rapamycin enhanced the antigen-specific T cell response following bacterial infection.
Figure 1. Rapamycin enhanced the antigen-specific T cell response to OVA in the context of a bacterial infection but not a transplant.
A–C, 104 Thy1.1+ OT-I T cells were adoptively transferred into naïve B6 recipients, which were then infected with 104 CFU of OVA-expressing LM-OVA. Rapamycin was administered intraperitoneally in 500 μl of sterile PBS from days 0–10 post-infection. Analysis of splenocytes indicated that the day 10 frequency (A, B) or % CD62Lhi (C) of the donor-reactive CD8+ population was significantly increased in the rapamycin treated recipients(p<0.005). D–F, 106 Thy1.1+ CD8+ OT-I and CD4+ OT-II T cells were adoptively transferred into B6 recipients two days prior to receiving an OVA-expressing skin graft in the presence or absence of rapamycin. Analysis of splenocytes indicated that the day 10 frequency (D, E) or % CD62Lhi (F) of the donor-reactive CD8+ population was not increased in the rapamycin treated recipients. Results are cumulative analyses of three independent experiments with five mice per treatment group.
Rapamycin treatment did not result in increased donor-reactive CD8+ T cell responses following skin transplantation
In order to determine whether rapamycin resulted in an increased frequency of donor-reactive memory T cells following transplantation, we used the same transgenic mouse model to identify and track T cells responding to the graft in the presence or absence of rapamycin. Briefly, mice received 106 Thy1.1+ OVA-specific CD8+ OT-I T cells two days prior to receiving an OVA-expressing skin graft. Mice were then left untreated or were treated with rapamycin, and were sacrificed on day 10 post-transplant. Results indicated that the frequency of the donor-reactive CD8+ populations was not increased in the rapamycin treated recipients at the peak of the response(Figures 1D, 1E). This result was also true for the draining LN (data not shown). The minimal effect of rapamycin was not due to failure of Thy1.1+ T cells to be recruited into the response, since all Thy1.1+ cells underwent division following engraftment as measured by CFSE dilution (data not shown). Furthermore, the differential effect of rapamycin shown in Figure 1B vs Figure 1D was not simply due to differences in precursor frequency, as increasing the number of adoptively transferred T cells to 106 in recipients of LM-OVA still resulted in an increase in the frequency of antigen-specific T cells following rapamycin treatment as compared to untreated controls (data not shown). Therefore, these results suggest an intrinsic difference in the effect of rapamycin on the T cell response to a graft vs. a pathogen.
Rapamycin altered CD62L expression on CD8+ T cells responding to a pathogen but not a graft
In addition to enhancing the quantity of antigen-specific T cells following Listeria infection, we found that treatment with rapamycin also altered the quality of the antigen-specific CD8+ T cell response. CD62L has been used as a marker to differentiate between effector and memory T cells at memory time points (11). Central memory T cells (CD62Lhi) possess increased proliferative capacity and increased ability to mount a secondary response as compared to CD62Llo cells. Therefore, the presence of more CD62Lhi cells in the rapamycin-treated recipients may signify an increase in the quality of these memory T cells. Specifically, treatment with rapamycin during Listeria infection resulted in an increased frequency of CD62Lhi Thy1.1+ cells (17.6 ± 1.6% CD62Lhi vs. 10.8 ± 1.1% in untreated controls, p=0.0046, Figure 1C). These results indicated that in addition to augmenting the frequency of pathogen-specific T cells, rapamycin also impacted the degree of differentiation of these cells. Therefore, we next sought to address whether treatment with rapamycin resulted in increased CD62L expression in donor-reactive CD8+ T cells stimulated by a skin graft. In contrast to our observations in the bacterial infection model, we found that splenocytes analyzed on day 10 post-transplant in rapamycin-treated recipients did not demonstrate an increase in CD62L expression (Figure 1F).
Rapamycin differentially impacted the absolute number of IFN-γ-secreting cells generated in response to LM-OVA vs. an mOVA skin graft
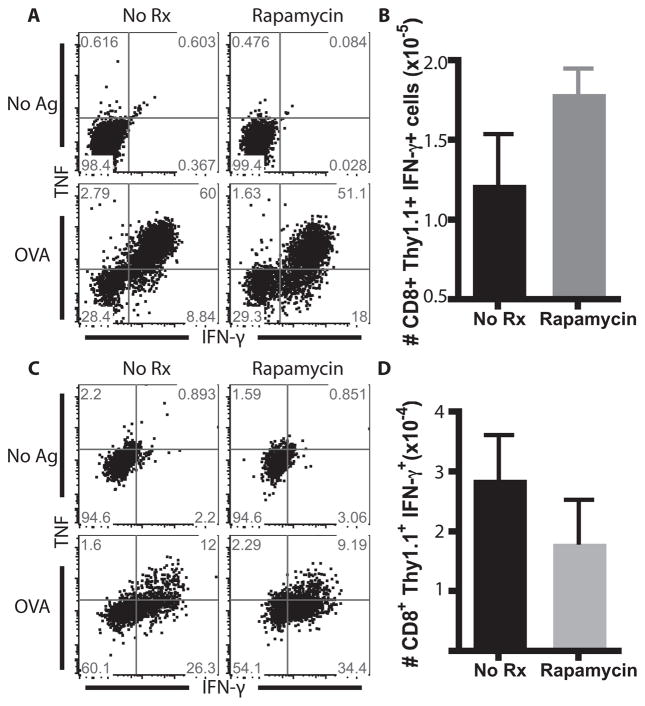
As shown in Figure 1, we observed increased quantity and quality of antigen-specific CD8+ T cell populations in response to a bacterial infection following treatment with rapamycin. To further assess the quality of antigen-specific T cell responses generated against a pathogen vs. a graft in the presence of rapamycin, we examined the ability of these antigen-specific T cells to produce cytokines following ex vivo restimulation (Figure 2). Following ex vivo restimulation with OVA peptide, CD8+ T cells derived from splenocytes of rapamycin-treated LM-OVA-infected recipients exhibited an increased absolute number of IFN-γ-secreting T cells as compared to untreated LM-OVA infected controls(Figure 2A, 2B). In sharp contrast, we observed a modest decrease in the absolute number of IFN-γ-producing cells isolated from rapamycin-treated mOVA skin graft recipients in response to antigen restimulation (Figure 2C,D). In summary, the immunostimulatory effect of rapamycin on antigen-specific T cell populations observed in the context of a pathogen was not observed in the context of a transplant.
Figure 2. Rapamycin altered IFN-γ production in antigen-specific T cell populations following pathogen infection.
A, Splenocytes from LM-OVA infected mice, which were either left untreated or treated with rapamycin were restimulated with OVA peptide and stained for the presence of IFN-γ or TNF. B, Treatment with rapamycin resulted in an increase in the number of total IFN-γ+ T cells. C, Splenocytes from mOVA grafted mice, which were untreated or treated with rapamycin, were restimulated with OVA peptide and stained for the presence of IFN-γ or TNF. D, Treatment with rapamycin did not result in an increase in the number of total IFN-γ+ T cells. Experiments were performed three times independently with 5 mice per group.
Simultaneous infection and transplantation did not result in increased donor-specific T cell immunity in rapamycin-treated recipients
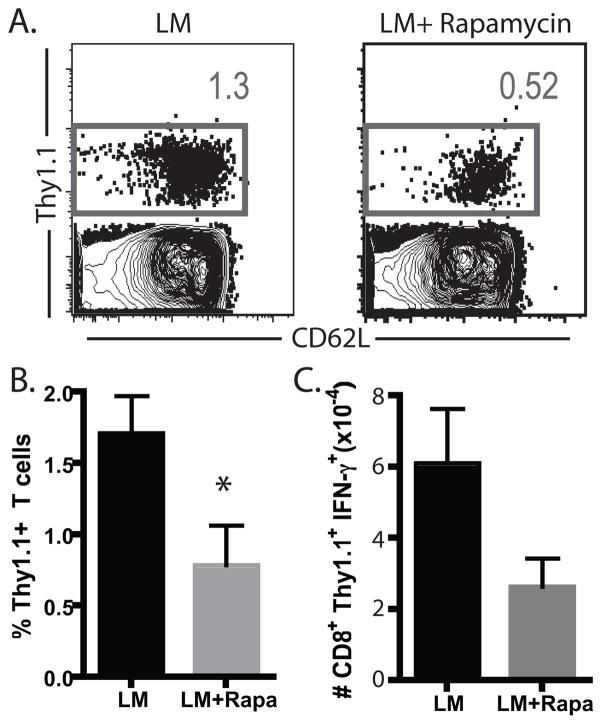
From the experiments presented above we concluded that rapamycin exhibited disparate effects on identical antigen-specific T cells that were responding in the context of a bacterial infection versus a transplant. Because mTOR has also been shown to be involved in signaling downstream of Toll-like receptors (TLRs) (12), we hypothesized that TLR signaling or other inflammatory signals associated with a bacterial pathogen might be required for the enhancing effects of rapamycin on antigen-specific T cell responses. As such, we examined the donor-reactive T cell response to a skin graft in the presence of a concomitant bacterial infection. Briefly, naïve B6 recipients were adoptively transferred with Thy1.1+ OT-I and OT-II T cells and received a mOVA skin graft. On the day of graft placement, mice also were infected with wild-type (non-OVA-expressing) LM. Results demonstrated that concomitant infection with LM in the presence of rapamycin failed to result in the augmentation of OVA-specific CD8+ T cell populations in response to the graft (Figure 3A, B). Furthermore, we observed no difference in the expression of CD62L as we had in OVA-specific T cell populations responding to LM-OVA (data not shown). Also in contrast to our observations of OT-I T cells stimulated by LM-OVA, we observed a modest decrease in the number of IFN-γ-producing donor-reactive T cells in rapamycin-treated mice receiving a concurrent LM infection and mOVA skin graft as compared to untreated controls (Figure 3C). These data therefore demonstrated that TLR-mediated stimulation or other pathogen-associated inflammation was not sufficient to explain the disparate effects of rapamycin on antigen-specific T cell responses following stimulation by a pathogen vs. a transplant.
Figure 3. Simultaneous infection and transplantation did not result in increased donor-specific immunity in rapamycin treated recipients.
106 Thy1.1+ OVA-specific CD8+ OT-I and CD4+OT-II T cells were adoptively transferred into B6 recipients. Recipients were infected with 104 CFU of wild-type Listeria in the presence or absence of rapamycin. Analysis of splenocytes indicated that the frequency of donor-reactive CD8+ population was not increased in the rapamycin treated recipients (A, B (p<0.01)). Absolute numbers of IFN-γ producing Thy1.1+ cells were calculated by TruCount analysis (C). Experiments were performed three times independently with five mice per group.
Discussion
Because rapamycin was widely appreciated to attenuate immune responses through mechanisms such as altered DC differentiation and increased Treg populations (13, 14), recent evidence demonstrating that exposure to rapamycin during the course of an immune response to viral infection increased the quantity and quality of antigen-specific T cells (7) poses an intriguing paradox for the field of immunology. In this report, we have attempted to reconcile these seemingly disparate findings by directly comparing the effects of rapamycin on an antigen-specific T cell response to a bacterial infection versus a skin graft. By employing a transgenic system in which both the antigen of interest and the responding monoclonal T cell population were identical in both models, we observed that treatment with rapamycin augmented the antigen-specific T cell response to bacterial infection, while failing to do so when the same antigen was presented in the context of a transplant. These results suggest a fundamental difference in the effects of rapamycin on the expansion of T cell populations in response to an infectious agent as compared to an allograft. They also serve to mitigate concern that treatment with rapamycin might paradoxically augment donor-reactive T cell responses.
In addition to its known role as a signaling component downstream of the T cell receptor/CD3 complex, mTOR has also been shown to participate in the signaling cascade downstream of many TLRs (12). Therefore, we speculated that the observed difference in the effects of rapamycin on pathogen-and donor-reactive T cell responses might be due to engagement of TLR/innate immune pathways during the bacterial infection, either on antigen-presenting cells (4, 13, 14) or on the T cells themselves (15). These pathways would presumably not be engaged following transplantation of a skin graft. However, results indicated that infection with non-OVA-expressing Listeria concurrently with transplantation of mOVA skin grafts did not result in augmentation of anti-OVA T cell responses. One potential caveat of this experiment might be the question of whether the Listeria was present in the same local environment as the graft-specific T cells during priming, in order to influence the effect of rapamycin on these cells. However, Chong and colleagues have demonstrated that Listeria infection concurrent with transplantation resulted in the prevention of tolerance induction (16), signifying that LM infection-derived inflammatory signals are likely to reach graft-specific T cells in this model, and that the presence of a concurrent bacterial infection and engagement of innate immune mechanisms does influence the quality of anti-donor T cell responses. Importantly, however, our results indicate that this effect was not impacted by the presence or absence of rapamycin. If activation of innate immune mechanisms is not responsible, what other factors could account for the observed discrepancy of the effect of rapamycin on pathogen-specific vs graft-specific T cell responses? One possibility is that duration of antigen presentation may modulate the impact of rapamycin on antigen-specific T cell responses. Further experiments are required to test this hypothesis.
In addition, these data may have relevance to the issue of heterologous alloreactivity. Our group and others have shown that prior or concurrent pathogen infection can augment the allospecific T cell response and alter the outcome of an allograft (16–18). This has been attributed in part to molecular mimicry (19), bystander activation (16)and other reasonably simple mechanisms. It would appear from our results that in the presence of rapamycin, presentation of the same antigen delivered either by a graft or a pathogen to the same T cell could evoke markedly different responses. As such, the outcome of stimulation with a cross-reactive antigen cannot be assumed to adversely effect allograft survival. Moreover, the order of presentation (pathogen or allograft first), and presence of immunosuppression at the time of initial presentation may significantly influence the ultimate outcome.
In summation, these results highlight disparate effects of rapamycin on T cell responses to pathogens and donor tissue, and underscore the fact that there are many facets to the mTOR signaling pathway in immune cells that are still poorly understood. Still, the fact that previous studies and data presented here indicate that rapamycin might paradoxically enhance the antigen-specific CD8+ T cell response to viral or bacterial pathogens suggests that treatment of transplant recipients with rapamycin monotherapy may simultaneously increase immunity to a virus or vaccine (7) while inhibiting the response to an allograft. The impact of rapamycin on pathogen-specific CD8+ T cell responses in the context of other immunosuppressive agents is an important area of future research.
References
- 1.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, Schmidt J, Zeier M. Sirolimus in renal transplantation. Nephrol Dial Transplant. 2007;22(Suppl 8):viii61–viii65. doi: 10.1093/ndt/gfm652. [DOI] [PubMed] [Google Scholar]
- 3.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 12.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 13.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 14.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, Wang Z, Thomson AW. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184:624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, Shen J, Xu H, Wang CR, Alegre ML, Chong AS. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991–5999. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MC, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, McCluskey J. T cell allorecognition via molecular mimicry. Immunity. 2009;31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]