Abstract
Background
Recent studies have suggested that a restrictive pattern assessed with a single spirometry is associated with increased morbidity and mortality. In this study, we sought to determine demographic, clinical, and mortality profiles of subjects with either a recurrent or inconsistent restrictive spirometric pattern assessed prospectively.
Methods
We analyzed data from 2048 adult participants in the population-based TESAOD study. Normal (FEV1/FVC ratio≥70% and FVC≥80% predicted), restrictive (FEV1/FVC≥70% and FVC<80% predicted), and obstructive (FEV1/FVC<70%) patterns were assessed at the enrollment survey (1972) and in eleven subsequent follow-up surveys up to 1996. Demographic and clinical characteristics were measured at enrollment and vital status and cause of death were assessed as of January 2005.
Results
Overall, 12% of participants had a restrictive spirometric pattern at enrollment. They were less likely to be males, to smoke and to have asthma, and had lower IgE levels as compared with subjects in the obstructive group. Among subjects with a restrictive pattern at enrollment, 38% developed an obstructive pattern during the follow-up. The remaining 62% had either a recurrent (restrictive pattern≥50% of follow-up surveys) or inconsistent (restrictive pattern<50% of follow-up surveys) longitudinal restrictive pattern. The recurrent and inconsistent restrictive groups had increased mortality risk for all-cause (adjHR: 1.7, 1.3–2.3; and 1.9, 1.4–2.6; respectively), heart disease (2.0, 1.3–3.1; and 2.7, 1.7–4.3), stroke (2.4, 0.9–6.3; and 3.5, 1.2–9.8), and diabetes (8.0, 2.9–21.8; and 6.0, 1.9–19.2).
Conclusions
The restrictive spirometric pattern identifies a pulmonary condition that is distinguishable from obstructive lung disease and associated with increased risk of life-threatening co-morbidities.
Keywords: spirometric restrictive pattern, lung function, mortality, FVC, FEV1, FEV1/FVC, TESAOD
Introduction
A reduction of the forced expiratory volume in one second (FEV1) has been associated with respiratory symptoms, functional limitation, and mortality risk1,2. These findings are partly explained by morbidity and mortality associated with obstructive lung diseases, particularly chronic obstructive pulmonary disease (COPD). COPD, which is defined functionally by a reduction of the ratio between FEV1 and forced vital capacity (FVC) below 70%3, is one of the leading causes of mortality and quality-adjusted life years lost in the US as well as worldwide4,5.
Yet, at the population level, a significant proportion of subjects with reduced FEV1 levels have also reduced FVC and normal FEV1/FVC ratio. Thus, COPD is not the only explanation for the excess mortality in individuals with low FEV1. Indeed, a strong link between low FVC and mortality risk has been long known6 and recent evidence suggests that a restrictive spirometric pattern is relatively common and accounts for an important portion of morbidity and mortality related to lung function impairment. Epidemiological studies have found that, in the general population, between 7% and 13% of adults have FVC values below 80% of expected for their sex, age, and height in the presence of an FEV1/FVC ratio ≥ 70%7–10 and that these individuals are at increased risk for all-cause and cardiovascular mortality8,9. This restrictive spirometric pattern is also associated with significant functional impairment7 and various comorbid conditions, including diabetes11,12, metabolic syndrome13,14, hypertension12,15, stroke15, and cardiovascular disease16. Although some of the above studies assessed the relation of lung function to morbidity and mortality prospectively, they used a single spirometric test at enrollment to measure lung function.
Low FVC values assessed by spirometry can also be found in a proportion of normal subjects who complete spirometry using sub-maximal inspiratory efforts and in subjects with air trapping associated with obstructive lung diseases, such as asthma and COPD. Although assessment of total lung capacity such as using a plethysmograph is the only conclusive test to differentiate truly restricted subjects from misclassified obstructed and normal individuals, this test – unlike spirometry – is time-consuming, costly, and needs to be completed in a specialized setting, precluding its potential use in screening programs on large populations. It is reasonable to hypothesize that a longitudinal follow-up based on serial spirometric tests on the same subjects, as contrasted with a single spirometric assessment, may help distinguish cases with a true restrictive spirometric pattern from those in which the restrictive pattern is a manifestation of an underlying airway obstruction. Yet, no previous study has attempted to characterize prospectively the restrictive spirometric pattern and to evaluate the specific morbidity and mortality burden associated with such longitudinal patterns.
In this study, in a large population-based prospective cohort we sought to determine demographic, clinical, and mortality profiles associated with recurrent and inconsistent restrictive longitudinal patterns and to compare them to those of obstructive longitudinal patterns.
Methods
Study population
The Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD) is a population-based prospective cohort study initiated in Tucson, AZ in 1972. Details of the enrollment process have been previously reported17. Briefly, using a multistage stratified cluster sample of anglo-white Tucson households (based on the 1970 Census block statistics), 2989 households were originally approached, of which 559 (18.7%) refused to participate, 350 (11.7%) were ineligible, 329 (11%) were vacant dwelling units, and 96 (3.2%) were classified as permanently not-at home. A total of 1655 households finally participated in the enrollment survey (survey 1, completed in 1972–73). At enrollment, 2754 white participants (age range: 6 to 95 years) from these households completed both a standardized respiratory questionnaire and spirometric lung function tests with a pneumotachygraphic device according to methods previously described18. Twelve additional follow-up surveys were completed approximately every two years up to 1996, in which participants completed the same standardized questionnaire and (with the only exception of survey 4) spirometric lung function tests. To reduce the loss-to-follow-up of participants who moved out of Tucson during the study, the study nurses traveled to various states in the US to perform lung function tests and collect questionnaire data from many of these participants.
For the present study, we used data from 2048 participants who were 21 to 80 years old, did not report having ever had lung / chest surgery, were not pregnant, and completed acceptable lung function tests at enrollment.
Baseline and longitudinal spirometric patterns and covariates
Baseline spirometric patterns at enrollment were defined as normal (FEV1/FVC ≥ 70% and FVC ≥ 80% predicted), restrictive (FEV1/FVC ≥ 70% and FVC < 80% predicted), or obstructive (FEV1/FVC < 70% independent of FVC values) using reference equations generated in the same population by Knudson and colleagues19.
In addition, among subjects who had at least one follow-up survey, the following six longitudinal groups were generated based on the combination of spirometric patterns at enrollment and follow-up surveys:
Consistent Normal (normal pattern at enrollment and in all completed follow-up surveys);
Recurrent Restrictive (restrictive pattern at enrollment and in at least 50% of the follow-up surveys, plus never obstructive pattern);
Inconsistent Restrictive (restrictive pattern at enrollment and in less than 50% of the follow-up surveys, plus never obstructive pattern);
Recurrent Obstructive (obstructive pattern at enrollment and in at least 50% of the follow-up surveys, plus never restrictive pattern);
Inconsistent Obstructive (obstructive pattern at enrollment and in less than 50% of the follow-up surveys, plus never restrictive pattern);
Mixed Restrictive/Obstructive (restrictive pattern at enrollment and obstructive pattern in at least one of the follow-up surveys; or obstructive pattern at enrollment and restrictive pattern in at least one of the follow-up surveys).
Two additional longitudinal spirometric patterns (Incident Restrictive: normal pattern at enrollment and development of a restrictive pattern during the follow-up; and Incident Obstructive: normal pattern at enrollment and development of an obstructive pattern during the follow-up) were also identified, but – because of the specific goals of the present study – they were not included in the main analyses. Results from these two incident groups are only presented in the online data repository.
At enrollment, in addition to an in-depth standardized respiratory questionnaire and lung function tests, height and weight were measured by research nurses, and skin prick tests and blood eosinophil counts performed (see Online Repository). Obesity was defined as a BMI ≥ 30 Kg/m2 and being overweight as a BMI ≥ 25 and < 30 Kg/m2. Positive skin prick tests were defined as a wheal ≥ 2 mm larger than the control wheal for at least one of five tested allergens and eosinophilia as eosinophils > 4%. Measurement of total serum IgE was also completed through the paper radioimmunosorbent test (PRIST) (Pharmacia Diagnostics, Piscataway, NJ) method20.
Assessment of mortality
Vital status of TESAOD participants as of January 2005 was determined through direct contact with the family or designated next of kin of the participant, linkage with the Social Security Death Index21, and – for deaths that occurred after 1978 – linkage with the National Death Index (NDI)22. Underlying causes of death were obtained from NDI records for deaths that occurred after 1978 and directly from death certificates for deaths that occurred earlier (Table E1).
Statistical analyses
Because spirometric patterns differed substantially by age, demographic and clinical characteristics were compared across different spirometric patterns using linear and logistic regression models adjusted by age. IgE values were log-transformed to achieve normalization.
The relation of longitudinal spirometric patterns to all-cause and cause-specific mortality was investigated in sex-, age-, and BMI-adjusted Cox proportional hazards models. Time to event was defined as the time between enrollment and the date of death for deceased participants and as the time between enrollment and January 2005 for alive subjects. In analyses for specific causes of death, cases were represented by participants who had that specific disease as underlying cause of death. However, because only 10 subjects had diabetes identified as underlying cause of death, in Cox models for diabetes mortality events were defined as cases with diabetes indicated as either underlying cause of death or co-existing condition on the death certificate.
Results
Comparison of participants included and excluded from the present study
At the enrollment survey, 2408 TESAOD participants were between 21 and 80 years old and did not report being pregnant or having ever had lung or chest surgery. Among them, 2048 (85%) completed lung function tests and were included in the present study. Participants included in the present study did not differ from those excluded in terms of age, sex, BMI, education, smoking, or skin test positivity. The only significant difference between the two groups was that subjects included in the present study were more likely to have physician confirmed asthma than subjects excluded from the present study (11.1% vs 7.5%, respectively; p = 0.04).
Demographic and clinical characteristics at enrollment (1972–73)
At enrollment, of the 2048 subjects included in the present study 1505 (74%) had normal lung function, 249 (12%) had a spirometric restrictive pattern, and 294 (14%) had an obstructive pattern. The three groups differed substantially by age distribution (p < 0.001). In order to reduce the impact of age differences across the three groups, demographic and clinical characteristics are compared in Table I after adjustment for age. The restrictive pattern was associated with obesity and asthma. However, less than 14% of subjects with the restrictive pattern were obese and no differences in the proportion of overweight subjects were found among the restrictive (35%), normal (34%), and obstructive (34%) pattern. Overall, 14% of subjects with the restrictive pattern had a physician-confirmed diagnosis of asthma versus 8% of subjects in the normal pattern. Yet, these two patterns did not differ in terms of skin prick tests, eosinophilia, or IgE levels. In contrast, subjects with the obstructive pattern were more likely to be males and smokers and to have asthma, eosinophilia, and elevated IgE, as compared with subjects with normal lung function. Overall, 70% of subjects with the obstructive pattern were heavy smokers (≥ 20 packyears) and/or had asthma, as compared with 42% of subjects with the restrictive pattern and 30% of subjects with normal lung function.
Table I.
Demographic and clinical characteristics at enrollment of TESAOD participants according to their lung function at enrollment. Vital status as of January 2005 is also presented. N is 2048, unless otherwise specified.
| LUNG FUNCTION AT ENROLLMENT |
||||
|---|---|---|---|---|
| Normal (N = 1505) | Restrictive Spirometric Pattern (N = 249) | Obstructive Spirometric Pattern (N = 294) | P value* | |
| Age in years: mean ± SD | 46 ± 17 | 60 ± 15 | 60 ± 15 | N/A |
| Sex: % female | 56.3 | 67.1 | 46.9 ^ ‡ | <0.001 |
| Obesity, (n = 1978): % with BMI ≥ 30 Kg/m2 | 6.8 | 13.6 ^ | 6.4 ‡ | 0.004 |
| Years of formal education: % with > 12 years | 45.8 | 37.8 | 32.0 | 0.27 |
| Ever smoking (n = 2046): % with ≥ 1 pack-year | 53.9 | 50.6 | 77.1 ^ ‡ | <0.001 |
| Pack-Years (n = 1163): geometric mean among ever smokers | 14.1 | 22.5 | 28.9 ^ | 0.006 |
| Physician confirmed asthma (n = 2046): positive % | 8.2 | 14.1 ^ | 23.5 ^ ‡ | <0.001 |
| Allergy skin tests (n = 2012): positive % | 39.7 | 25.9 | 31.6 | 0.28 |
| Eosinophilia (n = 1542): positive % | 7.4 | 9.6 | 12.8 ^ | 0.01 |
| Total serum IgE in IU/ml (n = 1836): geometric mean | 27.5 | 21.5 | 37.8 ^ ‡ | <0.001 |
| Chronic Cough and Phlegm: % positive | 7.7 | 13.7 ^ | 31.0 ^ ‡ | <0.001 |
| Shortness of Breath with Wheezing (n = 2043): % positive | 16.9 | 27.7 ^ | 42.8 ^ ‡ | <0.001 |
| ≥ Grade 2 on the Modified MRC Dyspnea Scale^^ (n = 2038): %positive | 9.5 | 24.0 ^ | 34.9 ^ ‡ | <0.001 |
| Ever Pneumonia: % positive | 23.0 | 40.6 ^ | 39.5 ^ | <0.001 |
| Ever “Heart Trouble”: % positive | 13.8 | 26.1 | 20.7 | 0.36 |
| Ever stroke: % positive | 1.1 | 6.0 ^ | 3.1 | 0.05 |
| Diabetes: % positive | 3.0 | 7.2 | 4.4 | 0.29 |
| Deceased as of January 2005: % deceased | 46.3 | 82.7 | 84.0 | N/A |
Statistical comparisons are completed across the three groups after adjustment for age (p value reported in the last column) as well as between pairs after adjustment for age and Bonferroni correction.
P value for the comparison across the three groups after adjustment for age.
Significantly different from Normal after adjustment for age and Bonferroni correction.
Significantly different from Restrictive Spirometric Pattern after adjustment for age and Bonferroni correction.
Both the restrictive and the obstructive pattern were associated with chronic bronchitis, shortness of breath with wheezing, dyspnea, and a positive history of pneumonia as compared with the normal pattern (Table I), although the majority of these associations were stronger among subjects with the obstructive than subjects with the restrictive pattern. The latter group, however, tended to have higher rates of heart disease, stroke, and diabetes.
Longitudinal spirometric patterns (1972 to 1996)
Among the 2048 subjects included in this study, 24 (1%) died before the first follow-up survey (10 subjects in the normal, 5 in the restrictive, and 9 in the obstructive spirometric pattern groups). Among the remaining 2024 subjects, 1798 (89%) had follow-up data, with an average 12.2 years of follow-up and an average 6.3 lung function tests completed during the study. Among them, 126 subjects had an incident restrictive and 304 an incident obstructive pattern. Findings on these groups are presented in Tables E2 and E3 in the online data repository. The remaining 1368 participants were divided into the six longitudinal spirometric patterns shown in Table II. Of note, among the 211 subjects with spirometric restriction at enrollment and available follow-up data, 69 (33%) had a recurrent restrictive pattern, 62 (29%) an inconsistent restrictive pattern, and 80 (38%) developed airflow limitation during the study follow-up and accounted for the majority of subjects included in the mixed restrictive/obstructive pattern.
Table II.
Demographic and clinical characteristics at enrollment and length of follow-up of subjects included in the six longitudinal spirometric patterns. Total N is 1368, unless otherwise specified.
| Longitudinal Spirometric Patterns |
|||||||
|---|---|---|---|---|---|---|---|
| Consistent Normal (N = 913) | Recurrent Restrictive (N = 69) | Inconsistent Restrictive (N = 62) | Recurrent Obstructive (N = 155) | Inconsistent Obstructive (N = 54) | Mixed Restr/Obstr (N = 115) | P * | |
| Age in years: mean ± SD | 44 ± 17 | 62 ± 15 | 55 ± 17 | 60 ± 13 | 55 ± 17 | 64 ± 11 | N/A |
| Sex: % female | 59.1 | 76.8 | 69.4 | 38.7 | 59.3 | 67.0 | <0.001 |
| Obesity (n = 1325): % with BMI ≥ 30 Kg/m2 | 6.3 | 15.4 | 16.7 | 3.3 | 9.6 | 12.5 | 0.006 |
| Ever smoking (n = 1367): % with ≥ 1 pack-year | 48.4 | 37.7 | 50.0 | 82.6 | 73.6 | 58.3 | <0.001 |
| Physician confirmed asthma (n = 1366): positive % | 6.4 | 10.3 | 4.8 | 33.5 | 13.0 | 21.7 | <0.001 |
| Eosinophilia (n = 1035): positive % | 7.1 | 6.3 | 10.9 | 14.0 | 14.0 | 13.8 | 0.05 |
| Total serum IgE in IU/ml (n = 1221): geometric mean | 25.2 | 19.3 | 29.1 | 52.2 | 25.7 | 23.3 | <0.001 |
| Total follow-up in years: mean ± SD | 12 ± 7 | 7 ± 7 | 10 ± 7 | 9 ± 7 | 10 ± 7 | 11 ± 7 | <0.001 |
P value for the comparison across the six groups after adjustment for age.
No statistical pair comparisons were completed because of the number of groups.
Demographic and clinical characteristics at enrollment of subjects in the six longitudinal spirometric patterns are shown in Table II. As compared with the recurrent obstructive group, subjects in the recurrent restrictive group appeared more likely to be females and obese and less likely to smoke and to have asthma, eosinophilia, and elevated IgE. The characteristics of subjects with the recurrent and inconsistent restrictive patterns were very similar, with the former differing from the latter only in terms of older age and lower FVC % predicted at baseline (data not shown, 68% versus 75%, p < 0.001).
All-cause and cause-specific mortality associated with longitudinal patterns (1972 to 2005)
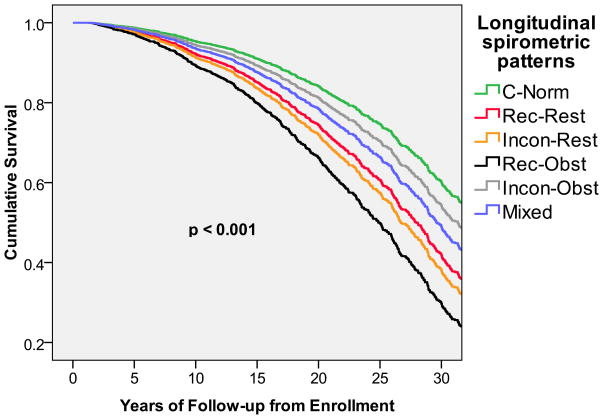
After adjusting for sex, age, and BMI, subjects with the recurrent restrictive pattern had a 70% (30–130%) increased mortality risk and subjects with the inconsistent restrictive pattern a 90% (40–160%) increased mortality risk, as compared with subjects who had consistently normal lung function during the study follow-up (Table III). Among the corresponding obstructive longitudinal patterns, only the recurrent obstructive group was associated with an increased all-cause mortality risk, whereas subjects with inconsistent obstructive pattern were not at increased risk for mortality. The mixed pattern was also associated with a significant adjusted HR for all-cause mortality of 1.4 (1.1–1.8). In Figure 1, survival curves adjusted for sex, age, and BMI are shown for the six longitudinal spirometric patterns. Unadjusted survival curves are shown in the online data repository (Figure E1). Because subjects had to complete at least one follow-up survey to be eligible for longitudinal analyses, sensitivity analyses were completed after defining survey 2 as the first time point in survival models. Results were unchanged.
Table III.
Sex-, age-, and BMI-adjusted Mortality Hazard Ratios and corresponding 95% Confidence Intervals associated with the six longitudinal spirometric patterns. Total N is 1325 because 43 subjects had missing information for BMI.
| Underlying Cause of Death |
|||||||
|---|---|---|---|---|---|---|---|
| Longitudinal Spirometric Patterns | All-cause adjHR (95 CI) | Heart Disease adjHR (95% CI) | Cancer adjHR (95% CI) | Lung Cancer adjHR (95% CI) | COPD adjHR (95% CI) | Stroke adjHR (95% CI) | Diabetes adjHR (95% CI) |
| Consistent Normal (N = 883) | reference | reference | reference | reference | reference | reference | reference |
| Recurrent Restrictive (N = 65) | 1.7 (1.3 – 2.3) | 2.0 (1.3 – 3.1) | 1.0 (0.5 – 2.2) | 0.7 (0.1 – 5.5) | 2.0 (0.2 – 17.3) | 2.4 (0.9 – 6.3) | 8.0 (2.9 – 21.8) |
| Inconsistent Restrictive (N = 60) | 1.9 (1.4 – 2.6) | 2.7 (1.7 – 4.3) | 1.4 (0.7 – 3.0) | 1.5 (0.4 – 6.6) | N/A | 3.5 (1.2 – 9.8) | 6.0 (1.9 – 19.2) |
| Recurrent Obstructive (N = 153) | 2.4 (1.9 – 2.9) | 2.1 (1.4 – 3.0) | 1.4 (0.8 – 2.2) | 3.2 (1.5 – 6.6) | 38.0 (15.6 – 92.6) | 6.2 (2.9 – 13.4) | 5.4 (2.0 – 15.2) |
| Inconsistent Obstructive (N = 52) | 1.2 (0.8 – 1.7) | 1.3 (0.8 – 2.3) | 0.7 (0.3 – 1.8) | N/A | 4.2 (0.8 – 21.1) | 1.5 (0.3 – 6.5) | 1.0 (0.1 – 7.7) |
| Mixed Restrict/Obstruct (N = 112) | 1.4 (1.1 – 1.8) | 1.6 (1.1 – 2.3) | 0.7 (0.3 – 1.3) | 1.1 (0.4 – 3.4) | 10.9 (4.0 – 30.0) | 1.0 (0.3 – 3.0) | 4.7 (1.9 – 11.9) |
| N events | 728 | 268 | 141 | 45 | 61 | 48 | 38* |
Because only 10 subjects had diabetes identified as underlying cause of death, in Cox models for diabetes mortality events were defined as “deaths with diabetes indicated as either underlying cause of death or co-existing condition on the death certificate”.
Figure 1.
Survival curves adjusted for sex, age, and BMI associated with the six longitudinal spirometric patterns.
C-Norm: Consistent Normal
Rec-Rest: Recurrent Restrictive
Incon-Rest: Inconsistent Restrictive
Rec-Obst: Recurrent Obstructive
Incon-Obst: Inconsistent Obstructive
Mixed: Mixed Restrictive/Obstructive
Similarly, when all-cause mortality risks were compared across the three cross-sectional spirometric patterns at enrollment, both the restrictive and the obstructive spirometric patterns at enrollment were associated with sex-, age-, and BMI-adjusted increased mortality risk as compared with subjects with normal lung function (adjHR: 1.4, 1.2–1.7; and 1.9, 1.6–2.2; respectively).
Profiles of specific causes of death differed substantially across longitudinal spirometric groups. Subjects with the recurrent obstructive pattern were at increased risk for dying of heart disease, lung cancer, COPD, stroke, and diabetes. In particular, as expected, their risk of dying of COPD was very high (adjHR: 38.0, 15.6 – 92.6). The risk of dying from COPD was also significantly increased among subjects with the mixed longitudinal pattern, but not among subjects with either recurrent or inconsistent restrictive pattern. Subjects in either the recurrent or inconsistent restrictive pattern groups were most likely to dye of heart disease and diabetes.
Discussion
In this study of the long-term population-based TESAOD cohort we found that: 1) twelve percent of the population had a restrictive spirometric pattern at enrollment; 2) this restrictive spirometric pattern was associated with respiratory symptoms, but not with smoking, serum IgE, allergy skin tests, or eosinophilia; 3) in longitudinal analyses, 38% of subjects with the restrictive pattern at enrollment developed airway obstruction during the follow-up; 4) the remaining 62% had either a recurrent or inconsistent chronic restrictive spirometric pattern and were at significantly increased risk of dying from all causes, including high risk of dying of heart disease and dying of or with diabetes.
Our finding that 12% of the TESAOD cohort had a restrictive spirometric pattern at the baseline survey is comparable with cross-sectional data reported by Mannino and colleagues from the NHANES I (9%)8, the Cardiovascular Health Study (10%)10, and the ARIC (13%)9 adult cohorts. However, when the TESAOD subjects were re-evaluated during the follow-up only one third of them showed a recurrent restrictive pattern, while 29% had an inconsistent restrictive pattern and 38% developed an obstructive pattern. The latter group was associated with both a positive smoking history and an asthma diagnosis at enrollment (data not shown).
These findings suggest that, although in about one third of cases a restrictive pattern on a single spirometry may convert into an obstructive pattern over time, in the majority of cases the restrictive spirometric pattern is not due to an underlying obstructive lung disease. Consistent with this scenario, subjects in the restrictive group had smoking rates comparable to those of participants in the normal group and significantly lower than those of subjects in the obstructive group, indicating that they did not have a form of cigarette-related COPD. They also had less asthma and lower IgE levels than did subjects in the obstructive group, which also argues against a link between the restrictive spirometric pattern and latent airway obstruction. Indeed, although subjects who had a restrictive pattern at enrollment had more diagnoses of asthma as compared with subjects in the normal group, this association was mainly due to subjects who developed a subsequent obstructive pattern since only 8% of subjects with either a recurrent or inconsistent longitudinal restrictive pattern had a physician confirmed diagnosis of asthma at enrollment (versus 6.4% of consistent normals and 33.5% of subjects with a recurrent obstructive pattern).
Factors that are causally linked to spirometric restriction remain largely unknown. Obesity and heart failure are potential common causes of spirometric restriction23,24. However, obesity is unlikely to play a major role in our study because less than 15% of subjects with the restrictive spirometric pattern were obese at enrollment and overweight rates were largely comparable across subjects with the restrictive, obstructive, and normal patterns. Data on heart failure were not available in our study, but heart disease was reported at enrollment by 26% of subjects in the restrictive group, only a minority of whom presumably suffered from heart failure.
In our study, both the recurrent and the inconsistent restrictive spirometric patterns were strongly associated with all-cause mortality. In addition, subjects in either the recurrent or inconsistent restrictive pattern groups had particularly high risks for dying of heart disease and diabetes. These findings suggest that spirometric restriction accounts for a substantial proportion of the association between lung function impairment and these co-morbidities, although the nature of these links remains to be determined. The reasons why subjects with an inconsistent restrictive pattern had a mortality risk largely comparable to that of subjects with a recurrent restrictive pattern, despite the greater lung function impairment of the latter, also remain to be determined. However, it is noteworthy that the recurrent and inconsistent restrictive groups included a similar number of subjects, whereas the recurrent to inconsistent obstructive pattern ratio was almost 3:1, suggesting that spirometric restriction may be more likely than obstruction to resolve and relapse inconsistently over time. Taken together, these observations indicate the importance of an early identification of subjects who present with a restrictive spirometric pattern, a phenotype whose sequelae are frequently underestimated in the clinical setting.
A limitation of our study is that, like most other large epidemiological cohorts, it lacks data on lung function after bronchodilator. Thus, we could not determine in what proportion of subjects in the restrictive and obstructive groups the abnormal spirometric pattern was reversible (i.e., it would resolve into a normal pattern following administration of a bronchodilator). Also, the possibility that clinical conditions such as heart failure may have developed in some participants over time and, in turn, influenced the likelihood of these subjects to be included in one of the restrictive longitudinal patterns cannot be ruled out. However, this potential limitation would apply to the longitudinal spirometric patterns and, thus, it could not explain the strong link between the restrictive pattern assessed at enrollment and subsequent mortality risk . Finally, differences in the length of follow-up may influence the likelihood of participants to have a recurrent versus inconsistent longitudinal pattern because subjects with longer follow-up have more chances to alternate normal and abnormal lung function tests during the study. To minimize the impact of such a potential artifact, we defined inconsistent and recurrent patterns based on the proportion of follow-up surveys with normal or abnormal lung function tests, a parameter that should be relatively independent of the length of follow-up. Subjects with recurrent restrictive or obstructive longitudinal spirometric patterns had a shorter mean length of follow-up than subjects with a consistently normal pattern (Table II). However, these differences may be due to the different mortality risk among these groups.
Strengths of our study are the population-based prospective nature of the TESAOD cohort, the extensive phenotypic data and long-term follow-up period, and the exhaustive search for information on vital status of participants.
In summary, our findings indicate that a restrictive spirometric pattern is present in a substantial proportion of the general adult population and that in most cases it is not associated with the subsequent development of an obstructive pattern. Subjects with the restrictive pattern show a profile of clinical characteristics and risk factors largely distinct from those of subjects with obstructive lung disease. Most importantly, they carry a significant burden of respiratory symptoms, functional impairment, and risk of mortality by co-morbidities, even among those with an inconsistent restrictive pattern. Clinical evaluations of potential interventions to reduce the morbidity and mortality burden associated with spirometric restriction are needed.
Supplementary Material
Acknowledgments
Funding
This study was funded by grants HL14136, HL085195, and HL095021 by the National Heart, Lung, and Blood Institute, a grant award by the American Thoracic Society / Alpha1 Foundation, grant 0660059Z by the American Heart Association, and an unrestricted grant from the Barry and Janet Lang Donor Advised Fund.
Dr Guerra was the recipient of a Parker B. Francis Fellowship.
Footnotes
Publisher's Disclaimer: Licence
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in THORAX editions and any other BMJPG Ltd products to exploit all subsidiary rights, as set out in the licence at (http://thorax.bmj.com/ifora/licence.pdf).
Competing interest
None declared.
References
- 1.Speizer FE, Fay ME, Dockery DW, Ferris BG., Jr Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140(3 Pt 2):S49–55. doi: 10.1164/ajrccm/140.3_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 2.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. Bmj. 1996;313(7059):711–5. doi: 10.1136/bmj.313.7059.711. discussion 715–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. Jama. 2005;294(10):1255–9. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 5.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley F, Kannel WB, Sorlie PD, Masson R. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med. 1975;82(6):739–45. doi: 10.7326/0003-4819-82-6-739. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254(6):540–7. doi: 10.1111/j.1365-2796.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–93. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100(1):115–22. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Davis KJ. Lung function decline and outcomes in an elderly population. Thorax. 2006;61(6):472–7. doi: 10.1136/thx.2005.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford ES, Mannino DM. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care. 2004;27(12):2966–70. doi: 10.2337/diacare.27.12.2966. [DOI] [PubMed] [Google Scholar]
- 12.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 13.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–16. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 14.Fimognari FL, Pasqualetti P, Moro L, et al. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol A Biol Sci Med Sci. 2007;62(7):760–5. doi: 10.1093/gerona/62.7.760. [DOI] [PubMed] [Google Scholar]
- 15.Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613–21. [PubMed] [Google Scholar]
- 16.Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63(7):599–605. doi: 10.1136/thx.2007.088112. [DOI] [PubMed] [Google Scholar]
- 17.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102(2):137–52. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 18.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 19.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 21.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis KB, Fisher L, Gillespie MJ, Pettinger M. A test of the National Death Index using the Coronary Artery Surgery Study (CASS) Control Clin Trials. 1985;6(3):179–91. doi: 10.1016/0197-2456(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 23.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128(3):501–6. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 24.Faggiano P. Abnormalities of pulmonary function in congestive heart failure. Int J Cardiol. 1994;44(1):1–8. doi: 10.1016/0167-5273(94)90060-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.