Abstract
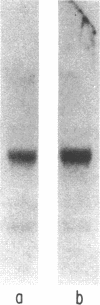
Previous studies performed in this laboratory have demonstrated somatostatin-containing cells in close proximity to gastrin cells in antral mucosa and have shown that somatostatin exerts a local regulatory effect on gastrin release. The present studies were directed to determine whether the effects of somatostatin on the antral gastrin cell involve pretranslational events. The effects of somatostatin on gastrin mRNA were determined by dot blot hybridization using a gastrin antisense RNA probe derived from human gastrin cDNA. Inclusion of somatostatin in the incubation medium caused a dose-dependent inhibition of steady-state gastrin mRNA. Conversely, when antral somatostatin was neutralized by the addition of specific somatostatin antibodies to the incubation medium, gastrin mRNA levels increased by 116 +/- 31% over control values (P less than 0.01). Northern blot hybridization of total antral RNA demonstrated a single major band with a molecular size of approximately 620 nucleotides, closely matching the predicted size of gastrin mRNA. The effect of somatostatin on the rate of gastrin gene transcription was examined using nuclear run-off transcription assays. Inclusion of antibodies to somatostatin in the incubation medium resulted in a 33.8 +/- 3.3% increase in gastrin gene transcriptional activity (P less than 0.01). These studies indicate that, in addition to its established effect on peptide release, somatostatin exerts inhibitory effects on antral gastrin cells at the pretranslational level. Although this inhibition appears to occur in part at the gene transcriptional level, the results also indicate that somatostatin may affect posttranscriptional processing of gastrin mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aponte G., Gross D., Yamada T. Capillary orientation of rat pancreatic D-cell processes: evidence for endocrine release of somatostatin. Am J Physiol. 1985 Nov;249(5 Pt 1):G599–G606. doi: 10.1152/ajpgi.1985.249.5.G599. [DOI] [PubMed] [Google Scholar]
- Barinaga M., Bilezikjian L. M., Vale W. W., Rosenfeld M. G., Evans R. M. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature. 1985 Mar 21;314(6008):279–281. doi: 10.1038/314279a0. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Mortimer C. H., Thorner M. O., Besser G. M., Hall R., Gomez-Pan A., Roy V. M., Russell R. C., Coy D. H., Kastin A. J. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet. 1974 Nov 9;2(7889):1106–1109. doi: 10.1016/s0140-6736(74)90869-1. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Campbell G., Gibbins I. L., Morris J. L., Furness J. B., Costa M., Oliver J. R., Beardsley A. M., Murphy R. Somatostatin is contained in and released from cholinergic nerves in the heart of the toad Bufo marinus. Neuroscience. 1982;7(9):2013–2023. doi: 10.1016/0306-4522(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Carr D., Gomez-Pan A., Weightman D. R., Roy V. C., Hall R., Besser G. M., Thorner M. O., McNeilly A. S., Schally A. V., Kastin A. J. Growth hormone release inhibiting hormone: actions on thyrotrophin and prolactin secretion after thyrotrophinreleasing hormone. Br Med J. 1975 Jul 12;3(5975):67–69. doi: 10.1136/bmj.3.5975.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Kadowaki S., Taminato T., Chihara K., Seino Y., Matsukura S., Fujita T. Effect of antisomatostatin gamma-globulin on gastrin release in rats. Gastroenterology. 1981 Aug;81(2):321–326. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chrapkiewicz N. B., Beale E. G., Granner D. K. Induction of the messenger ribonucleic acid coding for phosphoenolpyruvate carboxykinase in H4-II-E cells. Evidence for a nuclear effect of cyclic AMP. J Biol Chem. 1982 Dec 10;257(23):14428–14432. [PubMed] [Google Scholar]
- Colturi T. J., Unger R. H., Feldman M. Role of circulating somatostatin in regulation of gastric acid secretion, gastrin release, and islet cell function. Studies in healthy subjects and duodenal ulcer patients. J Clin Invest. 1984 Aug;74(2):417–423. doi: 10.1172/JCI111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancygier H., Klein U., Leuschner U., Hübner K., Classen M. Somatostatin-containing cells in the extrahepatic biliary tract of humans. Gastroenterology. 1984 May;86(5 Pt 1):892–896. [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Regulation of pancreatic insulin and glucagon secretion. Annu Rev Physiol. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- Gick G. G., Bancroft C. Glucocorticoid stimulation of growth hormone messenger ribonucleic acid levels in GH3 cells is inhibited by calcium but not by somatostatin. Endocrinology. 1987 May;120(5):1986–1990. doi: 10.1210/endo-120-5-1986. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Harty R. F., Maico D. G., McGuigan J. E. Postreceptor inhibition of antral gastrin release by somatostatin. Gastroenterology. 1985 Mar;88(3):675–680. doi: 10.1016/0016-5085(85)90136-2. [DOI] [PubMed] [Google Scholar]
- Harty R. F., Maico D. G., McGuigan J. E. Somatostatin inhibition of basal and carbachol-stimulated gastrin release in rat antral organ culture. Gastroenterology. 1981 Oct;81(4):707–712. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Jefferson L. S., Liao W. S., Peavy D. E., Miller T. B., Appel M. C., Taylor J. M. Diabetes-induced alterations in liver protein synthesis. Changes in the relative abundance of mRNAs for albumin and other plasma proteins. J Biol Chem. 1983 Jan 25;258(2):1369–1375. [PubMed] [Google Scholar]
- Kurokawa K., Aponte G. W., Fujibayashi S., Yamada T. Somatostatin-like immunoreactivity in the glomerulus of rat kidney. Kidney Int. 1983 Dec;24(6):754–757. doi: 10.1038/ki.1983.223. [DOI] [PubMed] [Google Scholar]
- Laverrière J. N., Tixier-Vidal A., Morin A., Buisson N., Muller M., Truong A. T., Martial J. A., Gourdji D. Régulations de l'expression des gènes de la prolactine et de l'hormone de croissance dans des cellules hypophysaires en culture. Ann Endocrinol (Paris) 1986;47(1):22–27. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Carter J. D., McDonald A. R. Dietary regulation of rat intestinal cholecystokinin gene expression. J Clin Invest. 1988 Jun;81(6):2015–2019. doi: 10.1172/JCI113552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Lyons R. T., Nordeen S. K., Young D. A. Effects of fasting and insulin administration on polyribosome formation in rat epididymal fat cells. J Biol Chem. 1980 Jul 10;255(13):6330–6334. [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H. E., Jefferson L. S., Wolpert E. B., Rannels D. E. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971 Apr 10;246(7):2163–2170. [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear estrogen receptor levels to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1977 Mar 25;252(6):2060–2068. [PubMed] [Google Scholar]
- Noguchi T., Inoue H., Tanaka T. Regulation of rat liver L-type pyruvate kinase mRNA by insulin and by fructose. Eur J Biochem. 1982 Nov 15;128(2-3):583–588. doi: 10.1111/j.1432-1033.1982.tb07004.x. [DOI] [PubMed] [Google Scholar]
- Plant P. W., Deeley R. G., Grieninger G. Selective block of albumin gene expression in chick embryo hepatocytes cultured without hormones and its partial reversal by insulin. J Biol Chem. 1983 Dec 25;258(24):15355–15360. [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Grimelius L., Bloom S. R. Growth-hormone release-inhibiting hormone in gastrointestinal and pancreatic D cells. Lancet. 1975 May 31;1(7918):1220–1222. doi: 10.1016/s0140-6736(75)92198-4. [DOI] [PubMed] [Google Scholar]
- Pry T. A., Porter J. W. Control of fatty acid synthetase mRNA levels in rat liver by insulin, glucagon, and dibutyl cyclic AMP. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1002–1009. doi: 10.1016/0006-291x(81)91923-9. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Secretion of somatostatin and its physiologic function. J Lab Clin Med. 1987 Mar;109(3):320–326. [PubMed] [Google Scholar]
- Riegel A. T., Martin M. B., Schoenberg D. R. Transcriptional and post-transcriptional inhibition of albumin gene expression by estrogen in Xenopus liver. Mol Cell Endocrinol. 1986 Mar;44(3):201–209. doi: 10.1016/0303-7207(86)90125-5. [DOI] [PubMed] [Google Scholar]
- Saffouri B., Weir G., Bitar K., Makhlouf G. Stimulation of gastrin secretion from the perfused rat stomach by somatostatin antiserum. Life Sci. 1979 Nov 12;25(20):1749–1753. doi: 10.1016/0024-3205(79)90478-8. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Sato F., Ignotz G. G., Ignotz R. A., Gansler T., Tsukada K., Lieberman I. On the mechanism by which insulin stimulates protein synthesis in chick embryo fibroblasts. Biochemistry. 1981 Sep 15;20(19):5550–5556. doi: 10.1021/bi00522a031. [DOI] [PubMed] [Google Scholar]
- Seal A., Yamada T., Debas H., Hollinshead J., Osadchey B., Aponte G., Walsh J. Somatostatin-14 and -28: clearance and potency on gastric function in dogs. Am J Physiol. 1982 Aug;243(2):G97–102. doi: 10.1152/ajpgi.1982.243.2.G97. [DOI] [PubMed] [Google Scholar]
- Sibrowski W., Seitz H. J. Rapid action of insulin and cyclic AMP in the regulation of functional messenger RNA coding for glucokinase in rat liver. J Biol Chem. 1984 Jan 10;259(1):343–346. [PubMed] [Google Scholar]
- Simard J., Labrie F., Gossard F. Regulation of growth hormone mRNA and pro-opiomelanocortin mRNA levels by cyclic AMP in rat anterior pituitary cells in culture. DNA. 1986 Aug;5(4):263–270. doi: 10.1089/dna.1986.5.263. [DOI] [PubMed] [Google Scholar]
- Straus D. S., Takemoto C. D. Insulin negatively regulates albumin mRNA at the transcriptional and post-transcriptional level in rat hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):1955–1960. [PubMed] [Google Scholar]
- Wolfe M. M., Jain D. K., Reel G. M., McGuigan J. E. Effects of carbachol on gastrin and somatostatin release in rat antral tissue culture. Gastroenterology. 1984 Jul;87(1):86–93. [PubMed] [Google Scholar]
- Wolfe M. M., Reel G. M., McGuigan J. E. Inhibition of gastrin release by secretin is mediated by somatostatin in cultured rat antral mucosa. J Clin Invest. 1983 Nov;72(5):1586–1593. doi: 10.1172/JCI111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P., Glover J. F., Martin S. C., Tenniswood M. P., Williams J. L., Tata J. R. Deinduction of transcription of Xenopus 74-kDa albumin genes and destabilization of mRNA by estrogen in vivo and in hepatocyte cultures. Eur J Biochem. 1985 Feb 1;146(3):489–496. doi: 10.1111/j.1432-1033.1985.tb08678.x. [DOI] [PubMed] [Google Scholar]
- Yamada T., Marshak D., Basinger S., Walsh J., Morley J., Stell W. Somatostatin-like immunoreactivity in the retina. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1691–1695. doi: 10.1073/pnas.77.3.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]