Abstract
Prion diseases are a unique category of illness, affecting both animals and humans, where the underlying pathogenesis is related to a conformational change of a normal self protein called cellular prion protein to a pathological and infectious conformer known as scrapie prion protein (PrPSc). Currently, all prion diseases lack effective treatment and are universally fatal. Past experiences with bovine spongiform encephalopathy and variant Creutzfeldt–Jakob disease mainly in Europe, as well as the current epidemic of chronic wasting disease in North America, have highlighted the need to develop prophylactic and/or therapeutic approaches. In Alzheimer’s disease that, like prion disease, is a conformational neurodegenerative disorder, both passive and active immunization has been shown to be highly effective in model animals at preventing disease and cognitive deficits, with emerging data from human trials suggesting that this approach is able to reduce amyloid-related pathology. However, any immunomodulatory approach aimed at a self-antigen has to finely balance an effective humoral immune response with potential autoimmune toxicity. The prion diseases most commonly acquired by infection typically have the alimentary tract as a portal of infectious agent entry. This makes mucosal immunization a potentially attractive method to produce a local immune response that partially or completely prevents prion entry across the gut barrier, while at the same time producing modulated systemic immunity that is unlikely to be associated with toxicity. Our results using an attenuated Salmonella vaccine strain expressing the prion protein showed that mucosal vaccination can protect against prion infection from a peripheral source, suggesting the feasibility of this approach. It is also possible to develop active and/or passive immunomodulatory approaches that more specifically target PrPSc or target the shared pathological conformer found in numerous conformational disorders. Such approaches could have a significant impact on many of the common age-associated dementias.
Keywords: amyloid-β, chronic wasting disease, conformational disorders, Creutzfeldt–Jakob disease, immunomodulation, neurodegenerative oligomers, prion, vaccine
Prion diseases are conformational neurodegenerative disorders characterized by the structural modification of the normal cellular prion protein (PrPC) into a pathological conformer, scrapie prion protein (PrPSc) [1–3]. They are a unique category of illness in that they can be inherited, infectious or sporadic in occurrence. Thus, the conversion of PrPC to PrPSc can be related to an exogenous infectious source of PrPSc, a mutation in the prion protein that predisposes to such a conformational change or a spontaneous conformational change, as occurs in sporadic prion disease. A comprehensive body of evidence has presented compelling data that the transmissible pathogen for these diseases is a proteinaceous infectious particle (hence the term ‘prion’) [4,3]. These diseases are also known as transmissible spongiform encephalopathies or prionoses. Currently, there is no effective therapy for this group of diseases [5–8]. The outbreak of bovine spongiform encephalopathy (BSE) and the resulting emergence of a new human prion disease, namely variant Creutzfeldt–Jakob disease (vCJD), highlight the public health threat from prion diseases. Although the original outbreak of vCJD is waning, there is the possibility of further outbreaks from current asymptomatic carriers and iatrogenically infected individuals. In North America, an ongoing threat from prion disease is from chronic wasting disease (CWD). High rates of infection among deer and elk populations have been reported, with experimental data indicating that this disease is transmissible to primates [9–11].
The human forms of prionoses include kuru, Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker disease, fatal familial insomnia, sporadic fatal insomnia and the more recently described ‘protease-sensitive prionopathy’ [1,2,12]. In animals these diseases include BSE in cattle, scrapie in sheep and goats, CWD in deer and elk, and transmissible mink encephalopathy [1,2]. Neuropathologically, these different forms of the disease are all characterized by spongiform change, neuronal loss and astrocytosis; in addition, amyloid deposition may occur (Figure 1). However, the regional pattern of brain lesions and the extent of prion amyloid deposition vary within and between species. Within species, these differences depend on the strain of prion causing the infection. A barrier exists limiting transmission of prions across species, but once this barrier is overcome, a new stable and distinct pattern of infection can develop in the new host species.
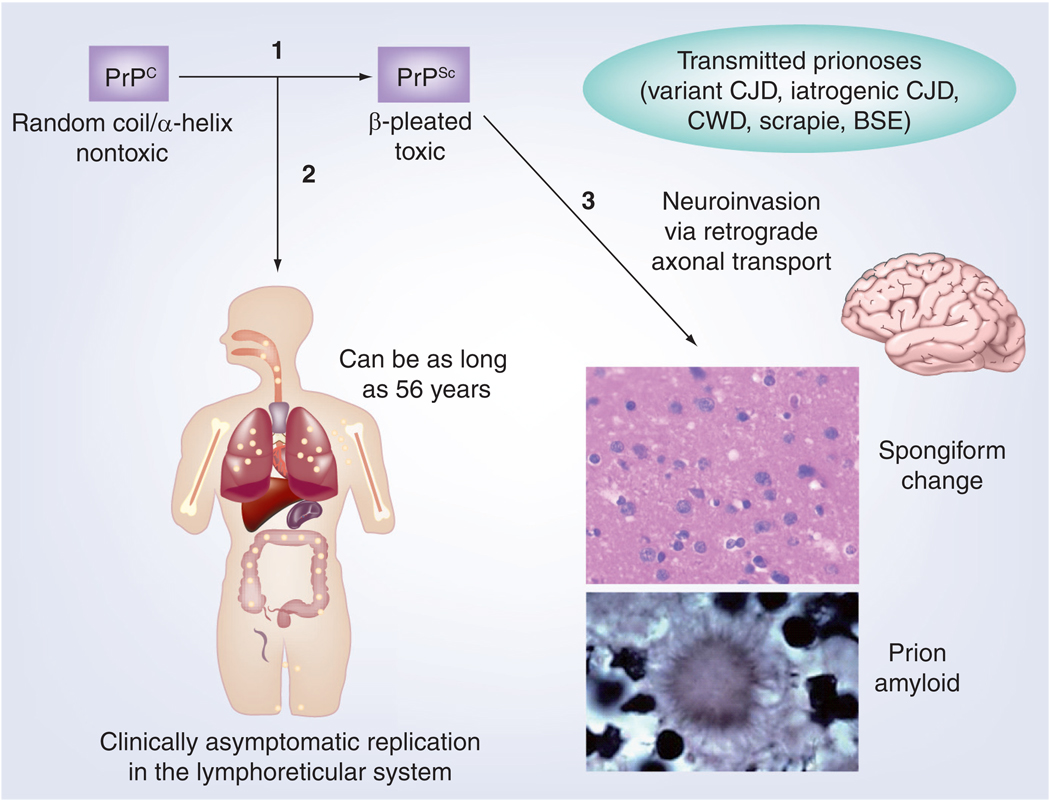
Figure 1. The progression of many forms of prion infection.
(1) The underlying pathogenesis is related to a conformational change of PrPC to PrPSc. (2) Many prion diseases have the gut as a point of entry followed by asymptomatic replication of the agent in the Peyer’s patches, lymph nodes and spleen of the lymphoreticular system. This typically occurs over long periods, which has been documented to last up to 56 years in humans [79]. Effective mucosal immunization can prevent prion agent entry, while an appropriate systemic humoral anti-PrP response can inhibit replication. (3) Ultimately, with PrPSc entry into the CNS, there is the development of the characteristic neuropathology of spongiform change, which may be associated with amyloid deposition. Once clinical symptoms start, progression in the most common human forms of prion disease is very rapid, producing death in approximately 6–9 months.
BSE: Bovine spongiform encephalopathy; CJD: Creutzfeldt–Jakob disease; CWD: Chronic wasting disease; PrPC: Cellular prion protein; PrPSc: Scrapie prion protein.
Adapted with permission from [162].
The protein-only hypothesis of prion disease
The protein-only hypothesis suggests that PrPSc is propagated by serving as a template for the autocatalytic recruitment and conversion of PrPC, with PrPSc being the principal or sole component of the infectious agent [4,13]. Definitive proof of this concept, using only synthetic or recombinant PrP (rPrP) to generate infectivity, has been lacking, despite a wealth of supportive experimental data [1–3,14]. Prion-like behavior has been well documented in a number of chromosomally encoded yeast and fungal proteins, including Ure2, Sup35, Rnq1, HET-s and Swi1, where each of these proteins can adopt distinct and inheritable prion states [15–18]. In these systems it has clearly been shown that β-sheet amyloid fibrils produced in vitro from bacterially-expressed yeast prion proteins introduced into living cells can act alone as self-propagating infectious agents [19,20]. For PrPSc, which has to replicate in a more complex biological environment, such definitive proof has been more elusive; however, a number of recent studies have provided strong suggestive evidence. It has been shown that in transgenic mice, which overexpress PrPC, amyloid fibrils formed from rPrP can cause a transmissible prion disease [21,22]. However, these studies are open to the caveat that the overexpression of PrPC could predispose these mice to a spontaneous transmissible encephalopathy, which is accelerated by the administration of fibrillar rPrP rather than this representing the true infectivity. Prion infectivity has also been propagated under cell free conditions using protein misfolding cyclic amplification (PMCA) [23–25]. This procedure uses successive rounds of sonication and incubation to continually amplify PrPSc employing PrPC present in brain homogenate as a substrate [26]. With each successive round of amplification, the PMCA product is diluted with fresh brain homogenate, with the original seed of PrPSc being virtually eliminated. It has been shown that the PrPSc generated in this manner remains infectious in animals [23]. Later studies have shown that infectious material can also be generated with PMCA using a variety of substrates, including purified brain or cell-derived PrPC with poly(A) RNA [27,28], rPrP mixed with mouse-derived RNA and acidic lipids [29] and recently with rPrP in the absence of cofactors but with proteinase K-digested PrPSc in the first round of amplification [30]. The de novo-generated infectious material from these PMCA experiments has a titer much less than brain extracted PrPSc, so these data cannot yet be considered definitive proof of the protein-only hypothesis. However, the data reinforce the primary importance of PrP to infection. The low titers of PMCA-generated PrPSc suggest that infection most likely depends on the availability of a specific low abundance species, as well as the likely participation of host factors for optimum infectivity. Furthermore, PMCA-generated PrPSc is produced under conditions of limited PrPC, contrasting with the conditions in infected brains. This provides another explanation for the lower titers of PMCA PrPSc. Therefore, immunotherapeutic approaches that limit the availability of PrPC as a substrate, prevent the entry of PrPSc, hinder the interaction of PrPC with PrPSc or increase the clearance of PrPSc are likely to be effective for either the infectious or sporadic forms of prionoses.
Biology of the prion protein
In its mature form following cleavage of N- and C-terminal signal peptides, PrPC is an approximately 210-amino acid protein, which is expressed in many types of cells; however, the highest level of expression is found in CNS neurons and in cells of the immune system [31–33]. The molecular anatomy of PrPC is crucial for understanding its malfunction in prion diseases. The whole protein is located on the outer surface of the cell anchored to the cell membrane by a phosphotidylinositol glycolipid attached to its C-terminus in lipid raft domains. PrPC has an unstructured N-terminus and a globular C-terminal domain, which contains three α-helices, two of which are linked by a disulphide bridge. The N-terminus harbors five octapeptide repeats. Histidines located within the octapeptides bind copper and manganese ions [34,35]. It has been postulated that one possible function of PrPC is in capture, storage and presentation of copper to the neuron [34,36,37]. The levels of copper and manganese influence the aggregation state of PrP. Increased manganese has been shown to enhance prion protein survival and increase infectivity [38], while manganese chelation has been shown to extend survival in a mouse model with prion infection [39]. Copper chelation has also been shown to have therapeutic effects in prion infection [40]; however, the roles of both copper and manganese in prion biology are complex [35,41]. The exact functions of PrPC remain to be fully elucidated. The protein is not essential since Prnp knock-out mice did not show a significant disease phenotype [42]. Minor abnormalities in synaptic physiology [43] and in circadian rhythm [44] using more detailed methods have been described in these knock-out mice. Hence, prion infection pathology is unlikely to be related to loss of PrPC function. Suggested PrPC functions have included anti-apoptotic activity, prevention of oxidative stress, cell adhesion, cell signaling and modulation of synaptic function, and recently antimicrobial activity has also been reported [45–47]. It is possible that PrPC conversion to PrPSc confers a gain of toxic function in one or more of these putative normal roles.
BSE, vCJD & CWD
Interest in prion disease has greatly increased since the emergence of BSE in the UK and the resulting appearance of vCJD in human populations. BSE arose from the feeding of cattle with prion-contaminated meat and bone-meal products, while vCJD developed following the entry of BSE into the human food chain [48,49]. Since the original report in 1995, a total of 217 probable or confirmed cases of vCJD have been diagnosed, 174 cases in the UK, 25 cases in France, five cases in Spain, four cases in Ireland, three in the USA and a few cases elsewhere (for the latest numbers see [201]). It has been difficult to predict the expected future numbers of vCJD. Mathematical analysis has given a broad range from 1000 to approximately 136,000 individuals who will eventually develop the disease [50,51]. This broad range reflects a lack of knowledge regarding the time of incubation and the number of patients who could be infected from a given dosage of a BSE agent. Because the vCJD agent is present at high levels in lymphatic tissue, screening for PrPSc was performed on sections from lymph nodes, tonsils and appendices archived in the UK. Out of 12,674 randomly selected cases, three showed evidence of subclinical infection, leading to a prediction that approximately 4000 further vCJD cases may occur in the UK [52]. However, there is much uncertainty surrounding such a prediction, as it is not known if all subclinical infections will progress to disease or whether such screening of lymphoid tissue would capture all subclinical cases. A complicating factor for estimating future numbers of vCJD is the documentation of several transfusion-associated cases. These occurred after incubation periods of 6–8 years. One of these disease-associated donations was made more than 3 years before the donor became symptomatic, suggesting that vCJD can be transmitted from silently infected individuals [53]. Currently, there is no method for routinely screening blood for possible prion contamination. An additional point of concern relates to the cases with a methionine/valine (MV) genotype at codon 129 of the Prnp gene. So far, all the clinically symptomatic cases of vCJD had the methionine/methionine codon 129 genotype. However, two clinically nonsymptomatic patients with the MV genotype were found to be infected (one from a blood transfusion and the other from a random appendix and tonsil specimen survey of the population) [6,51]. The discovery of such carriers raises the possibility of secondary spread of infection via blood transfusion, surgical procedures or tissue transplants from individuals who more than likely have much longer (or possibly lifelong) asymptomatic infections. Hence, the risk of vCJD originating elsewhere and spreading at least within Europe via blood transfusion remains a possibility. Current BSE surveillance methods are felt by many to be inadequate and there may be asymptomatic carriers of vCJD in the USA who probably acquired their infection elsewhere and are donating to the USA blood supply [54,55]. Furthermore, several atypical strains of scrapie and BSE have been documented with possibly greater transmissibility to humans [56]. It has been suggested that one atypical BSE strain may be responsible for a type of CJD (type MV2) previously thought to be sporadic [6]. Hence, the spectrum of infectious prion disease may widen.
In North America, an emerging prion infection in deer and elk populations, CWD, potentially represents an even greater significant threat to human populations. This disease is endemic in regions of Canada, Colorado (USA), Wyoming (USA) and Nebraska (USA) and continues to spread. Recently, it has been detected as far east as New York State (USA) [10,57,58]. Transmission of CWD is thought to be mainly horizontal via the mucosal/oral route [59,60]. The occurrence of CJD among three young deer hunters from this same region raised the speculation of transmission of CWD to humans [61]. Autopsy of these three subjects did not show the extensive amyloidosis characteristic of vCJD and CWD [62]. However, like BSE, CWD is transmissible to nonhuman primates (squirrel monkeys) [9,11]. CWD has also been shown to be transmissible to sheep, cattle and several North American rodents that can scavenge on CWD carcasses [63–65]. Each of these animals can enter the human food chain directly or, in the case of rodents, by accidental inclusion in grain and forage. To date, studies using transgenic mice expressing human PrPC have failed to show transmission of CWD, suggesting there is a significant species barrier that is greater than the BSE–human barrier [66–68]. On the other hand, two different strains of CWD have recently been identified and it is likely that there are more [69]. Whether any of these other strains for CWD have greater potential for human spread remains unknown. Future transmission studies using nonhuman primates and human PrP-expressing transgenic mice will need to be repeated with all known CWD strains and will also need to take into account PrP polymorphisms in the CWD source and human PrP [70]. Furthermore, CWD prions have been found not only in the brain of infected deer but also in the blood, muscle, feces, fat, urine, antler velvet and saliva [60,71–77]. Therefore, the possibility of transmission to humans needs to be closely monitored. The risk posed to humans by CWD is difficult to estimate [66]. The prevalence of CWD in free-range deer varies from up to 30% in some endemic areas to less than 1% in US states in which CWD has only recently been discovered, such as West Virginia and New York [57,10]. Over 370 hunter-killed deer and elk were identified as CWD-positive by the Colorado Department of Wildlife (USA) between 2003 and 2005 and over 240 CWD-positive hunter-harvested animals were identified by the Wisconsin Division of Natural Resources (USA) between 2002 and 2004 [78]. Overall, over 6.6 million deer and 6.9 million total cervid species are harvested annually in the USA. It is therefore certain that human exposure has occurred and continues to occur, either by direct contact in hunters and game processors, by consumption of venison or by contact with products from cervids. Furthermore, the preclinical period of human prion infection via an oral route can be very long; in the case of kuru, an incubation period of 56 years has been documented [79]. In contrast to the distribution of BSE-infected beef, which would be diluted in the food-processing chain, it is more typical that only a few family members and friends consume venison from a CWD-infected animal, thus leading to a proportionally greater potential exposure. Human exposure to CWD may also occur from contaminated environmental sources; however, there are no data available to estimate the significance of such exposure. The number of CWD-infected deer harvested without knowledge of the animal’s status can be estimated by considering the number of deer harvested annually in the three states in which CWD could be considered endemic (CO, USA; WY, USA; and WI, USA). For example, in 2006 (the year for which the most comprehensive data is available in these states), nearly 45,000 deer were harvested in CO, of which approximately 450 were CWD-positive (based on a statewide prevalence of ~1% in deer). Similar calculations for WI (0.71% of 506,947 deer harvested) and WY (~2% of 40,067 deer harvested) would add another 4400 positive animals, bringing this total to approximately 4900 CWD-infected animals annually (or between 9.3 and 160 CWD-positive deer per 100,000 residents or one CWD-positive deer for every 110–217 licensed hunters in these three states) [80]. A significant new finding is that CWD is able to transmit nasally with high efficacy by aerosol among cervid PrP transgenic mice [81]. This represents the first documentation of prion spread via this respiratory route. Hence, if CWD were to cross the species barrier to humans, it would pose a potentially grave threat, probably far greater than that of vCJD.
Prion disease & other conformational neurodegenerative disorders
The prion diseases belong to a broader category of conformational diseases [82], which include Alzheimer’s disease (AD), diffuse Lewy body disease, Parkinson’s disease, Huntington’s disease and frontotemporal lobar degeneration. In each of these disorders, a normal self-protein/peptide, which has a physiological function, undergoes a conformational change to a pathological conformer that has a high β-sheet content, is resistant to degradation and accumulates either in extracellular plaques or intracellular inclusion bodies, with the most toxic conformers being oligomeric [83]. In AD the normal soluble Aβ and tau are converted to oligomeric/fibrillar Aβ and abnormally phosphorylated tau in neurofibrillary tangles, respectively. A total of 11 different proteins are known to accumulate as oligomers, plaques and/or intracellular inclusions in the CNS leading to various neurodegenerative diseases, with the most common being Aβ, phosphorylated tau, α-synuclein and TAR DNA-binding protein-43 [83,84]. Among patients with a clinical diagnosis of dementia, neuropathological examination reveals that in the majority of cases there is an accumulation of a mixture of different pathological protein conformers, with the most common mix being Aβ, phosphorylated tau and α-synuclein [85]. CJD has also been reported to occur in association with the accumulation of one or more amyloid proteins [86,87] and some case of Gerstmann–Sträussler–Scheinker disease have widespread neurofibrillary tangles similar to AD [88,89]. One explanation for this frequent co-occurrence of age-associated pathologies in a given patient’s brain is that one type of pathological conformer can seed oligomerization/fibrillization in heterologous proteins that are prone to form amyloid, in what has been called abnormal conformational mimicry [90,91]. The spread of neuropathology in all the conformational neurodegenerative disorders has been suggested to be prion-like [92,93]. The major difference between PrPC and PrPSc in terms of its secondary structure is an increase in β-sheet content. Although the insolubility of PrPSc has prevented performance of crystallographic conformational studies, less precise structural methods such as circular dichroism and Fourier transform infrared spectroscopy have indicated a β-sheet content as high as 45% (compared with 3% in PrPC) and an α-helix content of 30% (40% in PrPC) [94,95]. During this conformational change, it is reasonable to assume that certain regions of PrP are exposed that are normally hidden in PrPC. For example, it has been demonstrated that the conversion of recombinant mouse PrP to a more β-sheet-rich form is associated with increased solvent exposure of tyrosine side chains [96]. Two bi-tyrosine pairs found in helix 1 and β-strand 2 in conjunction with a C-terminal arginine create a YYR motif [96]. It has recently been shown that through expansion of this motif and the use of a leukotoxin-based delivery system, it is possible to produce an active vaccine that induces a more PrPSc-specific immune response [97]. The increase in β-sheet content in PrPSc is also a critical characteristic of the pathological conformers of other cerebral amyloid proteins [84]. Thus, development of immunomodulatory therapeutic approaches that specifically target this particular shared pathological conformation of the numerous proteins that produce neurodegeneration has been suggested as a viable approach with many advantages [98,99].
Recently the link between PrP and the other conformational neurodegenerative disorders has been found to be even more extensive. PrPC has been reported to be a receptor for Aβ oligomers, in part mediating their toxicity [100,101]. It has been suggested that PrPC functions as a general receptor for multiple aggregated proteins [102]. Significantly, when AD model transgenic mice are crossed onto a Prnp knock-out background, there is rescue from the expected cognitive decline, despite the fact that the AD-related pathology in terms of amyloid deposition is unaffected [103]. It has recently been shown that the anti-PrP monoclonal antibody 6D11, which blocks the binding between Aβ oligomers and PrPC [100], can be used as a ‘treatment’ for cognitive deficits in an AD transgenic model [104]. Hence, therapeutic downregulation of PrPC may have other benefits besides inhibiting prion infection. However, this is a controversial and complex area of research, since it is likely that Aβ oligomers mediate toxicity via multiple, nonmutually exclusive pathways and the results obtained depend on the experimental setting [105–107].
The immune system & prion infection
Cellular prion protein is expressed in T and B lymphocytes, natural killer cells, platelets, monocytes, dendritic cells (DCs) and follicular DCs [108]. Owin to this expression pattern and the lack of an immune response to a self antigen, the immune system is involved in the peripheral replication of the prion agent and its ultimate access to the CNS [109,110]. Paradoxically, immune suppression with, for example, splenectomy or immunosuppressive drugs, increases the incubation period [109], while nonspecific immunostimulation has the opposite effect [111]. This incubation period during which time the prion agent replicates peripherally without producing any symptoms is quite long, lasting many months in experimental animals and up to 56 years in documented human cases associated with cannibalistic exposure to the prion agent [79]. Lymphatic organs, such as the spleen, tonsils, lymph nodes or gut-associated lymphoid tissue, contain high concentrations of PrPSc long before PrPSc replication starts in the brain (Figure 1) [59,112,113]. Cells found to be particularly important for peripheral PrPSc replication are the follicular DCs and the migratory bone marrow-derived DCs [59,113–115]. DCs from infected animals are capable of spreading the disease [115]. Immunotherapeutic approaches that can overcome the tolerance of these immune cells will probably inhibit prion replication in the lymphorecticular system and ultimately inhibit neuroinvasion; however, a delicate immunomodulation has to be accomplished in order to avoid potential autoimmune toxicity [110,116,117]. A further consideration is that while in most prion diseases infection and replication in the lymphorecticular system shortens the incubation times and facilitates neuroinvasion, this does not appear to be the case in most BSE cases, in sporadic CJD and in some types of scrapie such as the drowsy form of hamster scrapie [118–120]. Hence the potential beneficial effect of altering the immune response to PrP would have to be tailor made, depending on the specific type of prion disease.
Vaccination for prion infection
Currently there is no treatment that would arrest and/or reverse progression of symptomatic prion disease in patients or in non-genetically altered animals, although numerous approaches have been attempted [5,7,8,121]. In the AD mouse model, it has been definitively shown that immunotherapy can prevent the onset of cognitive deficits and the development of amyloid lesions [122]. Significantly, this method of treatment is associated with consistent cognitive benefits in the mice [99,123–126]. An antibody-mediated response is probably critical for a therapeutic response, since similar results have been obtained with passive immunization [127]. Active immunization has been tried in humans for AD by Elan (Dublin, Ireland), with significant toxicity resulting from the vaccine [128–130]. In the human Phase IIA clinical trial of the Elan AD vaccine (called AN-1792), 18 out of 372 patients worldwide developed symptoms of meningitis or meningoencephalitis with symptoms apparently responding to immunosuppression in most patients (12 patients out of the 18 responded fully)[128]. Evidence suggests that patients who developed anti-Aβ titers benefited cognitively from vaccination, including patients among the 12 that initially had complications, although these benefits were modest [128,131]. The lack of more dramatic clinical benefits in these patients has been suggested to be related to vaccination starting too late in the progression of the disease process [132]. Limited autopsy data documented that vaccination resulted in both amyloid clearance and in a reduction in tau-related pathology [133,134]. Hence, it appears that if future protocols can resolve safety issues and the timing at which treatment should begin, a vaccine approach will prove to have important therapeutic value in patients [129,135]. Numerous AD vaccination clinical trials are ongoing [136,137].
Partly because of this success in AD models, similar experiments with anti-PrP antibodies were initiated in prion infectivity culture models, along with active and passive immunization studies in rodent models. Earlier in vivo studies had shown that infection with a slow strain of PrPSc blocked expression of a more virulent fast strain of PrP, mimicking vaccination with a live-attenuated organism [138]. In tissue culture studies, anti-PrP antibodies and antigen-binding fragments directed against PrP have been shown to inhibit prion replication [139–142]. When we first demonstrated that active immunization with recombinant PrP delayed the onset of prion disease in wild-type mice, the therapeutic effect was relatively modest and eventually all the mice succumbed to the disease [143]. This limited therapeutic effect may be explained by the observation that antibodies generated against prokaryotic PrP often do not have a high affinity towards the critical portions of PrPC that are involved in binding and replication [144]; however, in our studies, the increase in the incubation period correlated well with the antibody titers against PrPC. Our follow-up passive anti-PrP immunization study confirmed the importance of the humoral response, showing that anti-PrP antibodies are able to prolong the incubation period [145]. In another study, using higher doses of anti-PrP antibodies, it was shown that prion infection from a peripheral source could be completely prevented if treatment began within 1 month of infection [146]. This type of approach could be used immediately following accidental exposure in humans to prevent future infection. However, passive immunization has not been found to be effective closer to the clinically symptomatic stages of prion infection. Furthermore, passive immunization would be an approach that is too costly for animal prion diseases. The possibility that immunological approaches that reduce levels of PrPC or limit its availability for conversion to PrPSc could be effective closer to the onset of symptomatic disease is supported by a study that showed that the behavioral deficits, impaired neurophysiological function and early hippocampal spongiform pathology of prion infection could be completely rescued by genetically knocking out the expression of endogenous neuronal PrPC [147].
The amalgamation of the aforementioned data suggest that by departing from the classical vaccination approach and by using some of the specific properties of antibodies it would be possible to interfere with one or more stages of the initiation or progression of prionoses. A critical issue is that the self nature of the precursor protein has to be taken into account. Specifically, immunomodulation for prion disease has to overcome tolerance to the original PrP to raise antibodies that will interfere with or neutralize PrP critical binding sites that are involved with one or more of the following functions: facilitation of invasion, promotion of conversion or interactions with host factors. Concurrently, any humoral or cellular autoimmune cytotoxic effect has to be avoided or minimized. A delicate balance between the quality and quantity of therapeutically active immunoglobulin molecules has to be accomplished without knowing a priori what would be the ideal nature of the required immunogen and what could be the best route by which to present it to the immune system.
A potentially ideal means of using immunomodulation to prevent prion infection is by mucosal immunization. One important reason for this is that the gut is the major route of entry for many prion diseases such as CWD, BSE, transmissible mink encephalopathy and vCJD. Furthermore, mucosal immunization can be designed to primarily induce a humoral immune response, avoiding the cell-mediated toxicity that was seen in the human AD vaccine trial [128,136,137]. Recently, we have been developing prion vaccines that target gut-associated tissue, the main site of entry of the prion agent. One of our approaches is to express PrP in attenuated Salmonella strains as a live vector for oral vaccination. Live-attenuated strains of Salmonella enterica have been used for many years as vaccines against salmonellosis and as a delivery system for the construction of multivalent vaccines with broad applications in human and veterinary medicine [148,149]. A main advantage for this system is that the safety of human administration of live-attenuated Salmonella has been extensively confirmed in humans and animals [149–151]. Ruminants and other veterinary species can be effectively immunized by the oral route using live Salmonella to induce humoral mucosal responses [152,153]. Salmonella targets M cells, antigen-sampling cells found in the intestines, which may also be important for the uptake of PrPSc [110,113,154]. Hence, this approach is more targeted than prior vaccination studies, probably explaining the improved efficacy [116,155]. The rationale takes into account that if tolerance is broken, the bulk of the B-cell response will be devoted to producing dimeric secretory IgA in the mucosa, with a discrete – in terms of conventional vaccination experience – systemic IgG level, which will help to maintain a desired number of antibodies with a low risk of autoimmune pathology. As the recombined V region genes would be the ones selected by the gut immune system through mesenteric lymph nodes, and typically produce neutralizing IgA molecules, it is probable that the genes selected and the recombinations would be different from the ones obtained by systemic immunization, with a lower possibility of autoimmune inflammatory complications. Results from experiments using 139A scrapie prions in wild-type CD-1 mice suggest that in animals that have a good anti-PrP mucosal IgA response and a systemic anti-PrP IgG response, full protection against oral challenge with the prion agent is possible [116,155]. Possible mechanisms of action of mucosal immunomodulation are illustrated in Figure 2. Further development of mucosal immunization, which aims for high specificity rather than high antibody response, is likely to lead to an effective means of preventing prion disease in animal and human populations at risk for prion exposure. Trials using this methodology to prevent CWD in mule deer are ongoing. However, this approach has so far not been effective when initiated after prion exposure has already occurred or during the symptomatic stages.
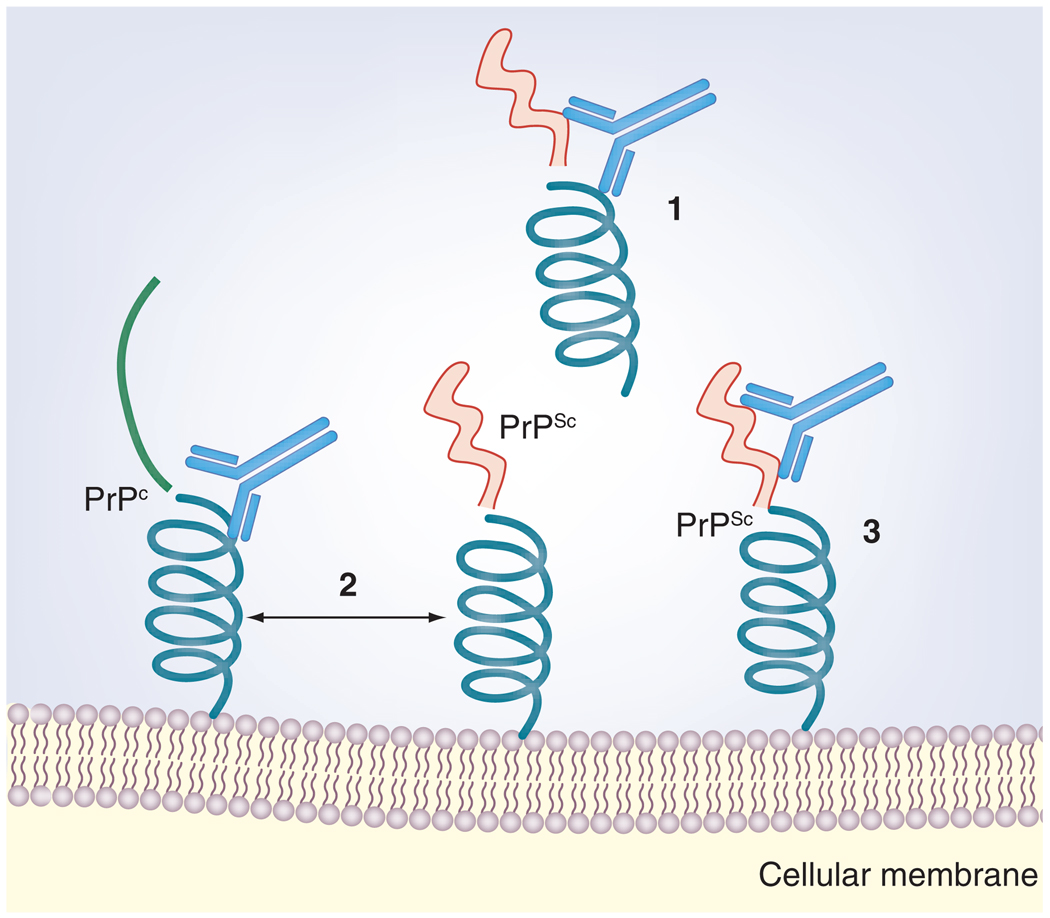
Figure 2. Some possible mechanisms of action of mucosal immunomodulation against PrP.
(1) A secretory IgA response in the gut may prevent entry of the infectious agent across the gut barrier. (2) Antibody binding to PrPC at critical sites may prevent an interaction with PrPSc by blocking the binding sites, steric hindrance, interference with host factors involved in the PrPC/PrPSc conversion and/or stabilization of the PrPC structure. (3) Binding to PrPSc can lead to increased clearance by the immune system, destabilization of the β-pleated sheet structure and/or interference with stabilizing host factors.
PrPC: Cellular prion protein; PrPSc: Scrapie prion protein.
As mentioned previously, passive immunization has also been shown to be effective at inhibiting prion infection when initiated immediately or within 30 days after peripheral prion infection [145,146,156]. Presumably, the lack of greater therapeutic efficacy of these passive immunization approaches is related to the poor blood–brain barrier permeability of the anti-PrP antibodies. Interestingly Song et al. showed therapeutic efficacy with anti-PrP antibodies up to 120 days post-inoculation, using direct intraventricular infusion [157]. However, a potential problem with such an approach is that neuronal apoptosis has been reported to occur in response to some anti-PrP antibodies being directly applied to the CNS [158,159], suggesting that the characteristics of the antibodies chosen for use will be an important factor determining the probability of toxic side effects. As some of the antibodies raised in a mucosal immunization might be effective to neutralize PrPSc invasion, binding, conversion or progression, successfully vaccinated animals can be used to produce monoclonal antibodies with the required therapeutic characteristics. Therapeutic monoclonal antibodies could be humanized and used to prevent potential infection or disease progression. Most probably, more than one monoclonal antibody would be necessary to obtain effective protection. This approach is being actively pursued in our laboratory, as well as by other groups.
Expert commentary
Currently, none of the conformational neurodegenerative disorders have a highly effective therapy. Many studies using AD models have shown that immunotherapeutic approaches can reduce both amyloid and tau-related pathology, which is associated with a cognitive rescue. Recent autopsy and imaging data from clinical trials also suggest that this approach can reduce AD pathology. The prion diseases are much less common than AD; however, the past outbreak of new vCJD originating from BSE and the current epidemic of CWD, with the potential of human transmission, highlight the importance of developing therapies for this group of disorders. Extensive in vitro data and studies using genetically manipulated animal models have suggested the importance of immune intervention and, more recently, a specific immunomodulatory approach was shown to be effective in prion disease, at least for prophylaxis against disease. Since many prion diseases have the mucosa of the alimentary tract as a point of entry, mucosal immunization may be particularly suitable for these forms of prion infection, with recent studies indicating that oral prion infection can be prevented by appropriate mucosal vaccination. Another approach with encouraging preliminary results is to induce active immunization that more specifically targets PrPSc or the specific shared β-sheet conformation of the pathological conformers found in prionoses and other neurodegenerative diseases [99].
Five-year view
Current developments in immunotherapies for prion infection suggest that development of mucosal immunomodulation, which can prevent or inhibit prion infection in at-risk animal or human populations, is a feasible short-term goal. Passive immunization for the prophylaxis of infection following accidental exposure is also a possible short-term goal. However, in the most common human prion disease, sporadic CJD, it is currently not possible to identify patients prior to symptomatic disease. For immunotherapy to be effective at a stage closer to symptomatic disease, and to be amenable for use in these more common cases, methods will need to be developed to more specifically target PrPSc and to obtain better CNS penetration [8,97,160,161]. A non-mutually exclusive possibility is to develop immunomodulation, which produces specific neutralizing antibodies that target the shared pathological conformation common to numerous proteins that accumulate in neurodegenerative disorders, but not to the normal precursor proteins, which could be associated with autoimmune toxicity [99]. This would have significant therapeutic implications for a wide range of common age-associated dementias.
Acknowledgments
This manuscript was supported by NIH grants: 5R01NS047433-06A1S1, 5R01NS047433-07 and 1R01NS073502.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Cobb NJ, Surewicz WK. Prion diseases and their biochemical mechanisms. Biochemistry. 2009;48(12):2574–2585. doi: 10.1021/bi900108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi A, Sigurdson C, Heikenwalder M. Molecular mechanisms of prion pathogenesis. Annu. Rev. Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Neurodegenerative diseases and prions. N. Eng. J. Med. 2001;344(20):1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 5.Trevitt CR, Collinge J. A systematic review of prion therapeutics in experimental models. Brain. 2006;129(Pt 9):2241–2265. doi: 10.1093/brain/awl150. [DOI] [PubMed] [Google Scholar]
- 6.Brown P. Transmissible spongiform encephalopathy in the 21st Century: neuroscience for the clinical neurologist. Neurology. 2008;70(9):713–722. doi: 10.1212/01.wnl.0000302186.10596.f6. [DOI] [PubMed] [Google Scholar]
- 7.Brazier MW, Wall VA, Brazier BW, Masters CL, Collins SJ. Therapeutic interventions ameliorating prion disease. Expert Rev. Anti. Infect. Ther. 2009;7(1):83–105. doi: 10.1586/14787210.7.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Napper S, Cashman NR. Immunotherapy for prion diseases: opportunities and obstacles. Immunotherapy. 2010;2(2):269–282. doi: 10.2217/imt.10.3. [DOI] [PubMed] [Google Scholar]
- 9. Marsh RF, Kincaid AE, Bessen RA, Bartz JC. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus) J. Virol. 2005;79(21):13794–13796. doi: 10.1128/JVI.79.21.13794-13796.2005. • Demonstrates that chronic wasting disease is transmissible to nonhuman primates.
- 10.Sigurdson CJ. A prion disease of cervids: chronic wasting disease. Vet. Res. 2008;39(4):41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 11.Race B, Meade-White KD, Miller MW, et al. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg. Infect. Dis. 2009;15(9):1366–1376. doi: 10.3201/eid1509.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gambetti P, Dong Z, Yuan J, et al. A novel human disease with abnormal prion protein sensitive to protease. Ann. Neurol. 2008;63:697–708. doi: 10.1002/ana.21420. • Description of a novel human prion disease where the scrapie prion protein (PrPSc) is more sensitive to protease digestion.
- 13.Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 14.Collinge J. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry. 2005;76:906–919. doi: 10.1136/jnnp.2004.048660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickner RB. A new prion controls fungal cell fusion incompatibility. Proc. Natl Acad. Sci. USA. 1997;94(19):10012–10014. doi: 10.1073/pnas.94.19.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patino MM, Liu JJ, Glover JR, Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273(5275):622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- 17.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: inherited structures and biological roles. Nat. Rev. Microbiol. 2007;5(8):611–618. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Park KW, Yu H, Fan Q, Li L. Newly identified prion linked to the chromatin-remodeling factor Swi1 in Saccharomyces cerevisiae. Nat. Genet. 2008;40(4):460–465. doi: 10.1038/ng.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428(6980):319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428(6980):323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 21.Legname G, Baskakov IV, Nguyen HO, et al. Synthetic mammalian prions. Science. 2004;305(5684):673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 22.Colby DW, Wain R, Baskakov IV, et al. Protease-sensitive synthetic prions. PLoS. Pathog. 2010;6(1) doi: 10.1371/journal.ppat.1000736. e1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121(2):195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Supattapone S, Deleault NR, Rees JR. Amplification of purified prions in vitro. Methods Mol. Biol. 2008;459:117–130. doi: 10.1007/978-1-59745-234-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000421. e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411(6839):810–813. doi: 10.1038/35081095. • Demonstration of the power of protein misfolding cyclic amplification to increase the level of PrPSc.
- 27.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc. Natl Acad. Sci. USA. 2007;104(23):9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geoghegan JC, Miller MB, Kwak AH, Harris BT, Supattapone S. Trans-dominant inhibition of prion propagation in vitro is not mediated by an accessory cofactor. PLoS Pathog. 2009;5(7) doi: 10.1371/journal.ppat.1000535. e1000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327(5969):1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JI, Cali I, Surewicz K, et al. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J. Biol. Chem. 2010;285(19):14083–14087. doi: 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jendroska K, Heinzel FP, Torchia M, et al. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology. 1991;41(9):1482–1490. doi: 10.1212/wnl.41.9.1482. [DOI] [PubMed] [Google Scholar]
- 32.Kretzschmar H, Prusiner SB, Stowring LE, DeArmond SJ. Scrapie prion protein are synthesized in neurons. Am. J. Pathol. 1986;122:1–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Cashman NR, Loertscher R, Nalbantoglu J, et al. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell. 1990;61(1):185–192. doi: 10.1016/0092-8674(90)90225-4. [DOI] [PubMed] [Google Scholar]
- 34.Brown DR, Qin K, Herms JW, et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 35.Mitteregger G, Korte S, Shakarami M, Herms J, Kretzschmar HA. Role of copper and manganese in prion disease progression. Brain Res. 2009;1292:155–164. doi: 10.1016/j.brainres.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Qin K, Yang Y, Mastrangelo P, Westaway D. Mapping Cu(II) binding sites in prion proteins by diethyl pyrocarbonate modification and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometric footprinting. J. Biol. Chem. 2002;277(3):1981–1990. doi: 10.1074/jbc.M108744200. [DOI] [PubMed] [Google Scholar]
- 37.Qin K, Yang DS, Yang Y, et al. Copper(II)-induced conformational changes and protease resistance in recombinant and cellular PrP. Effect of protein age and deamidation. J. Biol. Chem. 2000;275(25):19121–19131. doi: 10.1074/jbc.275.25.19121. [DOI] [PubMed] [Google Scholar]
- 38.Davies P, Brown DR. Manganese enhances prion protein survival in model soils and increases prion infectivity to cells. PLoS ONE. 2009;4(10):e7518. doi: 10.1371/journal.pone.0007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brazier MW, Volitakis I, Kvasnicka M, et al. Manganese chelation therapy extends survival in a mouse model of M1000 prion disease. J. Neurochem. 2010;14(2):440–451. doi: 10.1111/j.1471-4159.2010.06771.x. [DOI] [PubMed] [Google Scholar]
- 40.Sigurdsson EM, Brown DR, Alim MA, et al. Copper chelation delays the onset of prion disease. J. Biol. Chem. 2003;278:46199–46202. doi: 10.1074/jbc.C300303200. [DOI] [PubMed] [Google Scholar]
- 41.Brown DR. Brain proteins that mind metals: a neurodegenerative perspective. Dalton Trans. 2009;(21):4069–4076. doi: 10.1039/b822135a. [DOI] [PubMed] [Google Scholar]
- 42.Bueler H, Fischer M, Lang Y, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 43.Collinge J, Whittington MA, Sidle KC, et al. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 44.Tobler I, Gaus SE, Deboer T, et al. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380(6575):639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 45.Westergard L, Christensen HM, Harris DA. The cellular prion protein (PrPC): its physiological function and role in disease. Biochim. Biophys. Acta. 2007;1772(6):629–644. doi: 10.1016/j.bbadis.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol. Rev. 2008;88(2):673–728. doi: 10.1152/physrev.00007.2007. [DOI] [PubMed] [Google Scholar]
- 47.Pasupuleti M, Roupe M, Rydengard V, et al. Antimicrobial activity of human prion protein is mediated by its N-terminal region. PLoS ONE. 2009;4(10):e7358. doi: 10.1371/journal.pone.0007358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manson JC, Cancellotti E, Hart P, Bishop MT, Barron RM. The transmissible spongiform encephalopathies: emerging and declining epidemics. Biochem. Soc. Trans. 2006;34(Pt 6):1155–1158. doi: 10.1042/BST0341155. [DOI] [PubMed] [Google Scholar]
- 49.Butler R. Prion diseases in humans: an update. Br. J. Psychiatry. 2006;189:295–296. doi: 10.1192/bjp.bp.106.021832. [DOI] [PubMed] [Google Scholar]
- 50.Smith PG, Cousens SN, d’Huillard Aignaux JN, Ward HJ, Will RG. The epidemiology of variant Creutzfeldt–Jakob disease. Curr. Top. Microbiol. Immunol. 2004;284:161–191. doi: 10.1007/978-3-662-08441-0_7. [DOI] [PubMed] [Google Scholar]
- 51.Ironside JW. Variant Creutzfeldt–Jakob disease. Haemophilia. 2010;16 Suppl. 5:175–180. doi: 10.1111/j.1365-2516.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 52.Hilton DA. Pathogenesis and prevalence of variant Creutzfeldt–Jakob disease. J. Pathol. 2006;208(2):134–141. doi: 10.1002/path.1880. [DOI] [PubMed] [Google Scholar]
- 53.Brown P, Brandel JP, Preese M, Sato T. Iatrogenic Creutzfeldt–Jakob disease: the waning of an era. Neurology. 2006;67(3):389–393. doi: 10.1212/01.wnl.0000231528.65069.3f. [DOI] [PubMed] [Google Scholar]
- 54.Bishop MT, Hart P, Aitchison L, et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5(5):393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 55.Clarke P, Will RG, Ghani AC. Is there the potential for an epidemic of variant Creutzfeldt–Jakob disease via blood transfusion in the UK? J. R. Soc. Interface. 2007;4(15):675–684. doi: 10.1098/rsif.2007.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong Q, Zheng M, Casalone C, et al. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J. Virol. 2008;82(7):3697–3701. doi: 10.1128/JVI.02561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams ES. Chronic wasting disease. Vet. Pathol. 2005;42(5):530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- 58.Aguzzi A, Sigurdson CJ. Antiprion Immunotherapy: to suppress or to stimulate? Nat. Rev. Immunol. 2004;4(9):725–736. doi: 10.1038/nri1437. [DOI] [PubMed] [Google Scholar]
- 59.Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274(3):588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 60.Safar JG, Lessard P, Tamguney G, et al. Transmission and detection of prions in feces. J. Infect. Dis. 2008;198(1):81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belay ED, Maddox RA, Williams ES, Miller MW, Gambetti P, Schonberger LB. Chronic wasting disease and potential transmission to humans. Emerg. Infect. Dis. 2004;10:977–984. doi: 10.3201/eid1006.031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liberski PP, Guiroy DC, Williams ES, Walis A, Budka H. Deposition patterns of disease-associated prion protein in captive mule deer brains with chronic wasting disease. Acta Neuropathol. 2001;102(5):496–500. doi: 10.1007/s004010100417. [DOI] [PubMed] [Google Scholar]
- 63.Hamir AN, Kunkle RA, Cutlip RC, et al. Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J. Vet. Diagn. Invest. 2005;17(3):276–281. doi: 10.1177/104063870501700313. [DOI] [PubMed] [Google Scholar]
- 64.Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES, Richt JA. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J. Vet. Diagn. Invest. 2006;18(6):558–565. doi: 10.1177/104063870601800606. [DOI] [PubMed] [Google Scholar]
- 65.Heisey DM, Mickelsen NA, Schneider JR, et al. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J. Virol. 2010;84(1):210–215. doi: 10.1128/JVI.00560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong Q, Huang S, Zou W, et al. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J. Neurosci. 2005;25(35):7944–7949. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamguney G, Giles K, Bouzamondo-Bernstein E, et al. Transmission of elk and deer prions to transgenic mice. J. Virol. 2006;80(18):9104–9114. doi: 10.1128/JVI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandberg M, Al-Doujaily H, Sigurdson C, et al. Chronic wasting disease prions are not transmissible to transgenic mice over-expressing human prion protein. J. Gen. Virol. 2010;91(Pt 10):2651–2657. doi: 10.1099/vir.0.024380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angers RC, Kang HE, Napier D, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328(5982):1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collinge J. Prion strain mutation and selection. Science. 2010;328:1111–1112. doi: 10.1126/science.1190815. [DOI] [PubMed] [Google Scholar]
- 71.Angers RC, Browning SR, Seward TS, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311(5764):1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 72. Mathiason CK, Powers JG, Dahmes SJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314(5796):133–136. doi: 10.1126/science.1132661. • Demonstration that infectious prions are present at significant levels outside of the CNS in chronic wasting disease.
- 73.Mathiason CK, Hayes-Klug J, Hays SA, et al. B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J. Virol. 2010;84(10):5097–5107. doi: 10.1128/JVI.02169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Race B, Meade-White K, Race R, Chesebro B. Prion infectivity in fat of deer with chronic wasting disease. J. Virol. 2009;83(18):9608–9610. doi: 10.1128/JVI.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE. 2009;4(3):e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamguney G, Miller MW, Wolfe LL, et al. Asymptomatic deer excrete infectious prions in faeces. Nature. 2009;461(7263):529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angers RC, Seward TS, Napier D, et al. Chronic wasting disease prions in elk antler velvet. Emerg. Infect. Dis. 2009;15(5):696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joly DO, Samuel MD, Langenberg JA, et al. Spatial epidemiology of chronic wasting disease in Wisconsin white-tailed deer. J. Wildl. Dis. 2006;42(3):578–588. doi: 10.7589/0090-3558-42.3.578. [DOI] [PubMed] [Google Scholar]
- 79.Collinge J, Whitfield J, McKintosh E, et al. Kuru in the 21st Century – an acquired human prion disease with very long incubation periods. Lancet. 2006;367(9528):2068–2074. doi: 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- 80.Hamir AN, Kunkle RA, Miller JM, Greenlee JJ, Richt JA. Experimental second passage of chronic wasting disease (CWD[mule deer]) agent to cattle. J. Comp. Pathol. 2006;134(1):63–69. doi: 10.1016/j.jcpa.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 81. Denkers ND, Seelig DM, Telling GC, Hoover EA. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J. Gen. Virol. 2010;91(Pt 6):1651–1658. doi: 10.1099/vir.0.017335-0. • First demonstration that a prion disease may spread via aerosol, suggesting the high transmissible potential of chronic wasting disease.
- 82.Wisniewski T, Sigurdsson EM. Therapeutic approaches for prion and Alzheimer’s diseases. FEBS J. 2007;274:3784–3798. doi: 10.1111/j.1742-4658.2007.05919.x. [DOI] [PubMed] [Google Scholar]
- 83.Jellinger KA. Recent advances in our understanding of neurodegeneration. J. Neural. Transm. 2009;116(9):1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- 84.Rostagno A, Holton JL, Lashley T, Revesz T, Ghiso J. Cerebral amyloidosis: amyloid subunits, mutants and phenotypes. Cell Mol. Life Sci. 2010;67(4):581–600. doi: 10.1007/s00018-009-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 86.Haraguchi T, Terada S, Ishizu H, et al. Coexistence of Creutzfeldt–Jakob disease, Lewy body disease, and Alzheimer’s disease pathology: an autopsy case showing typical clinical features of Creutzfeldt–Jakob disease. Neuropathology. 2009;29(4):454–459. doi: 10.1111/j.1440-1789.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida H, Terada S, Ishizu H, et al. An autopsy case of Creutzfeldt–Jakob disease with a V180I mutation of the PrP gene and Alzheimer-type pathology. Neuropathology. 2010;30(2):159–164. doi: 10.1111/j.1440-1789.2009.01048.x. [DOI] [PubMed] [Google Scholar]
- 88.Ghetti B, Tagliavini F, Giaccone G, et al. Familial Gerstmann–Straussler–Scheinker disease with neurofibrillary tangles. Mol. Neurobiol. 1994;8(1):41–48. doi: 10.1007/BF02778006. [DOI] [PubMed] [Google Scholar]
- 89.Bugiani O, Giaccone G, Piccardo P, Morbin M, Tagliavini F, Ghetti B. Neuropathology of Gerstmann–Straussler–Scheinker disease. Microsc. Res. Tech. 2000;50(1):10–15. doi: 10.1002/1097-0029(20000701)50:1<10::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 90.Wisniewski T, Golabek AA, Kida E, Wisniewski KE, Frangione B. Conformational mimicry in Alzheimer’s disease. Role of apolipoproteins in amyloidogenesis. Am. J. Pathol. 1995;147(2):238–244. [PMC free article] [PubMed] [Google Scholar]
- 91.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and α-synuclein. Science. 2003;300(5619):636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 92.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010;11(3):155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010;11(4):301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan KM, Baldwin M, Njuyen J, et al. Conversion of α-helices into β-sheets features in the formation of scrapie prion poteins. Proc. Natl Acad. Sci. USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aucouturier P, Kascsak RJ, Frangione B, Wisniewski T. Biochemical and conformational variability of human prion strains in sporadic Creutzfeldt–Jakob disease. Neurosci. Lett. 1999;274:33–36. doi: 10.1016/s0304-3940(99)00659-x. [DOI] [PubMed] [Google Scholar]
- 96.Paramithiotis E, Pinard M, Lawton T, et al. A prion protein epitope selective for the pathologically misfolded conformation. Nat. Med. 2003;9(7):893–899. doi: 10.1038/nm883. [DOI] [PubMed] [Google Scholar]
- 97.Hedlin PD, Cashman NR, Li L, et al. Design and delivery of a cryptic PrPC epitope for induction of PrPSc-specific antibody responses. Vaccine. 2010;28(4):981–988. doi: 10.1016/j.vaccine.2009.10.134. [DOI] [PubMed] [Google Scholar]
- 98.Wisniewski T, Prelli F, Scholtzova H, et al. Immunotherapy targeting abnormal protein conformation. Alz. Dementia. 2009;5(4) Suppl. 1:P113. [Google Scholar]
- 99. Goni F, Prelli F, Ji Y, et al. Immunomodulation targeting abnormal protein conformation reduces pathology in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5(10):e13391. doi: 10.1371/journal.pone.0013391. • First demonstration of an effective active immunomodulation that generates an immune response that targets abnormal protein conformation.
- 100. Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. • First demonstration that the cellular prion protein (PrPC) may be a receptor for amyloid-β oligomers, mediating, in part, their toxicity.
- 101.Gunther EC, Strittmatter SM. β-amyloid oligomers and cellular prion protein in Alzheimer's disease. J. Mol. Med. 2010;88:331–338. doi: 10.1007/s00109-009-0568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aguzzi A, O’Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010;9(3):237–248. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 103.Gimbel DA, Nygaard HB, Coffey ET, et al. Memory impairment in transgenic alzheimer mice requires cellular prion protein. J. Neurosci. 2010;30(18):6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung E, Ji Y, Sun Y, et al. Anti-PrPC monoclonal antibody infusion as a novel treatment for Aβ oligomer cognitive cognitive deficits. BMC Neurosci. 2010;11:130. doi: 10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calella AM, Farinelli M, Nuvolone M, et al. Prion protein and Aβ-related synaptic toxicity impairment. EMBO Mol. Med. 2010;2(8):306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benilova I, De SB. Prion protein in Alzheimer’s pathogenesis: a hot and controversial issue. EMBO Mol. Med. 2010;2(8):289–290. doi: 10.1002/emmm.201000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-β. Nature. 2010;466(7308):E3–E4. doi: 10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aguzzi A, Heikenwalder M. Prions, cytokines, and chemokines: a meeting in lymphoid organs. Immunity. 2005;22(2):145–154. doi: 10.1016/j.immuni.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Aucouturier P, Carp RI, Carnaud C, Wisniewski T. Prion diseases and the immune system. Clin. Immunol. 2000;96:79–85. doi: 10.1006/clim.2000.4875. [DOI] [PubMed] [Google Scholar]
- 110.Sigurdsson EM, Wisniewski T. Promising developments in prion immunotherapy. Exp. Rev. Vaccines. 2005;4:607–610. doi: 10.1586/14760584.4.5.607. [DOI] [PubMed] [Google Scholar]
- 111.Bremer J, Heikenwalder M, Haybaeck J, et al. Repetitive immunization enhances the susceptibility of mice to peripherally administered prions. PLoS ONE. 2009;4(9):e7160. doi: 10.1371/journal.pone.0007160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown KL, Ritchie DL, McBride PA, Bruce ME. Detection of PrP in extraneural tissues. Microsc. Res. Tech. 2000;50(1):40–45. doi: 10.1002/1097-0029(20000701)50:1<40::AID-JEMT7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 113.Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat. Rev. Microbiol. 2006;4(3):201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 114.Kitamoto T, Muramoto T, Mohri S, Doh-ura K, Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt–Jakob disease. J. Virol. 1991;65(11):6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aucouturier P, Geissmann F, Damotte D, et al. Infected dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Invest. 2001;108:703–708. doi: 10.1172/JCI13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goni F, Prelli F, Schreiber F, et al. High titers of mucosal and systemic anti-PrP antibodies abrogates oral prion infection in mucosal vaccinated mice. Neuroscience. 2008;153:679–686. doi: 10.1016/j.neuroscience.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wisniewski T, Chabalgoity JA, Goni F. Is vaccination against transmissible spongiform encephalopathies feasible? OIE Sci. Tech. Rev. 2007;26(1):243–251. [PubMed] [Google Scholar]
- 118.Bartz JC, DeJoia C, Tucker T, Kincaid AE, Bessen RA. Extraneural prion neuroinvasion without lymphoreticular system infection. J. Virol. 2005;79(18):11858–11863. doi: 10.1128/JVI.79.18.11858-11863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bessen RA, Martinka S, Kelly J, Gonzalez D. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J. Virol. 2009;83(13):6435–6445. doi: 10.1128/JVI.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siso S, Gonzalez L, Jeffrey M. Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Neurosci. Interdiscip. Perspect. Infect. Dis. 2010;2010:747892. doi: 10.1155/2010/747892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muller-Schiffmann A, Korth C. Vaccine approaches to prevent and treat prion infection: progress and challenges. BioDrugs. 2008;22(1):45–52. doi: 10.2165/00063030-200822010-00005. [DOI] [PubMed] [Google Scholar]
- 122.Wisniewski T, Sigurdsson EM. Murine models of Alzheimer’s disease and their use in developing immunotherapies. Biochim. Biophys. Acta. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morgan D, Diamond DM, Gottschall PE, et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 124.Janus C, Pearson J, McLaurin J, et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 125.Sigurdsson EM, Knudsen EL, Asuni A, et al. An attenuated immune response is sufficient to enhance cognition in an Alzheimer’s disease mouse model immunized with amyloid-β derivatives. J. Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asuni A, Boutajangout A, Scholtzova H, et al. Aβ derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer’s model mice. Eur. J. Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of alzheimer disease. Nat. Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 128.Gilman S, Koller M, Black RS, et al. Clinical effects of Aβ immunization (AN1792) in patients with AD in an interupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 129.Wisniewski T, Frangione B. Immunological and anti-chaperone therapeutic approaches for Alzheimer’s disease. Brain Pathol. 2005;15:72–77. doi: 10.1111/j.1750-3639.2005.tb00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wisniewski T. Commentary on ‘Clinical effects of Aβ immunization (AN1792) in patients with AD in an interrupted trial’. Nat. Clin. Prac. Neurol. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 131.Hock C, Konietzko U, Straffer JR, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 132.Wisniewski T, Boutajangout A. Vaccination as a therapeutic approach for Alzheimer’s disease. Mt. Sinai J. Med. 2010;77:17–31. doi: 10.1002/msj.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boche D, Donald J, Love S, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Aβ42 immunisation in Alzheimer's disease. Acta Neuropathol. 2010;120(1):13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- 134.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled Phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 135.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol. 2006;6(5):404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 136.Wisniewski T, Konietzko U. Amyloid-β immunization for Alzheimer's disease. Lancet Neurol. 2008;7(9):805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nat. Rev. Neurol. 2010;6(2):108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manuelidis L. Vaccination with an attenuated Creutzfeldt–Jakob disease strain prevents expression of a virulent agent. Proc. Natl Acad. Sci. USA. 1998;95(5):2520–2525. doi: 10.1073/pnas.95.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heppner FL, Musahl C, Arrighi I, et al. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science. 2001;294(5540):178–182. doi: 10.1126/science.1063093. [DOI] [PubMed] [Google Scholar]
- 140.Enari M, Flechsig E, Weissmann C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl Acad. Sci. USA. 2001;98(16):9295–9299. doi: 10.1073/pnas.151242598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peretz D, Williamson RA, Kaneko K, et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature. 2001;412(6848):739–743. doi: 10.1038/35089090. [DOI] [PubMed] [Google Scholar]
- 142.Pankiewicz J, Prelli F, Sy MS, et al. Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur. J. Neurosci. 2006;24:2635–2647. doi: 10.1111/j.1460-9568.2006.04805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sigurdsson EM, Brown DR, Daniels M, et al. Vaccination delays the onset of prion disease in mice. Am. J. Pathol. 2002;161:13–17. doi: 10.1016/S0002-9440(10)64151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Polymenidou M, Heppner FL, Pellicioli EC, et al. Humoral immune response to native eukaryotic prion protein correlates with anti-prion protection. Proc. Natl. Acad. Sci. USA. 2004;101 Suppl. 2:14670–14676. doi: 10.1073/pnas.0404772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sigurdsson EM, Sy MS, Li R, et al. Anti-PrP antibodies for prophylaxis following prion exposure in mice. Neurosci. Lett. 2003;336:185–187. doi: 10.1016/s0304-3940(02)01192-8. [DOI] [PubMed] [Google Scholar]
- 146.White AR, Enever P, Tayebl M, et al. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature. 2003;422:80–83. doi: 10.1038/nature01457. [DOI] [PubMed] [Google Scholar]
- 147. Mallucci GR, White MD, Farmer M, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53(3):325–335. doi: 10.1016/j.neuron.2007.01.005. • Demonstrated that turning off the expression of PrPC can reverse cognitive deficits in prion-infected mice. This suggests that lowering the level of PrPC, even in symptomatic disease, is a viable therapeutic target.
- 148.Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet. J. 2001;161(2):132–164. doi: 10.1053/tvjl.2000.0502. [DOI] [PubMed] [Google Scholar]
- 149.Moreno M, Kramer MG, Yim L, Chabalgoity JA. Salmonella as live trojan horse for vaccine development and cancer gene therapy. Curr. Gene Ther. 2010;10(1):56–76. doi: 10.2174/156652310790945566. [DOI] [PubMed] [Google Scholar]
- 150.Tacket CO, Sztein MB, Wasserman SS, et al. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 2000;68(3):1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kirkpatrick BD, McKenzie R, O’Neill JP, et al. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine. 2006;24(2):116–123. doi: 10.1016/j.vaccine.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 152.Villarreal-Ramos B, Manser J, Collins RA, Dougan G, Chatfield SN, Howard CJ. Immune responses in calves immunised orally or subcutaneously with a live Salmonella typhimurium aro vaccine. Vaccine. 1998;16(1):45–54. doi: 10.1016/s0264-410x(97)00156-4. [DOI] [PubMed] [Google Scholar]
- 153.Chabalgoity JA, Moreno M, Carol H, Dougan G, Hormaeche CE. A dog-adapted Salmonella Typhimurium strain as a basis for a live oral Echinococcus granulosus vaccine. Vaccine. 2000;19:460–469. doi: 10.1016/s0264-410x(00)00197-3. [DOI] [PubMed] [Google Scholar]
- 154.Heppner FL, Christ AD, Klein MA, et al. Transepithelial prion transport by M cells. Nat. Med. 2001;7(9):976–977. doi: 10.1038/nm0901-976. [DOI] [PubMed] [Google Scholar]
- 155. Goni F, Knudsen EL, Schreiber F, et al. Mucosal vaccination delays or prevents prion infection via an oral route. Neuroscience. 2005;133:413–421. doi: 10.1016/j.neuroscience.2005.02.031. • This paper first demonstrated that mucosal immunization is able to prevent prion infection from an oral source in wild-type mice.
- 156.Sadowski MJ, Pankiewicz J, Prelli F, et al. Anti-PrP Mab 6D11 suppresses PrPSc replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo. Neurobiol. Dis. 2009;34:267–278. doi: 10.1016/j.nbd.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song CH, Furuoka H, Kim CL, et al. Effect of intraventricular infusion of anti-prion protein monoclonal antibodies on disease progression in prion-infected mice. J. Gen. Virol. 2008;89(Pt 6):1533–1544. doi: 10.1099/vir.0.83578-0. [DOI] [PubMed] [Google Scholar]
- 158.Solforosi L, Criado JR, McGavern DB, et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science. 2004;303(5663):1514–1516. doi: 10.1126/science.1094273. [DOI] [PubMed] [Google Scholar]
- 159.Lefebvre-Roque M, Kremmer E, Gilch S, et al. Toxic effects of intracerebral PrP antibody administration during the course of BSE infection in mice. Prion. 2007;1(3):198–206. doi: 10.4161/pri.1.3.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campana V, Zentilin L, Mirabile I, et al. Development of antibody fragments for immunotherapy of prion diseases. Biochem. J. 2009;418(3):507–515. doi: 10.1042/BJ20081541. [DOI] [PubMed] [Google Scholar]
- 161.Stanker LH, Serban AV, Cleveland E, et al. Conformation-dependent high-affinity monoclonal antibodies to prion proteins. J. Immunol. 2010;185(1):729–737. doi: 10.4049/jimmunol.0902930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scott KA, Daggett V. Folding mechanisms of proteins with high sequence identity but different folds. Biochemistry. 2007;46(6):1545–1556. doi: 10.1021/bi061904l. [DOI] [PubMed] [Google Scholar]
Website
- 201.Variant Creutzfeldt–Jakob disease :current data. 2010 October; www.cjd.ed.ac.uk/vcjdworld.htm.