Abstract
Objective: To explore the effects of exposure to subcutaneous (sc) interferon (IFN) beta-1a on efficacy in patients with relapsing–remitting multiple sclerosis (RRMS) enrolled in the PRISMS (Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) study.
Methods: Patients with RRMS received IFN beta-1a, 44 or 22 µg sc three times weekly (tiw), or placebo, for 2 years, at which point placebo recipients were re-randomized to IFN beta-1a, 44 or 22 µg sc tiw, for a further 2–4 years. Long-term follow-up visits occurred 7–8 years after enrolment and allowed participation of patients who had previously discontinued treatment. Post hoc descriptive analyses were conducted within the lower (MIN) and upper (MAX) quartiles of patients divided according to cumulative dose of IFN beta-1a and cumulative time on treatment. Outcomes were explored in patients initially randomized to IFN beta-1a, 44 µg sc tiw, who had received continuous or noncontinuous therapy during the study.
Results: For both cumulative dose and time analyses, the MIN and MAX groups comprised 96 and 95 patients, respectively. The continuous and noncontinuous groups included 45 and 91 patients, respectively. The MAX DOSE and MAX TIME groups had lower annualized relapse rates, lower rates of conversion to secondary progressive MS, lower percentages of patients with Expanded Disability Status Scale progression, higher percentages of relapse-free patients, and less T2 burden of disease than the MIN groups. The continuous therapy group had a lower annualized relapse rate and lower percentages of patients with Expanded Disability Status Scale progression or conversion to secondary progressive MS than the noncontinuous therapy group.
Conclusions: The findings of these post hoc analyses suggest that high exposure to sc IFN beta-1a may be associated with better clinical outcomes than low exposure, and also highlight the importance of maximizing adherence. Additional prospective investigation is warranted to evaluate further the effects of treatment exposure on outcomes and to determine the benefits of interventions to improve adherence.
Keywords: adherence, interferon beta-1a, long-term follow up, PRISMS, relapsing–remitting multiple sclerosis
Introduction
Multiple sclerosis (MS) affects an estimated 2.1 million people worldwide [National Multiple Sclerosis Society, 2010; McDonald, 2000] and is the most common disabling neurological disease among young adults, particularly those of Northern European origin [Murray, 2006; McDonald, 2000]. The primary goals of current treatments for MS are to prevent lesion formation in the central nervous system, to decrease the rate and severity of relapses and to delay the resulting disability [Hartung, 2009]. Progression of MS can be slowed by early treatment with disease-modifying drugs (DMDs) [Tintore, 2007; Kappos et al. 2006; PRISMS Study Group and University of British Columbia MS/MRI Analysis Group, 2001]. In particular, there is compelling evidence to support the use of high-dose, high-frequency interferon (IFN) beta-1a, administered subcutaneously (sc) [Kappos et al. 2006; Panitch et al. 2005, 2002; Schwid et al. 2005], which has been shown to provide an effective treatment option for relapsing–remitting MS (RRMS), to have a well-established long-term safety and tolerability profile [Kappos et al. 2006; PRISMS Study Group and University of British Columbia MS/MRI Analysis Group, 2001; PRISMS Study Group, 1998], and to be associated with a favourable benefit-to-risk profile [Freedman et al. 2008; Goodin et al. 2007]. However, long-term administration of injectable DMDs may be burdensome and lead to suboptimal treatment adherence. The main barriers to adherence are problems with injecting, perceived lack of efficacy and adverse events [Costello et al. 2008].
Among the various analyses that have examined issues related to adherence to DMD therapy in patients with MS, one retrospective study of patient records reported that the risk of severe relapse decreased with higher rates of adherence [Meletiche et al. 2008a, 2008b] and that gaps in DMD therapy of 90 or more days were associated with an increased risk of severe relapse compared with shorter gaps [Al-Sabbagh et al. 2008]. It has been estimated that approximately 50% of patients who discontinue DMD treatment do so within the first 2 years of therapy [Costello et al. 2008]. Indeed, a retrospective cohort analysis of patients at a US practice found that 26% of the patients studied discontinued DMDs within 4 months of initiating treatment, a percentage that increased to 43% within 14 months [Lafata et al. 2008]. In addition, ‘gap days’ (defined as days on which the patient did not have the medication on hand, as derived from supply information) accounted for 31% of the total number of days during the 24-month treatment period [Lafata et al. 2008]. The current lack of accurate, direct measures of treatment adherence means that such analyses have to rely on indirect measures and indicators of adherence to therapy, such as medication possession ratios.
Results from the PRISMS (Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) long-term follow-up (LTFU) study showed that sc IFN beta-1a provides sustained benefits in terms of relapse rate, disability, and magnetic resonance imaging (MRI) outcomes for up to 8 years [Kappos et al. 2006]. This LTFU dataset, therefore, provides an opportunity to examine the hypothesis that cumulative exposure to DMD therapy (as an indicator of treatment adherence) is an important predictor of treatment outcome. We conducted exploratory post hoc analyses to investigate clinical efficacy outcomes in patients with RRMS receiving sc IFN beta-1a for up to 8 years, according to total cumulative dose exposure, total cumulative time on therapy and continuous versus noncontinuous treatment.
Methods
Study design
The design and conduct of the PRISMS study have been described previously [Kappos et al. 2006; PRISMS Study Group and University of British Columbia MS/MRI Analysis Group, 2001; PRISMS Study Group, 1998]. In summary, during the initial 2-year, double-blind phase, patients with RRMS were assigned randomly to receive IFN beta-1a, either 44 or 22 µg sc three times weekly (tiw), or placebo (years 1–2). Patients originally randomized to placebo were then re-randomized to IFN beta-1a, 44 or 22 µg sc tiw, for an additional 2 years (years 3–4). At the end of year 4, all patients were given the choice of continuing to receive blinded or open-label treatment for a further 2 years (years 5–6). After withdrawal or completion of 6 years on study, treatment was open label such that patients could take any or no DMD as per standard clinical practice. Patients were eligible for enrolment in the LTFU phase if they had been randomized to treatment in the original study, regardless of whether they had subsequently withdrawn from the study. A single LTFU assessment took place close to the seventh or eighth anniversary of their baseline visit and included a neurological evaluation as well as a retrospective review of data (including treatment exposure and safety data) collected since the 4-year assessment [Kappos et al. 2006].
Clinical assessments throughout PRISMS included relapse rate and Expanded Disability Status Scale (EDSS) progression. Conversion to secondary progressive MS (SPMS) was defined as an endpoint and assessed at LTFU only. At LTFU, relapse counts were based on data collected during the first 4 years plus retrospective data collected at LTFU. EDSS progression was defined as an increase in score of ≥1.0 point (or 0.5 points for those with a baseline score of ≥6.0) that was not associated with a relapse. For progression to be confirmed, the increase had to be maintained for at least 3 months, although progression reported at the time of the LTFU assessment was assumed to be confirmed (as there was no subsequent follow-up visit). Conversion to SPMS was defined as progressive deterioration of disability for at least 12 months and confirmed EDSS progression of ≥1.0 point (0.5 point if the baseline score was ≥6.0) that was not associated with a relapse, following an initial relapsing–remitting disease course.
Post hoc exploratory analyses
Patient data from the three original study arms (placebo; IFN beta-1a, 44 µg sc tiw; or IFN beta-1a, 22 µg sc tiw) were pooled and ranked from lowest to highest cumulative dose exposure to sc IFN beta-1a, calculated as follows: (dose of IFN beta-1a) × (frequency of application) × (period of application, in weeks). Clinical and MRI outcomes were compared between the minimum (lowest quartile, MIN) and maximum (highest quartile, MAX) dose exposure groups, and included annualized relapse rate, proportion of patients free from relapse, proportion of patients with EDSS progression, proportion of patients who converted to SPMS and percentage change in T2 burden of disease.
Similar post hoc analyses were performed based on estimated MIN and MAX cumulative time on sc IFN beta-1a (from baseline to LTFU visit), which was estimated as the date of the last dose minus the date of the first dose (using data from the three original study arms). As these were exploratory post hoc analyses, no inferential statistical comparisons were made, and the results are summarized using descriptive statistics.
Exploratory analyses were also conducted to compare clinical outcomes between two subgroups of patients within the population randomized to IFN beta-1a, 44 µg sc tiw, on study day 1. To avoid confounding by cumulative dose, these subgroups consisted of patients who had received only the 44-µg dose. The ‘continuous’ subgroup consisted of all patients who had been randomized on study day 1 to IFN beta-1a, 44 µg sc tiw, and who had remained on that dose until the LTFU visit, with no interruptions and no other DMDs taken. A patient was considered to have had a treatment interruption if their time on treatment was less than their time on study. The ‘non-continuous’ subgroup consisted of all patients who had been randomized on study day 1 to IFN beta-1a, 44 µg sc tiw, and who had some medication interruptions, irrespective of other DMDs taken. Clinical outcomes were assessed in the two subgroups and included annualized relapse rate, proportion of patients free from relapse, proportion of patients with EDSS progression, proportion of patients who converted to SPMS and percentage increase in T2 burden of disease. As in the other exploratory analyses, only descriptive statistics were applied.
Results
Patient disposition, baseline characteristics and treatment exposure
The original PRISMS study enrolled 560 patients (31% male, 69% female), of whom 184 were randomized to IFN beta-1a, 44 µg sc tiw; 189 to IFN beta-1a, 22 µg sc tiw; and 187 to placebo. The LTFU visit was completed by 382 patients (68.2% of the original 560 randomized patients), of whom 105 (27.5%) were men and 277 (72.5%) were women. Of these 382 patients, 36 (9.4%) had been treated with other DMDs for any length of time after withdrawal or completion of 6 years on study. When ranked by cumulative IFN beta-1a dose exposure, there were 96 patients in the MIN quartile and 95 in the MAX quartile. A total of 15 (15.6%) and 63 (66.3%) patients, respectively, were still on treatment at the LTFU visit (Table 1). When ranked by cumulative time on therapy, there were also 96 patients in the MIN quartile and 95 in the MAX quartile. At LTFU, 4 (4.2%) and 71 (74.7%) patients, respectively, were still on therapy. The distribution of patients over the quartiles analyzed, and hence the overlap of patients between the cumulative dose quartiles and cumulative time quartiles, is shown in Table 2.
Table 1.
Numbers of patients on treatment within each of the lowest (MIN) and highest (MAX) quartiles, by cumulative dose or time on treatment in the population completing the LTFU visit.
| Randomized: n = 382 Male (M) n = 105 (27.5%); Female (F) n = 277 (72.5%) |
||||
|---|---|---|---|---|
| Cumulative dose analysis: patients on treatment |
Cumulative time analysis: patients on treatment |
|||
| MIN quartile (n) | MAX quartile (n) | MIN quartile (n) | MAX quartile (n) | |
| Day 1 | 96 M 20.8%; F 79.2% | 95 M 32.6%; F 67.4% | 96 M 19.8%; F 80.2% | 95 M 34.7%; F 65.3% |
| Year 2 | 80 (83.3%) | 94 (98.9%) | 78 (81.3%) | 93 (97.9%) |
| Year 4 | 49 (51.0%) | 94 (98.9%) | 45 (46.9%) | 93 (97.9%) |
| Year 6 | 23 (24.0%) | 69 (72.6%) | 12 (12.5%) | 72 (75.8%) |
| LTFU | 15 (15.6%) M 33.3%; F 67.7% | 63 (66.3%) M 34.9%; F 65.1% | 4 (4.2%) M 25.0%; F 75.0% | 71 (74.7%) M 33.8%; F 66.2% |
F, female; LTFU, long-term follow up; M, male; MAX, maximum (highest quartile); MIN, minimum (lowest quartile).
Table 2.
The distribution of patients over the quartiles analyzed, showing the overlap of patients between the cumulative dose quartiles and cumulative time quartiles.
| Cumulative dose of sc IFN beta-1a | Cumulative time on sc IFN beta-1a |
Total, n | |||
|---|---|---|---|---|---|
| 1st quartile [MIN], n | 2nd quartile, n | 3rd quartile, n | 4th quartile [MAX], n | ||
| 1st quartile [MIN], n | 69 | 26 | 1 | 0 | 96 |
| 2nd quartile, n | 18 | 27 | 25 | 25 | 95 |
| 3rd quartile, n | 9 | 32 | 31 | 24 | 96 |
| 4th quartile [MAX], n | 0 | 10 | 39 | 46 | 95 |
| Total, n | 96 | 95 | 96 | 95 | 382 |
IFN, interferon; MAX, maximum (highest quartile); MIN, minimum (lowest quartile); sc, subcutaneous.
Baseline demographic characteristics for the MIN and MAX cumulative dose groups were comparable, but with a higher percentage of women and a shorter time since MS onset in the MIN DOSE group (Table 3). Likewise, baseline demographic and clinical characteristics were generally similar between MIN and MAX cumulative time groups, although in the MIN TIME group there was a higher percentage of women, and the population was slightly younger, with a slightly shorter time since MS onset. For the analysis of cumulative dose, the MAX group predominantly comprised patients originally randomized to IFN beta-1a, 44 µg sc tiw (Table 3). For the analysis of cumulative time on treatment, the MIN TIME group comprised a greater proportion of patients originally randomized to the 22-µg dose of IFN beta-1a or placebo, whereas the MAX TIME group included only patients originally randomized to IFN beta-1a, 44 or 22 µg sc tiw.
Table 3.
Baseline demographic and clinical characteristics, original randomization groups, and treatment exposure in patients completing the long-term follow-up visit.
| Characteristic | Cumulative dose of sc IFN beta-1a |
Cumulative time on sc IFN beta-1a |
Noncontinuous versus continuous IFN beta-1a 44 µg sc tiw |
|||
|---|---|---|---|---|---|---|
| MIN | MAX | MIN | MAX | Noncontinuous | Continuous | |
| (n = 96) | (n = 95) | (n = 96) | (n = 95) | (n = 91) | (n = 45) | |
| Age, years | 33.8 ± 7.4 | 35.8 ± 7.7 | 33.8 ± 7.4 | 36.4 ± 6.6) | 34.9 ± 7.9 | 36.4 ± 7.8 |
| Median (range) | 32.9 (21–50) | 36.1 (19–49) | 33.4 (20–50) | 36.1 (21–50) | 34.7 (20–51) | 37.5 (19–49) |
| Women, n (%) | 76 (79.2) | 64 (67.4) | 77 (80.2) | 62 (65.3) | 70 (76.9) | 28 (62.2) |
| Race, n (%) | ||||||
| White | 95 (99.0) | 95 (100) | 95 (99.0) | 95 (100) | 90 (98.9) | 45 (100) |
| Other | 1 (1.0) | 0 | 1 (1.0) | 0 | 1 (1.1) | 0 |
| Time since first exacerbationa, years | 1.53 ± 0.42 | 1.57 ± 0.40 | 1.53 ± 0.47 | 1.58 ± 0.38 | 1.59 ± 0.43 | 1.54 ± 0.41 |
| Median (range) | 1.58 (0.4–2.4) | 1.61 (0.3–2.3) | 1.64 (0.4–2.4) | 1.65 (0.6–2.3) | 1.64 (0.3–2.3) | 1.60 (0.6–2.3) |
| Time since MS onset, years | 6.47 ± 5.25 | 7.72 ± 6.15 | 6.67 ± 5.38 | 7.56 ± 5.36 | 8.02 ± 6.16 | 8.06 ± 6.39 |
| Median (range) | 4.50 (1.1–23.2) | 6.20 (0.6–34.4) | 5.17 (1.3–25.7) | 6.38 (0.6–23.5) | 6.52 (0.6–25.7) | 7.58 (1.3–34.4) |
| EDSS score at baseline | 2.40 ± 1.24 | 2.42 ± 1.23 | 2.41 ± 1.24 | 2.39 ± 1.14 | 2.30 ± 1.27 | 2.59 ± 1.16 |
| Median (range) | 2.25 (0.0–5.5) | 2.0 (0.0–5.0) | 2.25 (0.0–5.5) | 2.0 (0.0–5.0) | 2.0 (0.0–5.0) | 2.50 (1.0–5.0) |
| MS relapses within past 2 years | 2.9 ± 1.2 | 2.9 ± 0.9 | 3.0 ± 1.2 | 2.9 ± 0.8 | 2.8 ± 1.1 | 2.9 ± 0.9 |
| Median (range) | 3.0 (2–8) | 3.0 (1–5) | 3.0 (2–8) | 3.0 (1–5) | 3.0 (2–8) | 3.0 (1–5) |
| Original randomization, n (%) | ||||||
| Placebo | 54 (56.3) | 4 (4.2) | 53 (55.2) | 0 | 0 | 0 |
| IFN beta-1a 22 µg | 28 (29.2) | 0 | 22 (22.9) | 42 (44.2) | 0 | 0 |
| IFN beta-1a 44 µg | 14 (14.6) | 91 (95.8) | 21 (21.9) | 53 (55.8) | 91 (100) | 45 (100) |
| Cumulative time exposure, weeks | 172.42 ± 102.55 | 375.25 ± 26.37 | 156.78 ± 87.66 | 390.27 ± 5.87 | 311.53 ± 105.06 | 385.70 ± 8.81 |
| Cumulative dose exposure, mg | 10.79 ± 5.98 | 46.60 ± 4.56 | 13.65 ± 9.37 | 38.28 ± 10.97 | 34.01 ± 13.53 | 49.43 ± 2.59 |
Values are means ± standard deviations unless indicated otherwise.
The length of time between the patient’s first MS exacerbation and their entry into the study.
EDSS, Expanded Disability Status Scale; IFN, interferon; MAX, maximum (highest quartile); MIN, minimum (lowest quartile); MS, multiple sclerosis; sc, subcutaneous; tiw, three times weekly.
The analyses of continuous versus noncontinuous treatment were conducted in a total of 136 patients who had been originally randomized to IFN beta-1a, 44 µg sc tiw. A total of 45 patients had received continuous treatment and 91 had received noncontinuous treatment during the course of the study. Baseline demographic and clinical characteristics were generally similar between these two subgroups (Table 3).
Patients in the MAX DOSE group had a mean cumulative dose exposure that was approximately 4.3-fold greater and a mean cumulative time exposure that was approximately 2.2-fold greater than in the MIN DOSE group (Table 3). The mean cumulative dose exposure was approximately 2.8-fold greater, and the mean cumulative time exposure was approximately 2.5-fold greater in the MAX TIME group compared with the MIN TIME group. Mean cumulative dose exposure was approximately 1.5-fold greater in the continuous group, compared with the noncontinuous group.
Clinical outcomes
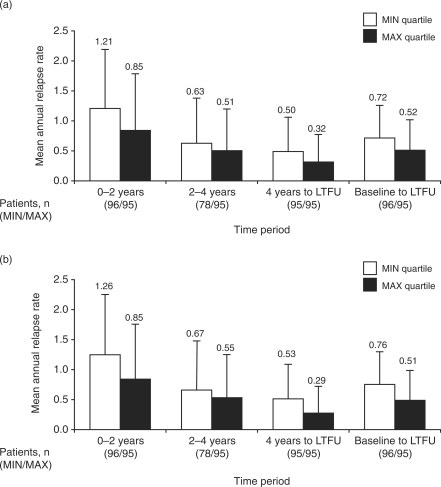
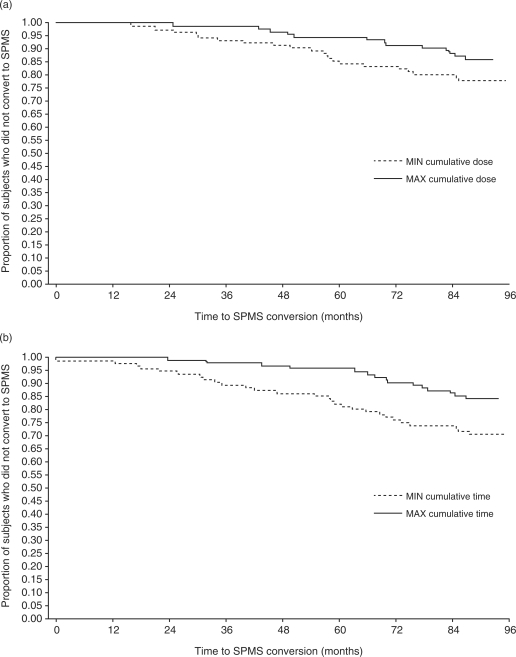
Mean annualized relapse rates were lower among patients in the MAX DOSE group than in the MIN DOSE group (Figure 1(a)). The proportion of patients who did not convert to SPMS was also greater in the MAX DOSE group than in the MIN DOSE group (Figure 2(a)). In addition, the MAX DOSE group had a higher percentage of relapse-free patients, lower percentage of patients with 3-month confirmed EDSS progression and a smaller mean increase in T2 burden of disease (Table 4). In the MIN DOSE group, conversion to SPMS occurred before treatment cessation in 5 patients and after treatment cessation in 16 patients, whereas progression to EDSS score ≥6 occurred before treatment cessation in 16 patients and after treatment cessation in 9 patients.
Figure 1.
Mean (standard deviation) annualized relapse rates in patients in the lowest (MIN) and highest (MAX) quartiles of (a) cumulative dose of subcutaneous (sc) interferon (IFN) beta-1a and (b) cumulative time on sc IFN beta-1a. LTFU, long-term follow up.
Figure 2.
Kaplan–Meier analysis of time conversion to secondary progressive multiple sclerosis (SPMS) in patients in the highest (MIN) and lowest (MAX) quartiles of (a) cumulative dose of subcutaneous (sc) interferon (IFN) beta-1a and (b) cumulative time on sc IFN beta-1a.
Table 4.
Measures of disease activity (baseline to LTFU) in the highest and lowest quartiles of patients, by cumulative dose or time, and continuous or noncontinuous sc IFN beta-1a treatment.
| Cumulative dose of sc IFN beta-1a |
Cumulative time on sc IFN beta-1a |
Noncontinuous versus continuous IFN beta-1a, 44 µg sc tiw |
||||
|---|---|---|---|---|---|---|
| MIN | MAX | MIN | MAX | Noncontinuous | Continuous | |
| (n = 96) | (n = 95) | (n = 96) | (n = 95) | (n = 91) | (n = 45) | |
| Mean ( ± SD) annualized relapse rate | 0.72 ± 0.55 | 0.52 ± 0.51 | 0.76 ± 0.55 | 0.51 ± 0.49) | 0.61 ± 0.56 | 0.51 ± 0.54 |
| Median (range) | 0.67 (0.00–2.34) | 0.396 (0.00–2.53) | 0.68 (0.00–2.57) | 0.394 (0.00–2.53 | 0.52 (0.00–2.34) | 0.27 (0.00–2.53) |
| Relapse-free, n (%) of patients | 7 (7.3) | 15 (15.8) | 7 (7.3) | 16 (16.8) | 15 (16.5) | 6 (13.3) |
| 3-month confirmed EDSS progression, n (%) of patients | 62 (64.6) | 51 (53.7) | 65 (67.7) | 51 (53.7) | 59 (64.8) | 21 (46.7) |
| Converted to SPMS, n (%) of patients | 21 (21.9) | 13 (13.7) | 28 (29.2) | 15 (15.8) | 23 (25.3) | 5 (11.1) |
| Mean ( ± SD) change in T2 burden of disease, % | 68.0 ± 159.0a | 28.2 ± 77.4b | 86.2 ± 183.1c | 44.3 ± 119.5d | 41.3 ± 145.9 | 32.8 ± 72.2 |
| Median (range) | 21.3 (−51.9–1054.8) | 3.7 (−64.7–413.9) | 23.5 (−51.9–1054.8) | 1.5 (−64.7–651.1) | 3.2 (−64.7–1054.8) | 6.2 (−54.3–304.2) |
Data are for patients completing the LTFU.
an = 82; bn = 92; cn = 78; dn = 91.
EDSS, Expanded Disability Status Scale; IFN, interferon; LTFU, long-term follow up; MAX, maximum (highest quartile); MIN, minimum (lowest quartile); sc, subcutaneous; SD, standard deviation; SPMS, secondary progressive multiple sclerosis; tiw, three times weekly.
Patients in the MAX TIME group had a lower mean annualized relapse rate than did patients in the MIN TIME group, over all time periods assessed (Figure 1(b)). Consistent with the analysis of cumulative dose exposure, the MAX TIME group had a higher percentage of patients who did not convert to SPMS (Figure 2(b)), higher percentage of relapse-free patients, lower percentage of patients with 3-month confirmed EDSS progression and a smaller mean increase in T2 burden of disease from baseline to LTFU (Table 4). In the MIN TIME group, conversion to SPMS occurred before treatment cessation in 8 patients and after treatment cessation in 20 patients, whereas progression to EDSS score ≥6 occurred before treatment cessation in 20 patients and after treatment cessation in 10 patients.
The mean annualized relapse rate was lower in the continuous group than in the non-continuous group (Table 4). In addition, the group receiving continuous therapy had a lower percentage of patients with 3-month confirmed EDSS progression and a lower percentage of patients converting to SPMS.
Discussion
The importance of adherence to long-term therapies in the optimal management of chronic diseases has long been recognized by the World Health Organization, which has stated that increasing the effectiveness of adherence interventions may have a far greater impact on population health than improvements in specific medical treatments [World Health Organization, 2003]. By extension, there is a clear need for reliable, accurate measures of treatment adherence, without which advances in biomedical technology would not be able to realize their potential in reducing disease burden. This is clearly exemplified by the challenges of measuring adherence in patients with MS. In the absence of more direct measures of adherence, our analytical approach suggests that that higher levels of sc IFN beta-1a exposure (in terms of both dose and time) and continuous treatment, both of which may provide an indication of adherence to therapy, may be associated with better outcomes than lower cumulative dose/time on treatment or noncontinuous therapy in patients with MS.
In this post hoc analysis of clinical and MRI outcomes data from the PRISMS LTFU study, patients in the upper quartiles (MAX groups) of cumulative dose and time on sc IFN beta-1a had more favourable outcomes than did patients in the corresponding lower quartiles (MIN groups). Specifically, patients in the MAX groups experienced lower annualized relapse rates, lower rates of conversion to SPMS, lower risk of EDSS progression, greater likelihood of freedom from relapse and less T2 burden of disease. Similarly, this post hoc analysis suggests that continuous therapy may be associated with better outcomes than noncontinuous therapy.
Our findings are consistent with those of published studies. For example, an analysis of a US managed care database of information on patients taking intramuscular IFN beta-1a, sc IFN beta-1a, sc IFN beta-1b or glatiramer acetate showed that patients with long gaps (≥90 days) in their treatment had almost a twofold greater risk of severe relapse compared with patients with a treatment gap of 0–10 days [Al-Sabbagh et al. 2008]. A separate analysis of the same database also found that decreased adherence was associated with increased risk of severe relapse, whether adherence was assessed by consistence, persistence or medication possession ratio [Meletiche et al. 2008a, 2008b]. Other studies have shown that high-dose, high-frequency treatment with IFN beta is associated with better outcomes than low-dose, low-frequency treatment [Schwid and Panitch, 2007; Kappos et al. 2006; Durelli and Clerico, 2005; Panitch et al. 2005, 2002; PRISMS Study Group and University of British Columbia MS/MRI Analysis Group, 2001; PRISMS Study Group, 1998]. Three recently published analyses of patients treated for up to 15 or 16 years with other DMDs (intramuscular IFN beta-1a, glatiramer acetate or IFN beta-1b) also support long-term DMD therapy in MS [Bermel et al. 2010; Ebers et al. 2010; Ford et al. 2010]. Our findings, combined with those of previous studies, illustrate the importance of adherence with DMD therapy in patients with MS in order to achieve high and consistent drug exposure and thus improved outcomes. No conclusions regarding high-frequency versus low-frequency treatment can be drawn from the present post hoc analysis, as dose frequency was not analyzed separately from cumulative dose exposure. It should also be noted that there was a large overlap between the MIN DOSE and MIN TIME groups and also between the MAX DOSE and MAX TIME groups: 69/96 (72%) of patients were in the lowest quartiles for both cumulative dose and cumulative time, while 48% of patients (46/95) were in the highest quartiles for both cumulative dose and cumulative time.
As the MIN groups included many patients originally assigned to placebo, the differences in outcome measures observed between MIN and MAX groups may also be due to early initiation of treatment in some patients (those originally randomized to active treatment) and later initiation in others (those originally randomized to placebo and subsequently switched to active treatment). Indeed, patients receiving early treatment with sc IFN beta-1a have been shown to have better outcomes than those initially given placebo and switching to active treatment at the end of year 2 [Oger et al. 2005].
Although these results are informative and supportive of previous evidence, a number of limitations of the present analyses should be noted. These were exploratory, post hoc analyses of historical data from nonrandomized patient groups of small samples; hence, statistical comparisons were not appropriate, and any conclusions can only be drawn with caution. Differences between groups may have been due to between-group differences in demographic characteristics or disease severity at baseline, or to other confounding factors. Notably, standard deviations around mean values at baseline were widely spread. Some differences in baseline characteristics were noted between the quartiles of patients with highest and lowest exposure to sc IFN beta-1a. In particular, patients in the MIN groups were more likely to be female and have a slightly shorter time since MS onset than were patients in the MAX groups; patients with a shorter time since MS onset are more likely to be in the early phase of MS. In addition, although the return rate was high, 31.8% of patients enrolled in the original study did not return for the LTFU visit; however, there were no apparently meaningful differences in baseline characteristics or behaviour during the PRISMS study between patients who returned for the LTFU visit and those who did not [Kappos et al. 2006].
In the total population of patients included in the LTFU analyses, the percentages of men and women (27.5% and 72.5%, respectively) were similar to those in the original population enrolled in the PRISMS study (31% and 69%, respectively) and representative of disease epidemiology in general [Noseworthy et al. 2000]. However, in the LTFU population, the percentage of women was lower in the MAX DOSE group (67.4%) and MAX TIME group (65.3%) than in the MIN groups (79.2% and 80.2%, respectively). A similar pattern was seen for the percentages of women in the continuous group (62.2%) and noncontinuous group (76.9%). Possible reasons for these differences could include differences in drug effects, disease state or attitudes toward therapy between men and women.
Another limitation that potentially confounds the observed association between treatment and clinical effect in LTFU studies is whether disease progression is a consequence or cause of cessation of therapy. In this post hoc analysis, most patients who converted to SPMS in the MIN groups did so after discontinuation of therapy, making it less likely that these groups were predominantly biased towards treatment nonresponders. However, the majority of EDSS progression in the MIN groups occurred before treatment cessation. For both parameters, the number of patients involved was too small to draw any conclusions in this respect.
To summarize, although the direction of causality cannot be established clearly, and other possible contributing factors may be involved, the findings from our exploratory analyses reinforce the importance of high levels of treatment exposure in patients with MS receiving IFN beta and maintaining uninterrupted treatment over the long term. Indeed, our analyses suggest that patients with high levels of exposure to sc IFN beta-1a (based on cumulative dose or cumulative time on treatment) had lower rates of relapse and EDSS progression and were less likely to convert to SPMS than were patients with low levels of exposure to sc IFN beta-1a. These findings have significant clinical, psychosocial and socioeconomic implications and, together with the results of previous studies, provide a strong rationale for attempting to maximize adherence to sc IFN beta-1a treatment in MS.
Strategies to help improve adherence will hinge upon the relationships between patients and their key healthcare professionals. Careful selection of the most appropriate starting therapy, close management of patient expectations and more accurate and systematic measures of adherence and monitoring of patient progress are all crucial to maximizing adherence to therapy. Treatments with flexible dosing regimens, convenient routes of administration and good tolerability profiles will further help to improve adherence.
Prospective, controlled long-term studies, in which adherence is measured systematically, specifically and objectively, are warranted to evaluate further the effects of treatment exposure on clinical outcomes in patients with RRMS.
Acknowledgements
The authors wish to thank Stephen Jones of ACUMED, Matthew Evans of Caudex Medical (both supported by Merck Serono S.A. – Geneva, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany), and Sarah-Jane Loveday (Merck Serono S.A. – Geneva, Switzerland) for editorial assistance in the development of this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The results presented here are from a post hoc analysis of clinical and MRI outcomes data from the PRISMS LTFU study, which was supported by Merck Serono S.A. – Geneva, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany.
The PRISMS Study Group comprised the following participating clinics and investigators: University Hospital, London, Canada (G.C. Ebers, G. Rice, J. Lesaux); Vancouver Hospital and University of British Columbia. Vancouver, Canada (D. Paty, J. Oger, D.K.B. Li, S. Beall, V. Devonshire, S. Hashimoto, J. Hooge, L. Kastrukoff, C. Krieger, M. Mezei, P. Seland, G. Vorobeychi, W. Morrison, J. Nelson); Ottawa General Hospital, Ottawa, Canada (M.S. Freedman, S. Chrisie, R. Nelson, H. Rabinovitch, C. Freedman); Neurologische Universitätsklinik u Poliklinik in Kopfklinikum, Würzburg, Germany (H.P. Hartung, P. Rieckmann, J. Archelos, S. Jung, F. Weilbach, P. Flachenecker, J. Sauer); Stichting MS Centrum, Nijmegen, Netherlands (O. Hommes, P. Jongen, S. Brouwer); University of Sydney, Sydney, Australia (J. McLeod, J. Pollard, R. Ng); Lund University Hospital, Lund, Sweden (M. Sandberg-Wollheim, K. Källén, P. Nilsson, R. Ekberg, A. Lundgren, G. Jadbäck); University Central Hospital, Helsinki, Finland (J. Wikström, J. Multanen, M. Valjakka); Gasthuisberg, Leuven, Belgium (H. Carton, F. Lissoir, I. Declerq, M. Vieren, E. Peeters, B. Dubois, E. Dekeersmaeker, A. Van Herle); Guy’s Hospital, London, UK (R.A.C. Hughes, B. Sharrack, S. Soudain); Turku University Central Hospital, Turku, Finland (M. Panelius, J. Erälinna, M. Soilu-Hänninen, S. Murto); Universitaire Campus, Dr L Willems Instituut, Diepenbeek, Belgium (R. Medaer, J. Broeckx, E. Vanroose, A. Bogaers); University Hospital, Queen’s Medical Centre, Nottingham, UK (L.D. Blumhardt, S. Edwards, C. Liu, V. Orpe); Atkinson Morley’s Hospital, London, UK (D. Barnes, M. Schwartz, N. Stoy, C. Harraghy); Free University Hospital, Amsterdam, Netherlands (F. Bertelsmann, B. Uitdehaag, K. Nasseri); Hôpital Cantonal Universitaire, Geneva, Switzerland (M. Chofflon, S. Roth); Kantonsspital Basel, Universitätskliniken, Basel, Switzerland (L. Kappos, S. Huber, Y. Bellaiche, C. Senn); Royal Melbourne Hospital, Melbourne, Australia (J. King, J. Joubert, S. Whitten); Radcliffe Infirmary, University of Oxford, Oxford, UK (J. M. Newsom-Davis, J. Palace, M. Lee, N. Evangelou, A. Pinto, A. Cavey); Clinique Universitaire St-Luc, Brussels, Belgium (C.J.M. Sindic, P. Monteyne, D. Verougstraete); Academisch Ziekenhuis Dijkzigt, Rotterdam, Netherlands (P.A. Van Doorn, W. Moll, L. Visser, M. Willems, I. Martina, D. Buljevac, L. Loman); Royal Victoria Infirmary, Newcastle upon Tyne, UK (D. Bates, D. Pandit, J. Irving); University of British Columbia, MS/MRI Analysis Research Group, Vancouver, Canada (D.K.B. Li, B. Rhodes, A. Riddehough, G.J. Zhao, X. Wang, Y. Cheng); Ares-Serono International SA, Geneva, Switzerland (N. Ammoury, F. Dupont, A. Galazka, R. Hyde, M. Olson, M.-O. Pernin, A.K. Abdul-Ahad).
Conflict of interest statement
Bernard Uitdehaag has, in the past 12 months, received personal compensation for consultancy activities from Novartis, Merck Serono, and Danone Research.
Cris Constantinescu has, in the past 12 months, served on the Teva advisory board and received research support from Teva, Merck Serono, Biogen Idec, Novartis, and UCB.
Peter Cornelisse is an employee of Merck Serono S.A. – Geneva, Switzerland (an affiliate of Merck KGaA, Darmstadt, Germany).
Douglas Jeffery has received research support and honoraria, for speaking and consulting, from Bayer, Biogen, Teva, Serono, Pfizer, GSK, Acorda, and Novartis.
Ludwig Kappos has, in the past 12 months, served as principal investigator, member or chair of steering committees or advisory boards in corporate-sponsored clinical trials in MS, and other neurological diseases for Acorda Therapeutics, Actelion Pharmaceuticals, Allozyne, BaroFold, Bayer Healthcare Pharmaceuticals, Bayer Schering Pharma, Bayhill, Biogen Idec, Boehringer-Ingelheim, Eisai, Elan, Genmab, GlaxoSmithKline, Merck Serono, MediciNova, Novartis, Sanofi-Aventis, Santhera Pharmaceuticals, Shire, Roche, Teva, UCB, and Wyeth. Dr Kappos has also lectured at medical conferences or in public on various aspects of the diagnosis and management of MS. In many cases, these talks were sponsored by non-restricted educational grants from one or more of the above listed companies. Honoraria and other payments from these activities have been exclusively used for funding of research in his department. Research and clinical operations (nursing and patient care services) of the MS Centre in Basel have been supported by non-restricted grants from one or more of these companies and by grants from the Swiss MS Society, The Swiss National Research Foundation, the European Union, Gianni Rubatto, Novartis, and Roche Research Foundations.
David Li has, in the past 12 months, been a consultant with Genzyme and a speaker for Teva Neurosciences Educational Symposium. Dr Li is the Director of the University of British Columbia, MS/MRI Research Group, which has been contracted to perform a central analysis of MRI scans for therapeutic trials with Bayer, Berlex-Schering, Bio-MS, Daiichi, Genzyme, Hoffmann–La Roche, Merck Serono, and Sanofi-Aventis.
Magnhild Sandberg-Wollheim has, in the past 12 months, received honoraria from Serono Symposia International Foundation (lectures), Merck Serono (lectures, work in data safety monitoring boards DSMB), Genentech (DSMB), Elan (advisory board), and SEB Enskilda (lectures; [a bank in Stockholm]). She has also received honoraria for serving on the board of directors of Active Biotech in Lund, Sweden (a biotech company), and as external reviewer of PhD thesis at the University of Copenhagen.
Anthony Traboulsee has, in the past 12 months, received personal compensation from Merck Serono for data safety monitoring board membership and for co-chairing a symposium; received personal compensation from Bayer Healthcare for speaking; received personal compensation from Teva Neurosciences for chairing a symposium; and received personal compensation from Neura as a member of an editorial advisory board.
Elisabetta Verdun is an employee of Merck Serono S.A. – Geneva, Switzerland (an affiliate of Merck KGaA, Darmstadt, Germany).
Victor Rivera has, in the past 12 months, received sponsorship from EMD Serono; received grants from Bayer, Biogen Idec, Teva Neurosciences, and the National MS Society; served as a consultant/advisor to Biogen Idec; and served as a member of the speakers' bureau for Bayer, Biogen Idec, EMD Serono, Stendahl Neuroscience, Teva Neuroscience, the National Multiple Sclerosis Society and the Consortium of MS Centers.
References
- Al-Sabbagh A., Bennet R., Kozma C., Dickson M., Meletiche D. (2008) Medication gaps in disease-modifying therapy for multiple sclerosis are associated with an increased risk of relapse: findings from a national managed care database. J Neurol 255(Suppl. 2): S79–S79 [Google Scholar]
- Bermel R.A., Weinstock-Guttman B., Bourdette D., Foulds P., You X., Rudick R.A. (2010) Intramuscular interferon beta-1a therapy in patients with relapsing–remitting multiple sclerosis: a 15-year follow-up study. Mult Scler 16: 588–596 [DOI] [PubMed] [Google Scholar]
- Costello K., Kennedy P., Scanzillo J. (2008) Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med 10: 225–225 [PMC free article] [PubMed] [Google Scholar]
- Durelli L., Clerico M. (2005) The importance of maintaining effective therapy in multiple sclerosis. J Neurol 252(Suppl. 3): iii38–iii43 [DOI] [PubMed] [Google Scholar]
- Ebers G.C., Traboulsee A., Li D., Langdon D., Reder A.T., Goodin D.S., et al. (2010) Analysis of clinical outcomes according to original treatment groups 16 years after the pivotal IFNB-1b trial. J Neurol Neurosurg Psychiatry 81: 907–912 [DOI] [PubMed] [Google Scholar]
- Ford C., Goodman A.D., Johnson K., Kachuck N., Lindsey J.W., Lisak R., et al. (2010) Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler 16: 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M.S., Hughes B., Mikol D.D., Bennett R., Cuffel B., Divan V., et al. (2008) Efficacy of disease-modifying therapies in relapsing remitting multiple sclerosis: a systematic comparison. Eur Neurol 60: 1–11 [DOI] [PubMed] [Google Scholar]
- Goodin D.S., Biermann L.D., Bohlega S., Boiko A., Chofflon M., Gebeily S., et al. (2007) Integrating an evidence-based assessment of benefit and risk in disease-modifying treatment of multiple sclerosis. Curr Med Res Opin 23: 2823–2832 [DOI] [PubMed] [Google Scholar]
- Hartung H.P. (2009) High-dose, high-frequency recombinant interferon beta-1a in the treatment of multiple sclerosis. Expert Opin Pharmacother 10: 291–309 [DOI] [PubMed] [Google Scholar]
- Kappos L., Traboulsee A., Constantinescu C., Eralinna J.P., Forrestal F., Jongen P., et al. (2006) Long-term subcutaneous interferon beta-1a therapy in patients with relapsing–remitting MS. Neurology 67: 944–953 [DOI] [PubMed] [Google Scholar]
- Lafata J.E., Cerghet M., Dobie E., Schultz L., Tunceli K., Reuther J., et al. (2008) Measuring adherence and persistence to disease-modifying agents among patients with relapsing remitting multiple sclerosis. J Am Pharm Assoc 48: 752–757 [DOI] [PubMed] [Google Scholar]
- McDonald W.I. (2000) Relapse, remission, and progression in multiple sclerosis. N Engl J Med 343: 1486–1487 [DOI] [PubMed] [Google Scholar]
- Meletiche D., Dickson M., Kozma C., Okuda D.T., Fincher C., Bennett R., et al. (2008a) Association between adherence with multiple sclerosis disease-modifying therapy and severe relapses using three measures of medication adherence. J Neurol 255(Suppl. 2): P717–P717 [Google Scholar]
- Meletiche D., Kozma C., Bennett R., Al-Sabbagh A. (2008b) Relationship between severe relapses and adherence to disease-modifying therapy in multiple sclerosis patients. J Neurol 255(Suppl. 2): P826–P826 [Google Scholar]
- Murray T.J. (2006) Diagnosis and treatment of multiple sclerosis. BMJ 332: 525–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society (2010). Who gets MS? http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx (accessed 28 June 2010).
- Noseworthy J.H., Lucchinetti C., Rodriguez M., Weinshenker B.G. (2000) Multiple sclerosis. N Engl J Med 343: 938–952 [DOI] [PubMed] [Google Scholar]
- Oger J., Francis G., Chang P. (2005) Prospective assessment of changing from placebo to IFN beta-1a in relapsing MS: the PRISMS study. J Neurol Sci 237: 45–52 [DOI] [PubMed] [Google Scholar]
- Panitch H., Goodin D., Francis G., Chang P., Coyle P., O'Connor P., et al. (2005) Benefits of high-dose, high-frequency interferon beta-1a in relapsing–remitting multiple sclerosis are sustained to 16 months: final comparative results of the EVIDENCE trial. J Neurol Sci 239: 67–74 [DOI] [PubMed] [Google Scholar]
- Panitch H., Goodin D.S., Francis G., Chang P., Coyle P.K., O'Connor P., et al. (2002) Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology 59: 1496–1506 [DOI] [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504 [PubMed] [Google Scholar]
- PRISMS Study Group and University of British Columbia MS/MRI Analysis Group (2001) PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 56: 1628–1636 [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Panitch H.S. (2007) Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther 29: 2031–2048 [DOI] [PubMed] [Google Scholar]
- Schwid S.R., Thorpe J., Sharief M., Sandberg-Wollheim M., Rammohan K., Wendt J., et al. (2005) Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing multiple sclerosis: the EVIDENCE study. Arch Neurol 62: 785–792 [DOI] [PubMed] [Google Scholar]
- Tintore M. (2007) Early MS treatment. Int MS J 14: 5–10 [PubMed] [Google Scholar]
- World Health Organization (2003). Adherence to long-term therapies: evidence for action. http://www.who.int/chp/knowledge/publications/adherence_report/en/index.html (accessed 1 December 2009).