Abstract
Myoclonus is a sudden, brief, involuntary muscle jerk. It is caused by abrupt muscle contraction, in the case of positive myoclonus, or by sudden cessation of ongoing muscular activity, in the case of negative myoclonus (NM). Myoclonus may be classified in a number of ways, although classification based on the underlying physiology is the most useful from the therapeutic viewpoint. Given the large number of possible causes of myoclonus, it is essential to take a good history, to clinically characterize myoclonus and to look for additional findings on examination in order to limit the list of possible investigations. With regards to the history, the age of onset, the character of myoclonus, precipitating or alleviating factors, family history and associated symptoms and signs are important. On examination, it is important to see whether the myoclonus appears at rest, on keeping posture or during action, to note the distribution of jerks and to look for the stimulus sensitivity. Electrophysiological tests are very helpful in determining whether myoclonus is cortical, subcortical or spinal. A single pharmacological agent rarely control myoclonus and therefore polytherapy with a combination of drugs, often in large dosages, is usually needed. Generally, antiepileptic drugs such as valproate, levetiracetam and piracetam are effective in cortical myoclonus, but less effective in other forms of myoclonus. Clonazepam may be helpful with all types of myoclonus. Focal and segmental myoclonus, irrespective of its origin, may be treated with botulinum toxin injections, with variable success.
Keywords: classification, clinical approach, myoclonus, treatment
Definition
Myoclonus is a movement disorder, which presents itself with sudden, brief, shock-like jerks. Most myoclonic jerks are due to a brief burst of muscular activity, resulting in positive myoclonus [Shibasaki and Hallett, 2005]. When jerks result from brief cessation of ongoing muscular activity, they are called negative myoclonus (NM). Positive myoclonus is generally more common, while NM frequently occurs in hospital settings, as a result of toxic–metabolic causes. A combination of both forms may be present in the same disease, as in posthypoxic myoclonus or progressive myoclonic epilepsies (PMEs).
Classification and clinical presentation
Myoclonus can be classified in a number of ways. By distribution, myoclonus is classified as focal, multifocal or generalized and by provoking factors as spontaneous and reflex. Myoclonus can also be divided in cortical, subcortical, spinal or peripheral, based on the presumed source of its generation. An alternative way of classifying myoclonus is based on the activity during which it occurs. It may occur at rest, when maintaining a posture or during action. A new category of ‘orthostatic myoclonus’ has recently been proposed by Glass and colleagues, who described a heterogeneous group of 15 patients in which myoclonic jerks occurred predominantly or exclusively on assuming an upright posture [Glass et al. 2007]. Seven of these patients had neurodegenerative disease and two had a systemic illness that could cause myoclonus. Based on aetiology, myoclonus may be classified as physiological, essential, epileptic, symptomatic or psychogenic [Marsden et al. 1982].
In a given patient, more than one form of myoclonus may occur. For instance, in posthypoxic myoclonus (Lance–Adams syndrome), cortical myoclonus may coexist with brainstem myoclonus [Borg, 2006].
Physiological classification of myoclonus is the most practical, since the presumed source of myoclonus (cortical, subcortical, spinal or peripheral) guides the physician towards the most effective treatment. For example, drugs that provide the best likelihood of treatment response in cortical myoclonus are not effective in segmental myoclonus [Caviness and Brown, 2004].
Individual diseases and conditions featuring myoclonus have been previously thoroughly reviewed [Caviness, 2007; Borg, 2006; Defebvre, 2006]. This review is focused mainly on clinical aspects of myoclonus and its physiological classification.
Classification by underlying physiology
Cortical myoclonus
Cortical myoclonus is the most common form of myoclonus, seen in both outpatient and inpatient clinical settings. Cortical myoclonus mainly affects the distal upper limbs and face, which reflects the largest cortical representations of these body areas [Caviness, 2009]. It is often focal, but may be multifocal, bilateral or generalized, as a consequence of intracortical and transcallosal spreading of abnormal activity [Brown et al. 1996, 1991a]. It typically occurs on voluntary action and may affect speech and gait. Cortical myoclonic jerks are stimulus sensitive, typically to touch, but sensitivity to visual stimuli is also described [Shibasaki and Neshige, 1987]. Most patients with cortical myoclonus have both positive myoclonus and NM, occurring either independently or together as a complex of the two kinds of myoclonus [Shibasaki and Hallett, 2005]. If cortical myoclonus is prolonged and lasts for hours, days or weeks, it is called epilepsia partials continua and is considered to be a rare form of focal epileptic status [Bien and Elger, 2008]. Focal cortical myoclonus almost always points to an underlining lesion of the sensori-motor cortex, which produces hyperexcitability (e.g. vascular, inflammatory or neoplastic). Recently, Alvarez and Caviness reported a case series of seven patients aged over 65 with progressive cortical myoclonus, but no cause was identified after detailed investigations and they termed the condition as ‘primary progressive myoclonus of aging’ [Alvarez and Caviness, 2008]. Examples of multifocal cortical myoclonus include posthypoxic myoclonus (Lance–Adams syndrome), progressive myoclonic epilepsies (PMEs), progressive myoclonic ataxias (PMAs) and neurodegenerative diseases.
Negative myoclonus
NM occurs when there is sudden interruption of ongoing muscle contraction (Figure 1). Clinically, it appears as a shock- like involuntary jerk that causes postural lapses. When trunk or lower limbs are involved, as for example in Lance–Adams syndrome, NM will cause a person to fall. NM may be of cortical or subcortical origin [Shibasaki, 1995]. NM of an epileptic nature, or epileptic negative myoclonus (ENM), is defined as an interruption of tonic muscle activity, which is time-locked to an epileptic EEG abnormality, without evidence of an antecedent positive myoclonus [Rubboli and Tassinari, 2006]. ENM can be observed in idiopathic, cryptogenic and symptomatic epilepsy, i.e. in PME. ENM is never an isolated sign, but occurs in association with other types of seizures, such as partial motor seizures (often of the rolandic type), absences or atonic seizures. NM may also be of subcortical origin. For example, asterixis is a type of subcortical NM that occurs in toxic–metabolic encephalopathies. It is usually bilateral and rhythmic (6–11 Hz) [Rubboli and Tassinari, 2006]. Unilateral asterixis may be seen in thalamic lesions [Tatu et al. 2000].
Figure 1.
Negative myoclonus: cortical negative myoclonus. There is a sudden interruption of the muscle activity when the patient is holding the left leg up against gravity. Duration of EMG silent period is 50–100 ms.
Subcortical myoclonus
Subcortical myoclonus has its origin between the cortex and the spinal cord. It may be divided into the nonsegmental and the segmental types.
Nonsegmental subcortical myoclonus
Startle/hyperekplexia and reticular reflex myoclonus are considered to be classical examples of brainstem myoclonus. In addition, myoclonus dystonia and drug-induced myoclonus are also believed to be of subcortical origin, due to the absence of cortical correlates of myoclonic jerks [Li et al. 2008].
Brainstem myoclonus is manifested by generalized jerks and its most striking clinical feature is sensitivity to auditory stimuli. Two main types are (i) startle response, which may be physiologic or pathologic (hyperekplexia), and (ii) reticular reflex myoclonus.
Physiologic startle is an example of physiological brainstem reflex, which places the body in defensive posture, following an unexpected stimulus such as sudden noise. Sensitivity to somatosensory stimuli delivered to the mantle area (e.g. touching head, face and or upper chest) and visual stimuli may also be present. In startle response, EMG activity starts in sternocleidomastoid muscles and is followed by face, trunk and limb involvement in an orderly fashion, as myoclonic activity spreads up the brainstem and down the spinal cord. Startle involves proximal and distal muscles, bilaterally and synchronously, and produces brief, shock-like movement comprising grimacing, arm abduction and flexion of the neck, trunk, elbows, hips and knees. Hyperekplexia is pathological exaggeration of the normal startle response [Brown et al. 1991b], which does not habituate on repeated stimuli. Hyperekplexia may be familial as a result of mutation in the alpha1 subunit of the glycine receptor [Shiang et al. 1993], idiopathic or symptomatic of brainstem encephalitis, vascular lesions [Kimber and Thompson, 1997] or multiple sclerosis [Ruprecht et al. 2002].
Brainstem reticular myoclonus is another rare form of generalized myoclonus. Clinically it may be distinguished from hyperekplexia by the frequent occurrence of spontaneous myoclonus and sensitivity to somatosensory stimuli delivered to distal limbs rather than to the mantle area. It may occur in posthypoxic encephalopathy, brainstem encephalitis and uraemia [Chadwick and French, 1979].
Segmental subcortical myoclonus–palatal myoclonus
Palatal myoclonus is a type of segmental brainstem myoclonus, although it is considered by some authors as a form of tremor [Deuschl et al. 1994]. It consists of rhythmic (1–2 Hz) contractions of the soft palate, presumably due to a dysfunction (essential palatal myoclonus [EPM]) or a lesion (symptomatic palatal myoclonus [SPM]) in the Guillain–Mollaret triangle (GMT). The GMT comprises connections between dentate nucleus, red nucleus and inferior olivary nucleus. EPM is a result of rhythmic contractions of the tensor veli palatini muscle, which arises from the lateral wall of the Eustachian tube. Repetitive opening and closing of the tube, as the result of its contraction, produce an audible ‘click’ [Deuschl et al. 1991], typical for EPM. EPM disappears in sleep. In SPM, the main muscle involved is the levator veli palatini. SPM is usually not accompanied by clicking and tends to persist in sleep [Pearce, 2008]. SPM is more common than EPM [Deuschl et al. 1990]. Important causes of SPM include vascular lesions, multiple sclerosis and brainstem tumours. Another well-recognized cause of SPT is progressive ataxia palatal tremor syndrome (PAPT) [Pareyson et al. 2008]. PAPT may be sporadic or familial. Familial PAPT is associated with marked brainstem and spinal cord atrophy and no evidence of olivary hypertrophy [Samuel et al. 2004]. Some familial cases of PAPT are due to a GFAP mutation and represent adult onset of Alexander disease [Howard et al. 2008; Pareyson et al. 2008]. A rare cause of SPT is autosomal dominant neuroferritinopathy due to ferritin light chain (NFL) gene mutation [Wills et al. 2002]. Clinically, palatal myoclonus may sometimes be confused with palatal tics [Adam et al. 2009].
Spinal myoclonus
Spinal myoclonus may be segmental or propriospinal, reflecting spinal segmental organization and the presence of propriospinal pathways which connect different spinal segments [Brown et al. 1994]. It is generally resistant to supraspinal influences such as sleep (therefore it may persist in sleep) or voluntary action (therefore it is present at rest, independently of activation) and may or may not be stimulus sensitive [Caviness and Brown, 2004].
Spinal segmental myoclonus is usually symptomatic of an underlying structural lesion such as syringomyelia, myelitis, spinal cord trauma, vascular lesion or malignancy [Brown et al. 1994; Jankovic and Pardo, 1986]. It is confined to one or few contiguous myotomes and may occur irregularly or quasirhythmically, with the frequency as low as 1–2 per minute or as high as 100–200 per minute. EMG myoclonic bursts are prolonged up to 1000 ms.
Propriospinal myoclonus is a form of spinal myoclonus where the spinal generator recruits axial muscles up and down the spinal cord via long propriospinal pathways [Brown et al. 1994]. Typically, there are axial flexion jerks involving the neck, trunk and hips with a frequency of 1–6 Hz. EMG bursts are long, lasting several hundred milliseconds. Clinically, it can be distinguished from brainstem myoclonus, which is also axial in distribution, by sparing of the face and insensitivity to auditory stimuli. It typically occurs spontaneously, especially in recumbent position or may be provoked by tapping of the abdomen or by eliciting tendon reflexes. As opposed to segmental myoclonus, most patients with propriospinal myoclonus have no clear aetiology. Symptomatic forms are reported in cervical trauma, tumour or viral myelitis [Brown, 1996]. Psychogenic forms of propriospinal myoclonus are now increasingly recognized [Williams et al. 2008]. One recent study on a large cohort of patients with idiopathic spinal myoclonus, showed that at least 30% of patients had a definite premovement (Bereitschaftspotential) potential, indicating that the aetiology was psychogenic [Esposito et al. 2009]. In another large series, a psychogenic cause was suggested in 34 out of 35 patients with axial jerks, who were initially thought to have propriospinal myoclonus [van der Salm et al. 2010].
Peripheral myoclonus
Peripheral myoclonus is characterized by rhythmic or semirhythmic jerks secondary to plexus, nerve, root lesion or rarely anterior horn cell disease. Hemifacial spasm is the most common example of peripheral myoclonus, while other causes are relatively rare.
Classification by aetiology
A classification of myoclonus is given in Table 1.
Table 1.
Classification of myoclonus.
Physiological myoclonus
Physiological myoclonus occurs in healthy people. Jerks on falling asleep (hypnagogic myoclonus), hiccups and physiological startle response are common examples.
Essential myoclonus (myoclonus dystonia)
In essential myoclonus, myoclonus is isolated or the most prominent finding from which the patient experiences some, even if mild disability [Caviness and Brown, 2004]. It may be sporadic or hereditary.
Hereditary essential myoclonus is synonymous with myoclonus dystonia (DYT11), an autosomal dominant disease with variable penetrance. Approximately 50% of clinically definitive cases [Ritz et al. 2009] are due to mutations of the epsilon-sarcoglycan gene on chromosome 7q21 [Zimprich et al. 2001]. Myoclonus dystonia is typically inherited from the father due to maternal genomic imprinting [Grabowski et al. 2003; Muller et al. 2002]. It typically starts in childhood, with myoclonic, ‘lightning’ jerks in combination with usually mild dystonia, while other neurological deficits are absent. In a proportion of cases, psychiatric features such as anxiety, depression and obsessive–compulsive disorders are part of the clinical picture [Hess et al. 2007; Misbahuddin et al. 2007; Saunders-Pullman et al. 2002]. Myoclonus and dystonia affect mainly the head, neck and arms, but occasionally falls caused by myoclonic jerks in the legs may be the main feature [Koukouni et al. 2008]. Typically, there is quite a dramatic response of myoclonic jerks to alcohol. Stimulus sensitivity is not an important characteristic of this condition. Pathophysiology of myoclonus dystonia is not clear. Cortical somatosensory evoked potentials are normal and back-averaging of EEG activity preceding jerks reveals no cortical correlate [Li et al. 2008; Roze et al. 2008].
Epileptic myoclonus
This term is used to denote conditions where myoclonus occurs in the setting of epilepsy. Epileptic myoclonus may be positive or negative (lapses of postural tone). Epileptic myoclonus is accompanied by generalized epileptiform discharges on EEG, but the myoclonus itself may be focal, segmental or generalized [Caviness and Brown, 2004]. Generalized myoclonus can occur in the syndromes of primary (idiopathic) generalized epilepsy (e.g. juvenile myoclonic epilepsy) or in the secondary (symptomatic) generalized epilepsies (e.g. PME). Focal myoclonus can occur in symptomatic epilepsy, in the setting of infection, inflammation, vascular disease, trauma or tumours.
Familial cortical tremor, also known as benign autosomal dominant familial myoclonic epilepsy (BADFME), is a rare, although interesting disorder, because it clinically resembles essential tremor. It is a benign condition characterized by fine, shivering-like tremor, which usually starts in the third or fourth decade. Generalized seizures are infrequent and there is no significant clinical progression. The condition has been mapped to chromosome 8q and to chromosome 2p [Guerrini et al. 2001; Plaster et al. 1999].
Secondary myoclonus
This type of myoclonus occurs in the context of an underlying neurological or nonneurological disorder and is the most common form of myoclonus. The aetiology includes posthypoxic myoclonus, drug-induced myoclonus, toxic–metabolic causes, myoclonus due to focal nervous system damage, neurodegenerative diseases and hereditary metabolic diseases. Myoclonus due to toxic–metabolic causes is usually accompanied by encephalopathy and additional neurological findings, such as ataxia or seizures. Borg has given an exhaustive review of symptomatic myoclonus [Borg, 2006].
It is important to recognize that the following metabolic derangements may cause symptomatic myoclonus: renal failure, hepatic failure, respiratory failure, glycaemic disturbances, electrolytic disturbances, hyperthyroidism, metabolic alkalosis or acidosis, vitamin E deficiency, Hashimoto encephalopathy and hypoxia [Borg, 2006]. Symptomatic myoclonus is usually cortical, focal or multifocal and sensitive to stimuli. However, NM (asterixis) and brainstem reticular myoclonus may also be seen.
Toxic causes of myoclonus include chronic abuse of alcohol and withdrawal, the dialysis syndrome due to aluminium toxicity, chronic toluene abuse, methyl bromide and gasoline sniffing [Gordon, 2002].
Drugs that may cause myoclonus include levodopa, antidiarrhoeal bismuth subsalicylate, benzodiazepines, antidepressants (cyclic antidepressants, selective serotonin uptake inhibitors, monoamine oxidase inhibitors), lithium, anti-infectious agents (quinolone antibiotics, cephalosporines), clozapine, opioids, anticonvulsants (particularly gabapentin, pregabalin, lamotrigine, phenytoin, phenobarbital), anaesthetic propofol, cardiac medications (calcium channel blockers, antiarrhythmics) and contrast media [Caviness and Brown, 2004].
Postanoxic myoclonus (Lance–Adams syndrome) is a distinct condition that may follow severe cerebral hypoxia, usually after respiratory rather than cardiac arrest [Werhahn et al. 1997]. Myoclonus is mainly cortical and multifocal and there is a combination of positive myoclonus and NM, but reticular reflex myoclonus and exaggerated startle may also occur. Action myoclonus is the main disabling feature of this condition, although a variable degree of cognitive impairment and seizures may be present in a proportion of patients. NM in proximal leg muscles (‘bouncy legs’) is very resistant to the treatment and may leave the patient wheelchair-bound. Some patients may show late improvement and eventually be able to walk unaided and to discontinue antimyoclonic drugs [Werhahn et al. 1997].
The progressive myoclonic epilepsy syndromes are a group of rare disorders, characterized by myoclonic epilepsy, generalized tonic clonic seizures, progressive ataxia and dementia. Six main categories are recognized: Unverricht–Lundborg disease, myoclonic epilepsy with ragged red fibres (MERRF), Lafora body disease, neuronal ceroid lipofuscinosis, sialidosis and dentato-rubro-paladal-Lysian atrophy (DRPLA). Myoclonus in PME is multifocal, typically involving the distal limbs and face and is provoked by posture or action. It is sensitive to touch, noise and light [Shahwan et al. 2005]. Patients are typically severely disabled by their action myoclonus.
Action myoclonus–renal failure syndrome is a distinct type of PME, described by Badhwar and colleagues, that is associated with renal impairment [Badhwar et al. 2004]. Mutation of the LIMP-2 gene has been recently identified as a cause [Blanz et al. 2010] and the condition is inherited in autosomal recessive fashion. It usually starts with the tremor (age 17–26), followed by action myoclonus, infrequent generalized seizures and cerebellar signs. Proteinuria is invariably present in the course of the disease and the condition progresses to renal failure.
Progressive myoclonic ataxias, also known as Rumsey–Hunt syndrome, include conditions with prominent myoclonus and ataxia, but little in the way of epilepsy or progressive dementia. PMA include coeliac disease, some cases of mitochondrial diseases, vitamin E deficiency and some cases of Unverricht–Lundborg disease.
Myoclonus is often linked to neurodegenerative disorders.
Cortical myoclonus is present in about 15% of patients with dementia with Lewy bodies (DLB) [Caviness et al. 2003] or Parkinson’s disease dementia, but is rare in Parkinson’s disease without dementia [Caviness et al. 2002].
Patients with multiple system atrophy (MSA) often display irregular, small-amplitude myoclonic movements (polyminimyoclonus) of the hands and/or fingers on keeping outstretched posture (jerky postural tremor). Polyminimyoclonus is stimulus-sensitive and accentuated during voluntary movements. A cortical origin can be demonstrated by back-averaging techniques, and somatosensory evoked potentials (SSEPs) are sometimes ‘giant’ [Rodriguez et al. 1994].
Myoclonus occurs in 50% patients with corticobasal degeneration (CBD) [Caviness, 2007]. It appears focally in the affected arm, together with apraxia, rigidity, dystonia and alien limb phenomenon. At the beginning of the illness, it occurs in repetitive rhythmic fashion (jerky tremor) on an attempt to activate the arm or following somatosensory stimulation (reflex myoclonus). As the disease progresses, spontaneous myoclonus adjoins. A cortical origin has been postulated [Thompson et al. 1994b], even though an additional subcortical origin is possible [Grosse et al. 2003].
In contrast to CBD, myoclonus is rare in progressive supranuclear palsy.
In relation to Huntington’s disease, myoclonus may be seen in individuals with a juvenile onset and longer CAG repeats [Thompson et al. 1994a].
In Alzheimer’s disease (AD), myoclonus may appear in the middle or late stages of disease, is usually multifocal, occurring both at rest and during action. In patients with early onset AD and in familial cases, it may be present early in the disease [Caviness, 2007].
Myoclonus is typical finding in sporadic, familial and new variant Creutzfeldt–Jakob disease. Jerks, often limited and sporadic at the disease onset, become diffuse, generalized and relatively rhythmic (0.6–1.5 Hz) as the disease progresses [Borg, 2006].
Psychogenic myoclonus
Psychogenic myoclonus may occur spontaneously or following an external trauma. It may be focal (restricted to a few muscles) or generalized. Jerks are commonly distractible and inconsistent over time, with sudden onset and offset and day-to-day variability. Usually, there is exaggerated stimulus sensitivity. Despite these characteristics, it may be difficult to distinguish psychogenic from organic myoclonus and electrophysiology may be helpful (as described in the following).
Approach to patients with myoclonus
On history taking, one should be interested in the age at onset of myoclonus, the character of onset (acute versus gradual), precipitating or alleviating factors, family history and associated symptoms such as epilepsy, ataxia and cognitive decline (present in symptomatic as opposed to essential myoclonus). It is also important to know whether the condition is static or progressive.
The age at onset is important as it may point out to the major disease category. The onset of myoclonus in childhood or young adult, together with generalized epileptic fits, cognitive decline and progressive ataxia suggest the syndrome of PME. On the other hand, in elderly patients, myoclonus and cognitive decline are seen DLB, CBD and later stages of AD or, if rapidly progressive, in prion diseases. Opsoclonus myoclonus syndrome in childhood is typically associated with neuroblastoma or medulloblastoma. In adulthood, it occurs as paraneoplastic manifestation in lung small-cell carcinoma or melanoma, but may be postinfectious, associated with coeliac disease or may be drug related.
Regarding the nature of onset, the acute onset of myoclonus is seen in toxic–metabolic disorders such as hepatic and renal failure, thyrotoxicosis, electrolyte disturbances (e.g. hyponatraemia, hypoglycaemia, nonketotic hyperglycaemia), some neuroinfectious diseases (herpes simplex encephalitis, neuroboreliosis), following hypoxic brain injury, in paraneoplastic disorders and with drugs. The recent introduction of a new drug or increase in dosage should always be considered as a possible cause of new onset myoclonus. More insidious onset followed by chronic progression is characteristic of neurodegenerative diseases and PME.
Precipitating factors are recognized in cases of drug-induced myoclonus, intoxication and metabolic disturbances. Spinal and peripheral myoclonus may follow cord/plexus/root/nerve injury. Dramatic response to alcohol in myoclonus dystonia is an example of a factor alleviating myoclonus.
The presence of additional neurological findings, such as dementia, cerebellar ataxia or epilepsy automatically rule out essential myoclonus and prompt a search for symptomatic causes.
Family history with an autosomal recessive mode of inheritance is present in syndromes of PME or in hereditary metabolic disorders (e.g. GM1 gangliosidosis, Gaucher disease). Autosomal dominant inheritance is seen in myoclonus dystonia, DRPLA or familial cortical tremor. Mitochondrial inheritance is characteristic for MERRF.
On examination, it is important to check whether myoclonus appears at rest, on posture (keeping the arms outstretched) or during action and to note its distribution. Myoclonus at rest indicates a spinal or brainstem source, whereas action-induced myoclonus points to a cortical origin. Focal and multifocal jerks, occurring during voluntary action, are typical of cortical myoclonus. Spinal segmental myoclonus is also focal, although contrary to cortical myoclonus, it is not action-induced and is occasionally stimulus sensitive. Generalized myoclonus is usually subcortical (brainstem or propriospinal myoclonus) or less frequently cortical. The amplitude of myoclonus varies considerably. Very small, hardly visible distal myoclonic jerks (mini polymyoclonus) are typical for MSA, whereas very large amplitudes are typical for PME.
The next step in the examination is to look for stimulus sensitivity. This can be done by gently touching the outstretched fingers to trigger myoclonus. Clapping the hands may induce myoclonus sensitive to auditory stimuli, but common sounds in the examination room (opening or closing doors, loud speech) may be sufficient to trigger myoclonus in susceptible patients.
Finally, it is important to look for other neurological signs, particularly for dementia, cerebellar features, eye movement abnormalities and any associated signs of systemic disease.
Given the extensive list of different causes of myoclonus, it is important to take a good history and to use additional clinical findings, in order to avoid numerous, expensive and sometimes unnecessary investigations. In unexplained cases of myoclonus, the following tests are routinely done: electrolytes, glucose, liver, renal and thyroid function, brain and spinal imaging and EEG. Additional testing depends on clinical presentation and may include spinal fluid examination, paraneoplastic antibody testing, genetic tests or enzyme activity assays.
Neurophysiologic assessment
Electrophysiology is very helpful to detect whether myoclonus is cortical, subcortical or spinal/segmental. Polymyography is the first step in the neurophysiologic assessment of myoclonus and includes recording of duration, distribution and stimulus sensitivity of muscle jerks. Further investigations include combined EEG–EMG recording, EEG back averaging and recording of SSEPs.
Cortical myoclonus (Figure 2) may have the following electrophysiological characteristics: (a) it is represented by brief EMG discharges lasting less than 70 ms, usually less than 50 ms [Shibasaki, 2006]; (b) an EEG spike precedes the myoclonus by a short interval (20 ms for hand muscles and about 35 ms for the calf muscles); (c) there is an enhancement of the early cortical component of the SSEPs, called ‘giant SSEPs’ [Kakigi and Shibasaki, 1987]. Also, long loop reflexes mediated by the sensory–motor cortex (C-reflexes) are enhanced and correspond to cortical reflex myoclonus [Hallett et al. 1979]. EMG recording from limb muscles may demonstrate spread of the jerks from proximal to distal muscles with the velocity compatible with that of alpha motor fibres. On conventional EEG–EMG recording, EEG spikes associated with EMG myoclonic bursts suggest cortical origin. The absence of EEG spikes does not exclude the possibility of a cortical aetiology and back averaging of EEG, time locked to the onset of myoclonic jerks may disclose a cortical spike, occurring approximately at an interval appropriate for conduction in the fastest corticospinal pathways.
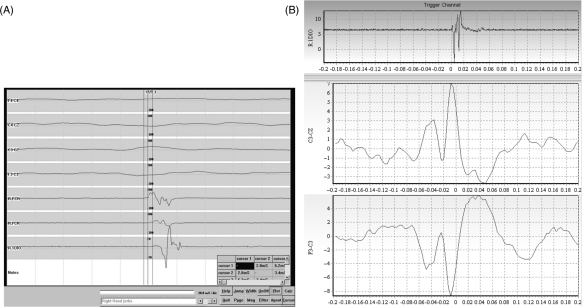
Figure 2.
Cortical myoclonus: EMG and EEG trace in a case of cortical myoclonus. (A) A magnification of a segment (20 ms/division) where myoclonic jerks are observed. Surface EMG shows brief bursts of activity (of approximately 20 ms duration) with a typical rostrocaudal pattern of muscle activation in the right upper limb. (B) EEG back averaging of the right first dorsal interossei muscle. EMG burst demonstrates cortical spikes in C3 derivation, starting 22 ms before the EMG myoclonic burst.
In contrast with cortical myoclonus, in subcortical myoclonus there are no signs of hyperexcitability on the EEG and SSEP recordings.
Simultaneous recording of surface EMG (multichannel surface EMG) from different muscles may give information on the distribution and mode of spread of myoclonus in the case of brainstem myoclonus (Figure 3). The first activated muscle is sternocleidomastoid or trapezius with subsequent spread of activity to rostral and caudal muscles.
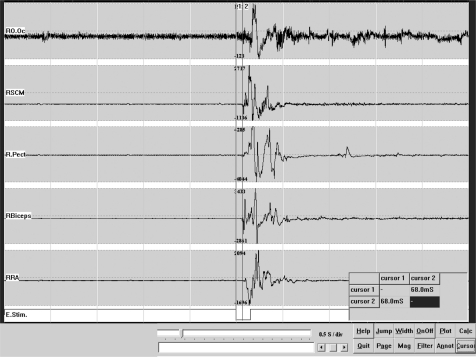
Figure 3.
Brainstem reticular myoclonus. Multichannel EMG recording: Following acoustic stimulation there was an initial activation of the right sternocleidomastoid muscle with a latency of 68 ms, followed by the spread to rostral and caudal muscles.
In propriospinal myoclonus (Figure 4), myoclonic bursts may last from 50 ms to 4 s. EMG jerks arise from abdominal or cervical spinal segments and spread slowly rostrally and caudally, sparing the cranial muscles.
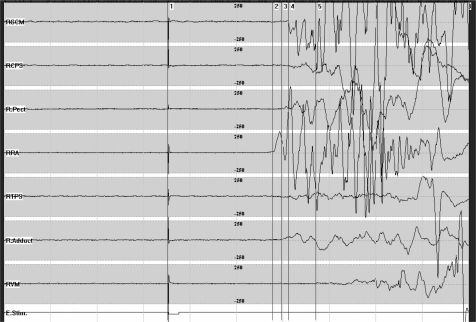
Figure 4.
Propriospinal myoclonus. With the patient in a recumbent position, surface multichannel EMG from right-sided muscles shows a jerk of approximately 400 ms duration. This jerk is electrically evoked, starts with a latency of 200 ms in the rectus abdominis muscle and is followed by activation of rostral and caudal muscles.
In spinal segmental myoclonus (Figure 5), myoclonic bursts are confined to one or two contiguous myotomes.
Figure 5.
Spinal segmental myoclonus. Multisurface EMG shows myoclonic bursts confined mainly to the right triceps but affecting also a few adjacent myotomes.
Simple EMG recording of myoclonic jerks may help to exclude psychogenic myoclonus. It is not possible to voluntarily produce an EMG burst of less than 50–75 ms and therefore bursts lasting less than this are strong evidence of organicity. In contrast, recording of premovement EEG potentials (Bereitschaftspotentials) just prior to a jerk is suggestive of a psychogenic cause (Figure 6).
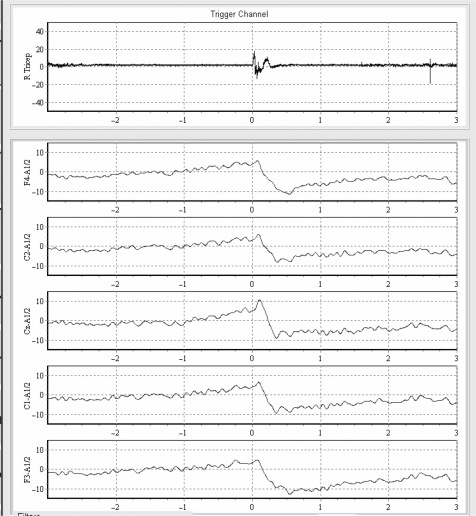
Figure 6.
Psychogenic myoclonus. A slow rising wave called the Bereitschafts potential is seen in EEG back averaging of the right triceps jerk (duration of the triceps jerk is 200 ms).
Most of these electrophysiological investigations are available only in specialized centres and do not form a part of everyday clinical practice.
Treatment of myoclonus
The treatment of myoclonus depends on the underlying disorder. Reversible causes of myoclonus include some toxic–metabolic states, drug intoxications or surgically treatable lesions, however in the majority of cases, the underlying cause is not correctable and symptomatic treatment is the only possibility. A useful approach to the treatment is to first establish the physiology of myoclonus (cortical versus subcortical or spinal), because different drugs will work in different types of myoclonus.
One single agent can seldom completely control myoclonus; therefore multiple drug trials and combination of drugs are necessary, often in large dosages. In general, antiepileptic drugs such as valproate, levetiracetam and piracetam are effective in cortical myoclonus, but ineffective in other forms of myoclonus. Clonazepam may be helpful in all types of myoclonus
Cortical myoclonus
Treatment of cortical myoclonus is aimed at enhancing deficient GABAergic inhibitory neurotransmission [Caviness and Brown, 2004]. As a rule, cortical myoclonus is treated with a combination of drugs. Sedation and ataxia are the main side effects of polytherapy, but they may be overcome with the ‘start low, go slow’ principle. Of the GABAergic drugs, sodium valproate is the most effective. It should be introduced slowly and titrated up to 1200–2000 mg daily. Benzodiazepines are also very useful, especially clonazepam in large doses (up to 15 mg a day). Tolerance may develop after several months, while rapid reduction or withdrawal can produce marked deterioration. Piracetam and levetiracetam are two related drugs, proven to be very useful in myoclonus [Genton and Gelisse, 2000; Ikeda et al. 1996], although their exact mechanism of action is poorly understood. Large doses of piracetam may be required (3200–4800 mg tds, maximum up to 20 g/day), but levetiracetam is a more potent drug (maximum 3000 mg daily). In cortical myoclonus, piracetam or levetiracetam can be combined with sodium valproate and clonazepam. Primidone and phenobarbital are rarely effective, whereas zonisamde has helped in some cases of PME [Leppik, 1999; Kyllerman and Ben-Menachem, 1998]. Phenytoin, carbamazepine, lamotrigine and vigabatrin are best avoided in cortical myoclonus, as they may paradoxically exacerbate myoclonus. This is particularly the case with phenytoin in Unverricht–Lundborg disease. Treatment of PME is very challenging, as drugs that help generalized seizures may worsen myoclonus and vice versa.
Negative myoclonus
ENM in children suffering from idiopathic partial epilepsy may respond to ethosuximide and levetiracetam [Gelisse et al. 2003; Capovilla et al. 1999]. ENM associated with symptomatic or cryptogenic epilepsies is usually less responsive to common antiepileptic drugs and may be worsened by carbamazepine, valproic acid, phenytoin, lamotrigine and oxcarbazepine. In posthypoxic myoclonus, distal action and reflex myoclonus of upper limbs respond to therapy much better than NM of proximal lower limbs, which causes gait disturbances and frequent falls.
Subcortical myoclonus
Antiepileptic drugs used in cortical myoclonus are not effective in subcortical myoclonus [Caviness and Brown, 2004]. Clonazepam is useful in hyperekplexia and partially in reticular reflex myoclonus. Myoclonus dystonia responds partially to clonazepam, although the response fails to match that from alcohol. In one report, alcohol-sensitive myoclonus dystonia was successfully treated with 6.125 g/day of gamma-hydroxybutyric acid [Priori et al. 2000]. Severe cases of myoclonic dystonia can be helped by bilateral pallidal [Magarinos-Ascone et al. 2005; Cif et al. 2004] or thalamic deep brain stimulation [Trottenberg et al. 2001].
Spinal myoclonus
In spinal myoclonus, pharmacological treatment is unsatisfactory. Clonazepam is the drug of first choice for both types of spinal myoclonus and dosages up to 6 mg are needed to diminish spinal segmental myoclonus. Levetiracetam was reported to be effective in a series of three patients with spinal segmental myoclonus [Keswani et al. 2002].
Segmental myoclonus
Segmental myoclonus, irrespective of its origin (palatal tremor, spinal segmental myoclonus) may be treated with botulinum toxin injections, with variable success [Penney et al. 2006; Lagueny et al. 1999].
Peripheral myoclonus
In peripheral myoclonus, drugs are usually ineffective, although carbamazepine may have some effect [Caviness, 2007]. Hemifacial spasm responds excellently to botulinum toxin injections [Costa et al. 2005].
Psychogenic myoclonus
Psychogenic myoclonus may improve as a result of placebo or psychotherapy.
Conclusion
Myoclonus is a clinical sign that may be found in a number of different diseases. To provide a framework to match a patient’s myoclonus to its aetiology, it is necessary to take a good history and to perform a detailed neurological examination, before deciding which additional tests are needed. It is important to establish the presumed origin of myoclonus (cortical, subcortical, spinal or peripheral) in order to choose the most effective treatment. Controlled evidence on the treatment of myoclonus is insufficient and therapy is mostly empirical.
Acknowledgments
We would like to thank Dr Joao Massano and Dr Marcello Esposito for their help in preparing the figures and the table.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- Adam O.R., Ferrara J.M., Jankovic J. (2009) Motor-phonic tic mimicking essential palatal myoclonus. Mov Disord 24: 2030–2032 [DOI] [PubMed] [Google Scholar]
- Alvarez M., Caviness J.N. (2008) Primary progressive myoclonus of aging. Mov Disord 23: 1658–1664 [DOI] [PubMed] [Google Scholar]
- Badhwar A., Berkovic S.F., Dowling J.P., Gonzales M., Narayanan S., Brodtmann A., et al. (2004) Action myoclonus-renal failure syndrome: characterization of a unique cerebro-renal disorder. Brain 127: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Bien C.G., Elger C.E. (2008) Epilepsia partialis continua: semiology and differential diagnoses. Epileptic Disord 10: 3–7 [DOI] [PubMed] [Google Scholar]
- Blanz J., Groth J., Zachos C., Wehling C., Saftig P., Schwake M. (2010) Disease-causing mutations within the lysosomal integral membrane protein type 2 (LIMP-2) reveal the nature of binding to its ligand beta-glucocerebrosidase. Hum Mol Genet 19: 563–572 [DOI] [PubMed] [Google Scholar]
- Borg M. (2006) Symptomatic myoclonus. Neurophysiol Clin 36: 309–318 [DOI] [PubMed] [Google Scholar]
- Brown P. (1996) Myoclonus. Curr Opin Neurol 9: 314–316 [DOI] [PubMed] [Google Scholar]
- Brown P., Day B.L., Rothwell J.C., Thompson P.D., Marsden C.D. (1991a) Intrahemispheric and interhemispheric spread of cerebral cortical myoclonic activity and its relevance to epilepsy. Brain 114: 2333–2351 [DOI] [PubMed] [Google Scholar]
- Brown P., Ridding M.C., Werhahn K.J., Rothwell J.C., Marsden C.D. (1996) Abnormalities of the balance between inhibition and excitation in the motor cortex of patients with cortical myoclonus. Brain 119: 309–317 [DOI] [PubMed] [Google Scholar]
- Brown P., Rothwell J.C., Thompson P.D., Britton T.C., Day B.L., Marsden C.D. (1991b) The hyperekplexias and their relationship to the normal startle reflex. Brain 114: 1903–1928 [DOI] [PubMed] [Google Scholar]
- Brown P., Rothwell J.C., Thompson P.D., Marsden C.D. (1994) Propriospinal myoclonus: evidence for spinal “pattern” generators in humans. Mov Disord 9: 571–576 [DOI] [PubMed] [Google Scholar]
- Capovilla G., Beccaria F., Veggiotti P., Rubboli G., Meletti S., Tassinari C.A. (1999) Ethosuximide is effective in the treatment of epileptic negative myoclonus in childhood partial epilepsy. J Child Neurol 14: 395–400 [DOI] [PubMed] [Google Scholar]
- Caviness J.N. (2007) Parkinsonism and related disorders. Myoclonus. Parkinsonism Relat Disord 13(Suppl. 3): S375–S384 [DOI] [PubMed] [Google Scholar]
- Caviness J.N. (2009) Pathophysiology and treatment of myoclonus. Neurol Clin 27: 757–777, vii [DOI] [PubMed] [Google Scholar]
- Caviness J.N., Adler C.H., Beach T.G., Wetjen K.L., Caselli R.J. (2002) Small-amplitude cortical myoclonus in Parkinson’s disease: physiology and clinical observations. Mov Disord 17: 657–662 [DOI] [PubMed] [Google Scholar]
- Caviness J.N., Adler C.H., Caselli R.J., Hernandez J.L. (2003) Electrophysiology of the myoclonus in dementia with Lewy bodies. Neurology 60: 523–524 [DOI] [PubMed] [Google Scholar]
- Caviness J.N., Brown P. (2004) Myoclonus: current concepts and recent advances. Lancet Neurol 3: 598–607 [DOI] [PubMed] [Google Scholar]
- Chadwick D., French A.T. (1979) Uraemic myoclonus: an example of reticular reflex myoclonus? J Neurol Neurosurg Psychiatry 42: 52–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cif L., Valente E.M., Hemm S., Coubes C., Vayssiere N., Serrat S., et al. (2004) Deep brain stimulation in myoclonus-dystonia syndrome. Mov Disord 19: 724–727 [DOI] [PubMed] [Google Scholar]
- Costa J., Espirito-Santo C., Borges A., Ferreira J.J., Coelho M., Moore P., et al. (2005) Botulinum toxin type A therapy for hemifacial spasm. Cochrane Database Syst Rev 1: CD004899–CD004899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defebvre L. (2006) Myoclonus and extrapyramidal diseases. Neurophysiol Clin 36: 319–325 [DOI] [PubMed] [Google Scholar]
- Deuschl G., Lohle E., Heinen F., Lucking C. (1991) Ear click in palatal tremor: its origin and treatment with botulinum toxin. Neurology 41: 1677–1679 [DOI] [PubMed] [Google Scholar]
- Deuschl G., Mischke G., Schenck E., Schulte-Monting J., Lucking C.H. (1990) Symptomatic and essential rhythmic palatal myoclonus. Brain 113: 1645–1672 [DOI] [PubMed] [Google Scholar]
- Deuschl G., Toro C., Hallett M. (1994) Symptomatic and essential palatal tremor. 2. Differences of palatal movements. Mov Disord 9: 676–678 [DOI] [PubMed] [Google Scholar]
- Esposito M., Edwards M.J., Bhatia K.P., Brown P., Cordivari C. (2009) Idiopathic spinal myoclonus: a clinical and neurophysiological assessment of a movement disorder of uncertain origin. Mov Disord 24: 2344–2349 [DOI] [PubMed] [Google Scholar]
- Gelisse P., Crespel A., Genton P., Baldy-Moulinier M. (2003) Dramatic effect of levetiracetam on epileptic negative myoclonus. Acta Neurol Scand 107: 302–303 [DOI] [PubMed] [Google Scholar]
- Genton P., Gelisse P. (2000) Antimyoclonic effect of levetiracetam. Epileptic Disord 2: 209–212 [PubMed] [Google Scholar]
- Glass G.A., Ahlskog J.E., Matsumoto J.Y. (2007) Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology 68: 1826–1830 [DOI] [PubMed] [Google Scholar]
- Gordon M.F. (2002) Toxin and drug-induced myoclonus. Adv Neurol 89: 49–76 [PubMed] [Google Scholar]
- Grabowski M., Zimprich A., Lorenz-Depiereux B., Kalscheuer V., Asmus F., Gasser T., et al. (2003) The epsilon-sarcoglycan gene (SGCE), mutated in myoclonus-dystonia syndrome, is maternally imprinted. Eur J Hum Genet 11: 138–144 [DOI] [PubMed] [Google Scholar]
- Grosse P., Kuhn A., Cordivari C., Brown P. (2003) Coherence analysis in the myoclonus of corticobasal degeneration. Mov Disord 18: 1345–1350 [DOI] [PubMed] [Google Scholar]
- Guerrini R., Bonanni P., Patrignani A., Brown P., Parmeggiani L., Grosse P., et al. (2001) Autosomal dominant cortical myoclonus and epilepsy (ADCME) with complex partial and generalized seizures: A newly recognized epilepsy syndrome with linkage to chromosome 2p11.1-q12.2. Brain 124: 2459–2475 [DOI] [PubMed] [Google Scholar]
- Hallett M., Chadwick D., Marsden C.D. (1979) Cortical reflex myoclonus. Neurology 29: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Hess C.W., Raymond D., Aguiar Pde C., Frucht S., Shriberg J., Heiman G.A., et al. (2007) Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 68: 522–524 [DOI] [PubMed] [Google Scholar]
- Howard K.L., Hall D.A., Moon M., Agarwal P., Newman E., Brenner M. (2008) Adult-onset Alexander disease with progressive ataxia and palatal tremor. Mov Disord 23: 118–122 [DOI] [PubMed] [Google Scholar]
- Ikeda A., Shibasaki H., Tashiro K., Mizuno Y., Kimura J. (1996) Clinical trial of piracetam in patients with myoclonus: nationwide multiinstitution study in Japan. The Myoclonus/Piracetam Study Group. Mov Disord 11: 691–700 [DOI] [PubMed] [Google Scholar]
- Jankovic J., Pardo R. (1986) Segmental myoclonus. Clinical and pharmacologic study. Arch Neurol 43: 1025–1031 [DOI] [PubMed] [Google Scholar]
- Kakigi R., Shibasaki H. (1987) Generator mechanisms of giant somatosensory evoked potentials in cortical reflex myoclonus. Brain 110: 1359–1373 [DOI] [PubMed] [Google Scholar]
- Keswani S.C., Kossoff E.H., Krauss G.L., Hagerty C. (2002) Amelioration of spinal myoclonus with levetiracetam. J Neurol Neurosurg Psychiatry 73: 457–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber T.E., Thompson P.D. (1997) Symptomatic hyperekplexia occurring as a result of pontine infarction. Mov Disord 12: 814–816 [DOI] [PubMed] [Google Scholar]
- Koukouni V., Valente E.M., Cordivari C., Bhatia K.P., Quinn N.P. (2008) Unusual familial presentation of epsilon-sarcoglycan gene mutation with falls and writer’s cramp. Mov Disord 23: 1913–1915 [DOI] [PubMed] [Google Scholar]
- Kyllerman M., Ben-Menachem E. (1998) Zonisamide for progressive myoclonus epilepsy: long-term observations in seven patients. Epilepsy Res 29: 109–114 [DOI] [PubMed] [Google Scholar]
- Lagueny A., Tison F., Burbaud P., Le Masson G., Kien P. (1999) Stimulus-sensitive spinal segmental myoclonus improved with injections of botulinum toxin type A. Mov Disord 14: 182–185 [DOI] [PubMed] [Google Scholar]
- Leppik I.E. (1999) Zonisamide. Epilepsia 40(Suppl. 5): S23–S29 [DOI] [PubMed] [Google Scholar]
- Li J.Y., Cunic D.I., Paradiso G., Gunraj C., Pal P.K., Lang A.E., et al. (2008) Electrophysiological features of myoclonus-dystonia. Mov Disord 23: 2055–2061 [DOI] [PubMed] [Google Scholar]
- Magarinos-Ascone C.M., Regidor I., Martinez-Castrillo J.C., Gomez-Galan M., Figueiras-Mendez R. (2005) Pallidal stimulation relieves myoclonus-dystonia syndrome. J Neurol Neurosurg Psychiatry 76: 989–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C.D., Hallett M., Fahn S. (1982) The nosology and pathophysiology of myoclonus, In: Marsden C.D., Fahn S. (eds), Movement Disorders. Butterworths: London, pp. 196–196–248 [Google Scholar]
- Misbahuddin A., Placzek M., Lennox G., Taanman J.W., Warner T.T. (2007) Myoclonus-dystonia syndrome with severe depression is caused by an exon-skipping mutation in the epsilon-sarcoglycan gene. Mov Disord 22: 1173–1175 [DOI] [PubMed] [Google Scholar]
- Muller B., Hedrich K., Kock N., Dragasevic N., Svetel M., Garrels J., et al. (2002) Evidence that paternal expression of the epsilon-sarcoglycan gene accounts for reduced penetrance in myoclonus-dystonia. Am J Hum Genet 71: 1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareyson D., Fancellu R., Mariotti C., Romano S., Salmaggi A., Carella F., et al. (2008) Adult-onset Alexander disease: a series of eleven unrelated cases with review of the literature. Brain 131: 2321–2331 [DOI] [PubMed] [Google Scholar]
- Pearce J.M. (2008) Palatal Myoclonus (syn. Palatal Tremor). Eur Neurol 60: 312–315 [DOI] [PubMed] [Google Scholar]
- Penney S.E., Bruce I.A., Saeed S.R. (2006) Botulinum toxin is effective and safe for palatal tremor: a report of five cases and a review of the literature. J Neurol 253: 857–860 [DOI] [PubMed] [Google Scholar]
- Plaster N.M., Uyama E., Uchino M., Ikeda T., Flanigan K.M., Kondo I., et al. (1999) Genetic localization of the familial adult myoclonic epilepsy (FAME) gene to chromosome 8q24. Neurology 53: 1180–1183 [DOI] [PubMed] [Google Scholar]
- Priori A., Bertolasi L., Pesenti A., Cappellari A., Barbieri S. (2000) gamma-hydroxybutyric acid for alcohol-sensitive myoclonus with dystonia. Neurology 54: 1706–1706 [DOI] [PubMed] [Google Scholar]
- Ritz K., Gerrits M.C., Foncke E.M., van Ruissen F., van der Linden C., Vergouwen M.D., et al. (2009) Myoclonus-dystonia: clinical and genetic evaluation of a large cohort. J Neurol Neurosurg Psychiatry 80: 653–658 [DOI] [PubMed] [Google Scholar]
- Rodriguez M.E., Artieda J., Zubieta J.L., Obeso J.A. (1994) Reflex myoclonus in olivopontocerebellar atrophy. J Neurol Neurosurg Psychiatry 57: 316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E., Apartis E., Clot F., Dorison N., Thobois S., Guyant-Marechal L., et al. (2008) Myoclonus-dystonia: clinical and electrophysiologic pattern related to SGCE mutations. Neurology 70: 1010–1016 [DOI] [PubMed] [Google Scholar]
- Rubboli G., Tassinari C.A. (2006) Negative myoclonus. An overview of its clinical features, pathophysiological mechanisms, and management. Neurophysiol Clin 36: 337–343 [DOI] [PubMed] [Google Scholar]
- Ruprecht K., Warmuth-Metz M., Waespe W., Gold R. (2002) Symptomatic hyperekplexia in a patient with multiple sclerosis. Neurology 58: 503–504 [DOI] [PubMed] [Google Scholar]
- Samuel M., Torun N., Tuite P.J., Sharpe J.A., Lang A.E. (2004) Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 127: 1252–1268 [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R., Shriberg J., Heiman G., Raymond D., Wendt K., Kramer P., et al. (2002) Myoclonus dystonia: possible association with obsessive-compulsive disorder and alcohol dependence. Neurology 58: 242–245 [DOI] [PubMed] [Google Scholar]
- Shahwan A., Farrell M., Delanty N. (2005) Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol 4: 239–248 [DOI] [PubMed] [Google Scholar]
- Shiang R., Ryan S.G., Zhu Y.Z., Hahn A.F., O’Connell P., Wasmuth J.J. (1993) Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet 5: 351–358 [DOI] [PubMed] [Google Scholar]
- Shibasaki H. (1995) Pathophysiology of negative myoclonus and asterixis. Adv Neurol 67: 199–209 [PubMed] [Google Scholar]
- Shibasaki H. (2006) Neurophysiological classification of myoclonus. Neurophysiol Clin 36: 267–269 [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Hallett M. (2005) Electrophysiological studies of myoclonus. Muscle Nerve 31: 157–174 [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Neshige R. (1987) Photic cortical reflex myoclonus. Ann Neurol 22: 252–257 [DOI] [PubMed] [Google Scholar]
- Tatu L., Moulin T., Martin V., Monnier G., Rumbach L. (2000) Unilateral pure thalamic asterixis: clinical, electromyographic, and topographic patterns. Neurology 54: 2339–2342 [DOI] [PubMed] [Google Scholar]
- Thompson P.D., Bhatia K.P., Brown P., Davis M.B., Pires M., Quinn N.P., et al. (1994a) Cortical myoclonus in Huntington’s disease. Mov Disord 9: 633–641 [DOI] [PubMed] [Google Scholar]
- Thompson P.D., Day B.L., Rothwell J.C., Brown P., Britton T.C., Marsden C.D. (1994b) The myoclonus in corticobasal degeneration. Evidence for two forms of cortical reflex myoclonus. Brain 117: 1197–1207 [DOI] [PubMed] [Google Scholar]
- Trottenberg T., Meissner W., Kabus C., Arnold G., Funk T., Einhaupl K.M., et al. (2001) Neurostimulation of the ventral intermediate thalamic nucleus in inherited myoclonus-dystonia syndrome. Mov Disord 16: 769–771 [DOI] [PubMed] [Google Scholar]
- van der Salm S.M., Koelman J.H., Henneke S., van Rootselaar A.F., Tijssen M.A. (2010) Axial jerks: a clinical spectrum ranging from propriospinal to psychogenic myoclonus. J Neurol 257: 1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J., Brown P., Thompson P.D., Marsden C.D. (1997) The clinical features and prognosis of chronic posthypoxic myoclonus. Mov Disord 12: 216–220 [DOI] [PubMed] [Google Scholar]
- Williams D.R., Cowey M., Tuck K., Day B. (2008) Psychogenic propriospinal myoclonus. Mov Disord 23: 1312–1313 [DOI] [PubMed] [Google Scholar]
- Wills A.J., Sawle G.V., Guilbert P.R., Curtis A.R. (2002) Palatal tremor and cognitive decline in neuroferritinopathy. J Neurol Neurosurg Psychiatry 73: 91–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A., Grabowski M., Asmus F., Naumann M., Berg D., Bertram M., et al. (2001) Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet 29: 66–69 [DOI] [PubMed] [Google Scholar]