Abstract
Over the past 5 years, there has been a rapid resurgence of interest in vitamin D outside of its traditional role in metabolic bone disease. Some nontraditional roles ascribed to vitamin D include anti-inflammatory and immune-modulating effects. These effects have led to possible implications in the pathophysiology of immune-mediated diseases including multiple sclerosis and inflammatory bowel disease (IBD). In addition, vitamin D insufficiency has been linked to higher rates of cancers including colon, prostate and breast cancers. Given these diverse associations of vitamin D and disease states, this review describes recent advances with regard to vitamin D and gastrointestinal diseases, in particular IBD and colorectal cancer.
Keywords: vitamin D, inflammatory bowel disease, colorectal cancer, immunology
Introduction
Over the past 5 years, there has been a rapid resurgence of interest in vitamin D outside of its traditional role in metabolic bone disease. Vitamin D is a hormone precursor present in two forms, ergocalciferol and cholecalciferol. Ergocalciferol or vitamin D2 is present in plants, yeast and fungi. In contrast cholecalciferol, vitamin D3, is synthesized in the skin upon exposure to sunlight. Vitamin D requirements may be fulfilled either by adequate ingestion and absorption or sun exposure. Historically, interest in vitamin D revolved around its major role in metabolic bone disease. However, novel insights into additional roles for vitamin D are being established, and interest in this vitamin is gaining popularity for many other reasons apart from those associated with bone disease.
Some nontraditional roles ascribed to vitamin D include anti-inflammatory and immune-modulating effects. These effects have led to possible implications in the pathophysiology of immune-mediated diseases including multiple sclerosis (MS) and inflammatory bowel disease (IBD). In addition, vitamin D insufficiency has been linked to higher rates of cancers including colon, prostate and breast cancers. For example, one group found that vitamin D and calcium supplementation reduced all cancer risk [Lappe et al. 2007]. In this study patients were randomized to receive 1400–1500 mg of supplemental calcium daily, supplemental calcium plus vitamin D3 1100 IU daily or placebo. Follow up was for 4 years. Patients receiving both supplemental calcium and vitamin D had a significant reduction in development of all cancers including colorectal cancer (CRC). When the analysis was confined to cancers diagnosed after the first 12 months, the reduction in cancer risk was even greater.
Given these diverse associations of vitamin D and disease states, our purpose in this review is to describe recent advances with regard to vitamin D and gastrointestinal diseases, in particular IBD and CRC.
The various roles of vitamin D
Vitamin D plays a key endocrine role in calcium and phosphate homeostasis. Most human vitamin D is derived endogenously upon exposure of the skin to UV light, leading to photochemical conversion of 7-dehydrocholesterol to cholecalciferol (vitamin D3). The skin production of vitamin D in response to UV exposure is self-limited, and sunlight exposure cannot cause vitamin D toxicity in normal vitamin D physiology.
Although vitamin D can be absorbed from the intestine, most foods contain insignificant amounts (except oily fish). Cholecalciferol is subsequently converted by hepatic vitamin D 25-hydroxylase to 25-hydroxyvitamin D (25OHD), the major circulating vitamin D metabolite. 25OHD is largely inactive, but a long half-life makes it the best indicator of overall vitamin D status. Finally, renal conversion of 25OHD by 25-hydroxyvitamin D 1α-hydroxylase produces the active vitamin D metabolite, 1,25-dihydroxyvitamin D3 (calcitriol or 1,25(OH)2D3). Serum calcium regulates 1α-hydroxylase, as do fibroblast growth factor 23 (FGF-23), phosphate and 1,25(OH)2D3 itself.
1,25(OH)2D3 stimulates intestinal absorption of orally ingested calcium and phosphate, tubular reabsorption of calcium filtered in the kidney, and mobilization of calcium and phosphate stores from the skeleton by stimulating maturation of osteoclasts (Figure 1). Chronic vitamin D deficiency results in undermineralization of bones, leading to rickets and osteomalacia [Holick, 2007].
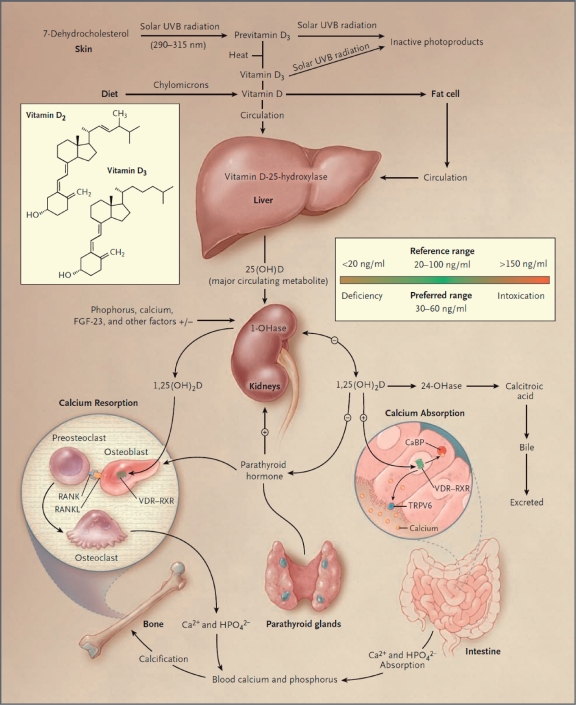
Figure 1.
Classical calcium and vitamin D homeostasis pathways. Vitamin D metabolism and functions. Under ultraviolet B light exposure, 7-dehydroxycholesterol is converted to vitamin D3 in the shin. Vitamin D3 is transported to the liver where it is converted to 25-hydroxyvitamin D3 (25-OHD) by 25-hydroxylase (25-OHase). 25-hydroxyvitamin D3 (25-OHD) is further converted to 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), the hormonal metabolite, by renal 1α-hydroxylase (1-OHase). 1α-hydroxylase. the rate-limiting enzyme, is stimulated by parathyroid hormone and feedback inhibited by 1,25(OH)2D3. 25-OHD and 1,25(OH)2D3 are further metabolized by 24-hydroxylase (24-OHase) to initiate their catabolism, which is stimulated by 1,25(OH)2D3. 1,25(OH)2D3 feedback inhibits parathyroid homione production. 1,25(OH)2D3 targets the intestine, kidney and bone to regulate calcium and phosphate homeostasis. The hormone also has other noncalcemic physiologic functions. 1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; 25-OHD, 25-hydroxyvitamin D3; 1-OHase, 1α-hydroxylase; 24-OHase, 24-hydroxylase; 25-OHase, 25-hydroxylase; PTH, parathyroid hormone; UVB, ultraviolet B light. [Holick, M.F. (2007) Vitamin D deficiency. N Engl J Med 357: 266-281] Copyright© [2007] Massachusetts Medical Society. All rights reserved.
Active 1,25(OH)2D3 is now known to exert its biological functions via the vitamin D receptor (VDR), a member of a superfamily of nuclear hormone receptors. This family includes the perioxisome proliferator-activated receptor and glucocorticoid receptor, implicated in the therapeutic action of the 5-aminosalicylates and glucocorticosteroids, respectively, in IBD [Probert et al. 1992]. Upon entering the target cell, 1,25(OH)2D3 binds with the VDR inducing a conformational change and heterodimerization with the retinoid X receptor (RXR). This increases the affinity of the VDR/RXR complex for a specific promoter region, the vitamin D responsive element, in vitamin D responsive genes leading to transcription [Carlberg and Polly, 1998] (Figure 2).
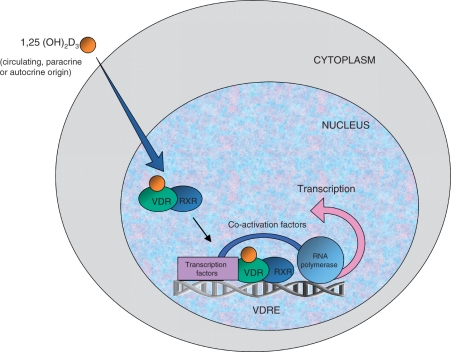
Figure 2.
Ligand-dependent gene transcription by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). Lipid soluble 1,25(OH)2D3 from serum, autocrine or paracrine sources enters the target cell and binds to the nuclear vitamin D receptor (VDR). This induces a conformational change and promotes heterodimerization with the retinoid X receptor (RXR). The VDR/RXR has an increased affinity for the vitamin D responsive element (VDRE). VRDE is a specific sequence of nucleotides in the promoter region of the vitamin D responsive gene. Binding of the VDR/RXR complex to the VDRE attracts a complex of co-activator proteins connecting VDRE with RNA polymerase II. Gene transcription then occurs, producing mRNA transcripts, which leave the nucleus for translation into the coded protein in the cytoplasm.
Importantly, many other tissues and cells in the body, including the immune system, not involved in calcium homeostasis, have been found to express VDR and possess the enzymes necessary to produce local 1,25(OH)2D3. This extrarenal enzyme activity is not subject to the same regulatory endocrine feedback mechanisms, but appears to be induced by other factors. These findings suggest vitamin D may have actions beyond simple endocrine activity, and may explain the far-reaching functions of vitamin D including its role in tuberculosis (TB) infection, diabetes mellitus, cardiovascular disease, congestive heart failure [Zittermann, 2006; Zittermann et al. 2003], and the regulation of blood pressure homeostasis through the renin–angiotensin system [Li et al. 2002]. In addition, vitamin D exhibits antiproliferative, cell differentiation and apoptotic effects in malignant cell lines, which may be protective against breast, colon and prostate cancer [Nagpal et al. 2010].
Vitamin D in colorectal cancer and inflammatory bowel disease
Colorectal cancer
The mechanisms by which vitamin D status may alter cancer development are still being delineated. Hundreds of genes contain vitamin D response elements [Carlberg, 2003], which encode for proteins important in the regulation of cell proliferation, differentiation and apoptosis [Diaz et al. 2000; Vanderwalle et al. 1994; Meggouh et al. 1991]. When vitamin D status is suboptimal, these activities are impaired. Similar to the prevalence of autoimmune diseases, it has long been noted that cancer mortality including mortality from CRC increases with geographical latitude [Garland and Garland, 1980]. In 1980, Garland and colleagues proposed that lower levels of vitamin D resulting from weaker UV-B radiation seen at higher latitudes may account for the geographical pattern of cancer mortality [Garland and Garland, 1980]. The latitudinal effect on serum 25OHD status, in combination with the potential biological effects of vitamin D deficiency on cancer incidence, resulted in numerous studies exploring the relationship between vitamin D and cancer risk; however until recently, the results had remained somewhat inconclusive. Recently, Yin and colleagues aimed to provide an updated review and meta-analysis of longitudinal epidemiological studies evaluating the association between 25OHD levels and CRC risk with a particular focus on potential variation by anatomic site [Yin et al. 2009]. Eight studies comprising 3556 patients were included in the analysis and support previous evidence that serum 25OHD levels are inversely associated with CRC risk (odds ratio (OR) 0.69; 95% confidence interval (CI) 0.55–0.86, p < 0.001) for patients with 25OHD levels in the highest compared with lowest quintiles. An increase of 25OHD by 20 ng/ml was associated with a risk reduction of 59% for rectal cancer and 22% for colon cancer. Analyses stratified by anatomical site suggest a stronger risk reduction for rectal cancers compared with colon cancers, however, this finding did not reach statistical significance.
Previous work suggested a 51% (OR 0.49; 95% CI 0.35–0.68) lower risk of CRC associated with the highest serum 25OHD quintile compared with the lowest quintile [Gorham et al. 2007]. An inverse association between plasma 25OHD and risk for colon cancer was seen in the Nurses Health Study (NHS), a predominantly female cohort, between levels in the highest quintile compared with those in the lowest quintile [Feskanich et al. 2004]. In the Third National Health and Nutrition Examination Survey, higher mortality from colon cancer was observed in patients with serum 25OHD levels lower than 50 nmol/l compared with those with levels greater than 80 nmol/l [Freedman et al. 2007].
Similarly, the relationship between serum 25OHD levels and colon cancer was investigated in a nested case–control study within the Health Professionals Follow-up Study (HPFS) [Wu et al. 2007]. As this was a male cohort, results were pooled with results from the NHS, to increase statistical power. The combined results from the HPFS and NHS studies showed that higher plasma 25OHD concentrations were statistically significantly associated with decreased risks of CRC, without any predilection for location of tumour (proximal or distal colon).
One of the largest studies to date and one of the first based on European populations showed that compared with a mid-range concentration of 50–75 nmol/l, 25OHD levels lower than 50 nmol/l was associated with an increased risk of CRC [Jenab et al. 2010]. Patients with 25OHD levels greater than 100 nmol/l had a significant 40% lower risk of CRC than those patients with levels lower than 25 nmol/l.
Previous studies have also shown a significant 30% reduction in formation of colorectal adenomas among patients with higher versus lower 25OHD levels, further supporting biological plausibility for a potential role of vitamin D in colorectal carcinogenesis.
The data regarding decreased cancer mortality observed in randomized clinical trials with vitamin D supplementation is somewhat conflicting. The first study exploring the relationship between vitamin D supplementation and CRC risk was conducted by Wactawski-Wende and colleagues [Wactawski-Wende et al. 2006]. Patients were randomized to receive 1000 mg of elemental calcium carbonate and 400 IU of vitamin D3 or placebo for 7 years. Results of this study did not show a significant reduction in the incidence of CRC in the 7-year follow-up period. During the time period that this study was conducted, doses of 400 IU/day were considered adequate and perhaps high. However, studies published since then have led to some recommendations of higher vitamin D daily intakes [Gorham et al. 2005]. In addition, a follow-up period of 7 years may not have been of sufficient duration to identify cancer development.
Inflammatory bowel disease
The pathogenesis of IBD such as Crohn’s disease (CD) and ulcerative colitis (UC) is complex and incompletely understood. It is thought to involve a complex interplay between genetic, environmental and microbial environments in the context of an inappropriate and abnormal activation of the mucosal immune system. Particularly, a dysregulated intestinal mucosal T-cell-mediated immune response, specifically CD4+ T helper type-1 (Th1) lymphocytes, leads to production of Th1-associated pro-inflammatory cytokines such as interferon-γ (IFN-γ) and tumour necrosis factor-alpha (TNF-α). Another potential pathogenic factor in CD is impaired mucosal barrier function and intestinal hyperpermeability [Gibson, 2004]. A relatively high number of first-degree relatives of patients with CD have increased intestinal permeability in the absence of clinical symptoms suggesting that permeability issues may precede clinical symptoms [Peeters et al. 1997]. The genetics of CD demonstrate that nucleotide-binding oligomerization domain containing 2 (NOD2) insufficiency contributes to development of the disease. Recently, one group observed that 1,25D signalling is a direct inducer of NOD2 expression arguing strongly that vitamin D insufficiency/deficiency does play a causative role in the prevalence of CD [Wang et al. 2010].
Epidemiological evidence for vitamin D in the pathogenesis of inflammatory bowel disease
The association of temperate climates (e.g. northern latitudes with lower sunlight exposures) with higher incidences and prevalence of autoimmune diseases has led to the implication of vitamin D in their pathogenesis. The classical example of such a geographical association is the higher incidence of MS in temperate climates, interestingly with the acquisition of indigenous risk by young immigrants [Warrell, 1996].
A similar observation has been demonstrated in the higher incidence and prevalence of IBD in northern European countries (e.g. UK and Scandinavia) compared with their sunnier southern counterparts (e.g. Croatia) [Loftus and Sandborn, 2002]. In addition, people living near the equator are at low risk of developing IBD, however, upon migration to developed countries in temperate climates, the risk of IBD increases [Carr and Mayberry, 1999; Probert et al. 1990]. Furthermore, a seasonal variation in onset and exacerbations of IBD has been noted. For example, symptomatic onset of UC seems to peak in December [Moum et al. 1996; Sellu, 1986], whereas higher CD relapse rates have been noted in autumn and winter [Zeng and Anderson, 1996]. In keeping with these findings, a seasonal variation in vitamin D levels has been demonstrated in patients with CD [Vogelsang et al. 1989] and a high prevalence of vitamin D deficiency exists in patients with established CD [Vogelsang et al. 1989; Harries et al. 1985; Driscoll et al. 1982], but also remains common even when the disease is in remission [Andreassen et al. 1998, 1997]. In addition, IBD patients are also prone to vitamin D intestinal malabsorption, especially following small-bowel resection or the use of cholestyramine for postresectional diarrhoea, both of which deplete bile acids essential for vitamin D absorption.
One recent group sought to describe the clinical disease characteristics based on serum 25OHD levels in patients with CD [Joseph et al. 2009]. They found that disease activity as assessed by the Harvey Bradshaw score correlated negatively with the serum 25OHD levels. Interestingly, lower vitamin D levels were seen in patients with jejunal involvement. The only predictors of 25OHD levels in this study not surprisingly were disease severity and sunlight exposure. One needs to ask however whether pre-existing vitamin D deficiency was the initiating event leading to disease severity, or whether vitamin D deficiency was the consequence of severe underlying illness. It is likely that the truth is somewhere in between as patients with more active disease are likely to receive less sunlight and not absorb adequate amounts of vitamin D received in the diet. Although, this was one of the first studies describing the correlation between serum 25OHD levels and disease severity, conclusions regarding the role of vitamin D deficiency as a predictor of disease severity cannot be generalized from these limited data.
Vitamin D and the immune system
The observation that VDR is expressed significantly in the immune system, including peripheral blood monocytes, leucocytes, antigen-presenting cells and activated CD4+ T cells has raised the possibility that VDR agonists may have immunomodulatory activity [Veldman et al. 2000; Brennan et al. 1987; Manolagas et al. 1986; Bhalla et al. 1983; Provvedini et al. 1983]. In addition, the demonstration that dendritic cells (DCs) and, to a lesser extent, activated T lymphocytes have the capacity to synthesize 1,25(OH)2D3 from sunlight-derived precursors suggests immune autocrine/paracrine activity [Sigmundsdottir et al. 2007]. Although vitamin D has no direct antimicrobial activity, there is evidence that 1,25(OH)2 can modulate host response while deficiency increases susceptibility and severity in Mycobacterium tuberculosis infection. Novel experiments have shown that monocytes and macrophages exposed to TB upregulate both the VDR and 25(OH)D-1α-hydroxylase. In addition, 1,25(OH)2D3, enhances the ability of mononuclear phagocytes to suppress the intracellular growth of TB in vitro, particularly in American-African individuals known to have an increased susceptibility to both TB and vitamin D deficiency [Liu et al. 2006]. Furthermore, a recent study has also shown that a single high oral dose of vitamin D3 (100,000 IU) significantly enhances the antimycobacterial immunity of tuberculosis contacts by restricting recombinant M. bovis in vitro [Martineau et al. 2007]. Interestingly, these recent findings of modern science add credence to the historical TB treatment of sunlight and cod liver oil (rich in vitamin D) back in the 1800s [Liu et al. 2006].
Effect of vitamin D on T-cell-mediated immunity
Monocyte-derived DCs are highly specialized antigen-presenting cells (APCs), which play a critical and central gatekeeping role in the initiation of mucosal CD4+ T-cell responses. Following the uptake of antigen, DCs mature into potent APCs and present antigen, in association with major histocompatibility complex (MHC) class II molecules, to the T-cell receptor (TCR) of naïve T cells. T-cell activation then occurs in the presence of required additional DC (CD80/CD86/CD40) and T-cell (CD28 and CD154) costimulatory signals [Banchereau et al. 2000]. Several studies have demonstrated inhibition of precursor monocyte differentiation into DCs by 1,25(OH)2D3 or its analogues [van Halteren et al. 2002; Canning et al. 2001; Berer et al. 2000; Griffin et al. 2000; Penna and Adorini, 2000; Piemonti et al. 2000]. This has been confirmed in both in vitro studies performed on peripheral blood monocyte-derived DCs obtained from healthy individuals and IBD patients [Stio et al. 2005; Rigby et al. 1984]. There is also substantial evidence that 1,25(OH)2D3 directly inhibits T-cell activation and proliferation [Bhalla et al. 1984; Rigby et al. 1984]. The expression of the essential costimulatory markers is also inhibited [van Halteren et al. 2002; Canning et al. 2001; Berer et al. 2000; Griffin et al. 2000; Penna and Adorini, 2000; Piemonti et al. 2000]. Furthermore, it is now recognized that vitamin-D-cultured DCs have tolerogenic properties (characterized by decreased costimulatory expression of CD40, 80, 86 and class II MHC molecules), which in the mixed lymphocyte reaction demonstrated a reduced ability to activate allogenic T cells, which themselves were hyporesponsive with limited IFN-γ production. These tolerogenic DCs also induce T cells with suppressive activity [Canning et al. 2001; Berer et al. 2000; Griffin et al. 2000; Jonuleit et al. 2000; Penna and Adorini, 2000; Piemonti et al. 2000]. Importantly, vitamin-D-treated DCs retained an immature phenotype despite the withdrawal of vitamin D, a feature not seen in the response to corticosteroids, which also impair DC maturity and proliferation [Griffin et al. 2001]. Also peripheral blood monocyte cells cultured in vitro with dexamethasone and/or 1,25(OH)2D3 demonstrated reduced lymphocyte proliferation with 1,25(OH)2D3 alone, but with an additive antiproliferation effect in combination with steroids. These tolerogenic effects have also been demonstrated in vivo during treatment with 1,25(OH)2D3 and mycophenolate mofetil, which induced tolerance to fully mismatched islet allografts in mice. This finding is associated with an increased percentage of CD4+CD25+ regulatory T cells which, when transferred, induced transplant tolerance in the recipient [Gregori et al. 2001].
The phenotype of T-cell-mediated response upon DC-modulated activation is also under the direction of specific DC-derived cytokines [Mannon et al. 2004]. Interleukin (IL)-12 is an important cytokine that plays a major role in driving pro-inflammatory Th1 differentiation implicated in the pathogenesis of IBD. IL-12 is also suspected of inhibiting T-cell apoptosis [Marth et al. 1999]. 1,25(OH)2D3 has been convincingly demonstrated to inhibit IL-12 production [Penna and Adorini, 2000; Lemire et al. 1995], probably by interfering with nuclear factor kappa B (NFκβ)-induced IL-12 transcription. Conversely, 1,25(OH)2 upregulates DC-derived IL-10 production, promoting the anti-inflammatory Th2 cell phenotype [Canning et al. 2001; Penna and Adorini, 2000], whilst inhibiting Th1 pathways by both downregulating IL-12 production and by blocking IFN-γ synthesis by differentiated Th1 T cells [Moore et al. 2001]. IL-10 also induces regulatory T cells and strongly inhibits production of other pro-inflammatory monokines, such as IL-1, IL-6 and TNF-α. Furthermore, when antigen-stimulated peripheral blood monocytes from CD patients were cultured in vitro with a vitamin D analogue, not only was there a significant reduction in cell proliferation, production of TNF-α and the associated inflammatory transcription factor NFκβ were also impaired [Stio et al. 2007]. T-cell production of IFN-γ is also directly inhibited by reduced 1,25(OH)2D3-mediated IFN-γ gene transcription [Cippitelli and Santoni, 1998].
An important observation with potential therapeutic implications is the apparent synergistic effect of vitamin D when combined with other conventional treatments and immunomodulators. A combination of steroids with vitamin D was more effective in reducing the Th1 cytokine IFN-γ and increasing Th2 cytokines IL5/IL10/IL13 [Barrat et al. 2002; Jirapongsananuruk et al. 2000]. Pretreatment in vivo with anti-TNF-α treatment (infliximab) is synergistic with vitamin D in reducing TNF-α [Stio et al. 2005]. However, unlike other immunosuppressants, which also appear able to induce a tolerogenic DC phenotype (e.g. MMF, sirolimus and glucocorticosteroids), only vitamin D and its analogues appear able to specifically increase IL-10 [Penna and Adorini, 2000]. Interestingly, a vitamin D analogue also worked synergistically with the calcineurin inhibitor cyclosporin, often used in the treatment of UC, when used in a mouse model of experimental autoimmune encephalitis and with T cells from UC patients, by potent inhibition of APC antigen presentation and inhibition of TCR-mediated T-cell activation and proliferation, but possibly also by suppression of IL-2 transcription, an autocrine T-cell growth factor [Van Etten, 2007; Stio et al. 2002; Alroy et al. 1995].
Chemotactic cytokines (or chemokines) and their cell receptors are important in determining the tissue-specific homing and microenvironmental destination of immune cells [Kunkel and Butcher, 2002]. There is also recent evidence that vitamin D can influence DC-mediated homing marker expression on activated T cells, including integrin intestinal-homing receptor α4β7, chemokine receptor (CCR) type 9 (CCR9) (gut homing) and CCR10 (skin homing). Vitamin D has been shown to inhibit the spontaneous upregulation of α4β7 during T-cell activation and prevent upregulation of CCR (in response to retinoic acid. Vitamin D also upregulates CCR10. This modulation of homing marker expression has obvious implications for redirection of immune cells implicated in IBD [Sigmundsdottir et al. 2007].
Overall 1,25(OH)2D3 appears to inhibit Th1 immune responses, promote desirable Th2 responses and influence immune cell homing marker expression. This in vitro laboratory data is supported by several novel in vivo animal models of IBD, which demonstrate powerful evidence of therapeutic immunomodulation by vitamin D.
Vitamin D and maintenance of the intestinal mucosal barrier
The integrity of the intestinal mucosal barrier is preserved by the enormous regenerating capacity of the mucosal epithelium. One potential pathogenic factor in the etiopathology of IBD is impaired mucosal barrier function [Gibson, 2004]. Recent animal data suggest that maintenance of the epithelial barrier integrity of the large intestine by vitamin D is critical in preventing IBD development [Kong et al. 2008]. In this study, it appeared that the VDR was required for mucosal repair in the mouse model of colitis, as supported by the observation that VDR expression was markedly induced in colonic mucosa during mucosal recovery in mice. In vitro studies have consistently demonstrated that vitamin D stimulates epithelial cell migration, suggesting that vitamin D is involved in the regulation of epithelial restitution in wound healing. These observations may explain at least in part the associations between vitamin D deficiency and IBD.
Animal models of inflammatory bowel disease
The IL-10 knockout (IL10-KO) mouse develops spontaneous severe panenterocolitis due to lack of IL-10 (a regulatory anti-inflammatory Th2 cytokine) [Kühn et al. 1993]. Vitamin-D-deficient IL10-KO mice develop an accelerated form of enterocolitis with early mortality, while vitamin-D-deficient normal (wild-type) mice do not develop enterocolitis. Interestingly, when vitamin-D-deficient IL10-KO mice received dietary vitamin D or 1,25(OH)2D3 enterocolitis did not develop. Furthermore, 1,25(OH)2D3 supplementation ameliorated and blocked progression of IBD symptoms in IL10-KO mice with established IBD [Cantorna et al. 2000]. This is strong evidence for vitamin D as an anti-inflammatory immunomodulator in IBD.
The importance of VDR in immunomodulation of the inflammatory response is highlighted in other models of IBD. In a T-cell transfer model of IBD, CD4+/CD45RBhigh cells are transferred into immunodeficient mice (recombinase-activated gene 2 (Rag-2) knockout mice that lack mature T and B cells) inducing enterocolitis. CD4+/CD45RBhigh T cells from VDR knockout (VDR-KO) mice induced a more severe form of IBD than CD4+/CD45RBhigh T cells from wild-type mice. In addition, in comparison to IL10-KO mice, VDR/IL-10 double knockout mice developed more severe colitis of more rapid onset with an increased 100% mortality [Froicu et al. 2003].
VDR-KO mice are extremely sensitive to chemically induced colitis and fail to recover spontaneously upon withdrawal of the chemical insult. Expression of several pro-inflammatory cytokines is also increased (e.g. TNF-α, IL-1α, IL-1β, IL-12, IFN-γ) and injection of lipopolysaccharide leads to a hyperactive inflammatory response. Dietary supplementation with 1,25(OH)2D3 reduced severity of dextran sulphate sodium-induced colitis in wild-type mice but unsurprisingly not in VDR-KO mice, demonstrating a requirement for a functional VDR for vitamin D efficacy [Froicu and Cantorna, 2007]. The contribution of 1,25(OH)2D3 in immune responses and maintenance of tolerance to self antigens is also suggested by the enlarged lymph nodes in VDR-deficient mice, which contain higher numbers of mature DCs, presumably as a result of the loss of vitamin-D-associated modulation of DC maturation and proliferation [Griffin et al. 2001].
Finally, an important study of 2,4,6-trinitrobenzenesulphonic acid-induced colitis (chemical model of Crohn’s colitis in mice) compared the benefits of corticosteroids and 1,25(OH)2D3 in vivo administered before and after induction of colitis. 1,25(OH)2D3 alone reduced clinical severity significantly (p < 0.05), but combination treatment (dexamethasone plus 1,25(OH)2D3) demonstrated the most effective reduction of IBD severity (p < 0.001). This was effective in both preventing severe colitis and ameliorating effects if given in established colitis. Both treatments independently downregulated Th1 (reduced Il-12, TNF-α, IFN-γ, IF-1β and T-bet expression) and upregulated Th2 responses (increased GATA3 and IL-4), as well as downregulating DCs responsible for pro-inflammatory differentiation of Th1 cells. In addition, Th17 responses (implicated in inflammation) were also downregulated. Interestingly, 1,25(OH)2D3 alone, but reinforced by additional dexamethasone, also promoted a regulatory T-cell profile [Daniel et al. 2008]. This is important as it implies a potential therapeutic steroid-sparing clinical application of 1,25(OH)2D3 derivatives in active IBD.
It should also be noted, that in addition to vitamin D, dietary calcium also appears to have an independent effect on IBD severity in animal models. Dietary calcium plus 1,25(OH)2D3 resulted in maximal suppression in murine experimental IBD. This finding is also documented in other animal models of autoimmune disease [Zhu et al. 2005].
What 25-hydroxyvitamin D level is sufficient for immunomodulatory actions?
Serum 25OHD is the accepted biomarker of vitamin D status. It has been demonstrated that 25OHD concentrations greater than 20–25 nmol/l indicate severe vitamin D deficiency, which will lead to rickets and osteomalacia [Holick, 2007]. However, there is no formal consensus of optimal 25OHD status at present, although expert opinion ranges from 50 to 100 nmol/l. These target 25OHD levels are based on the rationale that only patients with 25OHD levels above 50 nmol/l show no significant change in circulating parathyroid hormone (PTH) levels subsequent to vitamin D therapy. Furthermore, if based on attaining 25OHD levels at which a functional effect is achieved, the inverse relationship between 25OHD and PTH levels, suggests PTH suppression beneficial to bones and based on fracture prevention studies occurs at 25OHD levels 75–100 nmol/l. Nevertheless, the circulating serum 25OHD level that is optimal for the immune system is not known, however, there is some suggestion that 25OHD levels of around 75 nmol/l may be optimal as evidenced in TB defence [Liu et al. 2006].
The daily vitamin D dosage required to achieve adequate 25OHD levels are also unclear. Vitamin D is potentially toxic and can lead to hypercalcaemia and hypercalciuria, however, the current recommended safe daily dose limits remain both controversial and conservative (1000 IU/day and 2000 IU/day in the UK and North America, respectively [Vieth, 2006; Food Standards Agency, 2003]. The optimum dose and 25OHD levels for bone protection have not yet been established.
Several recent expert reviews now suggest traditional doses of 800 IU–1000 IU/day used for bone protection are woefully inadequate and based on outdated and insufficient evidence [Holick, 2004; Lips, 2004; Vieth, 2004; Heaney et al. 2003]. Indeed, these guidance doses of 1000–2000 IU/day seem somewhat lacking when compared with the suggested physiological daily limit of 10,000 IU vitamin D/day generated by total-body sunlight exposure in the absence of toxicity [Vieth, 1999; Barger-Lux et al. 1996; Davie et al. 1982; Stamp, 1975].
A single high oral dose of vitamin D3 (100,000 IU) given to individuals with TB contacts, induced a 91% increase in mean serum 25OHD, correcting any pre-existing vitamin D deficiency (<20 nmol/l) for at least 6 weeks, without causing hypercalcaemia [Martineau et al. 2007]. Recent studies in healthy subjects have shown that intakes of vitamin D at 4000–11,000 IU/day over 5–6 months in healthy subjects are safe and do not result in hypercalciuria, which occurs when circulating levels of 25OHD3 are above with no reported toxicity up to 25OHD levels of 250 nmol/l [Hollis and Wagner, 2004; Heaney et al. 2003; Vieth et al. 2001; Vieth, 1999]. Indeed, Aloia and colleagues recently demonstrated doses of 3800 and 5000 IU/day are required over a 6-month period to raise baseline 25OHD levels of >55 nmol/l and <55 nmol/l, respectively, to over 75 nmol/l [Aloia et al. 2008].
Trials of vitamin D interventions for immune modulation in humans
There are now many documented animal studies of the immunomodulatory properties of vitamin D in IBD, however to date, there are no published clinical trials in human patients. Although fish oils, when naturally derived, are a good source of vitamin D3, studies of efficacy in IBD (UC) have used largely purified or manufactured forms likely to contain negligible amounts of vitamin D. Fish oils also contain polyunsaturated fatty acids, namely docosahexaenoic acid and eicosapentaenoic acid, which are also thought to exhibit anti-inflammatory activity. Nevertheless, despite trials of fish oils in UC suggesting some benefit, a recent Cochrane analysis of six papers of differing quality concluded there was insufficient evidence to recommend clinical use in IBD [De Ley et al. 2007].
It is important to highlight that basic physiological effects using interventions with vitamin D are designed specifically to target 25OHD levels, in contrast to treatments using the ‘active’ vitamin D hormone 1,25(OH)2 cholecalciferol or its analogues designed to achieve an immunomodulatory effect. In the first case, one is treating a deficiency, whereas in the second case, one is testing a potential therapy. The active form of vitamin D, 1,25(OH)2D3 plays an important role in calcium homeostasis, cell differentiation and proliferation, immunity and cardiovascular function. Given its immunomodulatory properties, 1,25(OH)2D3 or its analogues might be clinically useful for the treatment of inflammatory and autoimmune diseases. It is believed that the active vitamin D hormone exerts physiological and pharmacological effects by binding to the VDR and then sustains a conformational change. Although 1,25(OH)2D3 could be a potentially useful immunomodulatory agent for clinical use, it can cause some serious adverse effects, in particular the induction of hypercalcaemia and bone resorption. Therefore, multiple drug development efforts are aimed at finding 1,25(OH)D3 analogues that exert immunomodulatory effects without causing these complications and are well under way.
In the appropriate context, physiological treatment of vitamin D deficiency does not necessarily guarantee the immunomodulatory benefits of higher pharmacological doses. In fact, it is more likely that the pharmacological benefits of supraphysiological doses of vitamin D are necessary to achieve immunomodulatory properties, in the face of vitamin D sufficiency.
There are a few limited clinical trials of vitamin D in autoimmune disease with Th1-mediated aetiology. Kimball and colleagues gave increasing oral doses of vitamin D (28,000–280,000 IU/week) over 4 weeks in 12 patients with an active phase of MS (together with 1.2 g calcium/day), safely achieving mean serum 25OHD concentrations twice the upper physiological range [Kimball et al. 2007]. Although disease progression and activity did not appear to be affected, the number of gadolinium-enhanced lesions on magnetic resonance imaging significantly decreased [Kimball et al. 2007]. Other studies using lower doses of vitamin D (1000–5000 IU/day) have suggested significant increases in anti-inflammatory cytokine (transforming growth factor beta 1) [Mahon et al. 2003] and reported a reduction in exacerbations and clinical severity in MS [Nordvik et al. 2000; Goldberg et al. 1986]. In a small case–control study, rheumatoid arthritis patients supplemented with a synthetic 1,25(OH)2D3 precursor (alphacalcidiol or 1α-hydroxyvitamin D3) also demonstrated a reduction in the severity of symptoms [Andjelkovic et al. 1999].
Summary
In summary, there is rapidly increasing epidemiological and strong experimental evidence, suggesting a role for vitamin D in IBD and CRC. Although data to date have been demonstrated in largely in vitro studies and murine models of IBD, it is clear that vitamin D potentially has potent immunomodulatory actions on the T-cell-mediated processes implicated in the pathogenesis of IBD, both at DC and T-cell level. In light of this evidence, well-conducted clinical trials of vitamin D or its analogues in human IBD patients are strongly indicated to assess further the potential therapeutic immunomodulatory properties of this much underestimated nutrient. Consideration to conducting randomized controlled clinical trials with vitamin D treatment using both physiological and pharmacological doses, in conjunction with standard of care therapy for CD should be provided. Of particular interest would be the effect of pre-existing serum 25OHD levels on the magnitude of response to therapy. Strong consideration for appropriate vitamin D supplementation should be provided to patients at risk for CRC.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest statement
No conflicts of interest exist.
References
- Aloia J.F., Patel M., DiMaano R., Li-Ng M., Talwar S.A., Mikhail M., et al. (2008) Vitamin D intake to attain a serried serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87: 1952–1958 [DOI] [PubMed] [Google Scholar]
- Alroy I., Towers T.L., Freedman L.P. (1995) Transcriptional repression of the interleukin-2 gene by vitamin D3: Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol 15: 5789–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic Z., Vojinovic J., Pejnovic N., Popovic M., Dujic A., Mitrovic D., et al. (1999) Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 17: 453–456 [PubMed] [Google Scholar]
- Andreassen H., Rix M., Brot C., Eskildsen P. (1998) Regulators of calcium homeostasis and bone mineral density in patients with Crohn’s disease. Scand J Gastroenterol 33: 1087–1093 [DOI] [PubMed] [Google Scholar]
- Andreassen H., Rungby J., Dahlerup J.F., Mosekilde L. (1997) Inflammatory bowel disease and osteoporosis. Scand J Gastroenterol 32: 1247–1255 [DOI] [PubMed] [Google Scholar]
- Banchereau F., Briere C., Caux J., Davoust S., Lebecque Y.J., Liu B. (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811 [DOI] [PubMed] [Google Scholar]
- Barger-Lux M.J., Heaney R.P., Lanspa S.J., Healy J.C., DeLuca H.F. (1996) An investigation of sources of variation in calcium absorption efficiency. J Clin Endocrinol Metab 80: 406–411 [DOI] [PubMed] [Google Scholar]
- Barrat F.J., Cua D.J., Boonstra A., Richards D.F., Crain C., Savelkoul H.F., et al. (2002) In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. Combination of calcitriol and dexamethasone induced high nos of IL10 producing T cells. J Exp Med 195: 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer A., Stockl J., Majdic O., Wagner T., Kollars M., Lechner K., et al. (2000) 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp Hematol 28: 575–583 [DOI] [PubMed] [Google Scholar]
- Bhalla A.K., Amento E.P., Clemens T.L., Holick M.F., Krane S.M. (1983) Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab 57: 1308–1310 [DOI] [PubMed] [Google Scholar]
- Bhalla A.K., Amento E.P., Serog B., Glimcher L.H. (1984) 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 133: 1748–1754 [PubMed] [Google Scholar]
- Brennan A., Katz D.R., Nunn J.D., Barker S., Hewison M., Fraher L.J., et al. (1987) Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology 61: 457–461 [PMC free article] [PubMed] [Google Scholar]
- Canning M.O., Grotenhuis K., deWit H., Ruwhof C., Drexhage H.A. (2001) 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol 145: 351–357 [DOI] [PubMed] [Google Scholar]
- Cantorna M.T., Munsick C., Bemiss C., Mahon B.D. (2000) 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine IBD. J Nutr 130: 2648–2652 [DOI] [PubMed] [Google Scholar]
- Carlberg C. (2003) Current understanding of the function on the nuclear vitamin D receptor in response to its natural and synthetic ligands. Recent Results Cancer Res 164: 29–42 [DOI] [PubMed] [Google Scholar]
- Carlberg C., Polly P. (1998) Gene regulation by vitamin D3. Crit Rev Eukaryot Gene Expr 8: 19–42 [DOI] [PubMed] [Google Scholar]
- Carr I., Mayberry J.F. (1999) The effects of migration on ulcerative colitis: A three-year prospective study among Europeans and first and second-generation South Asians in Leicester (1991–1994). Am J Gastroenterol 94: 2918–2922 [DOI] [PubMed] [Google Scholar]
- Cippitelli M., Santoni A. (1998) Vitamin D3: A transcriptional modulator of the interferon-gamma gene. Eur J Immunol 28: 3017–3030 [DOI] [PubMed] [Google Scholar]
- Daniel C., Sartory N.A., Zahn N., Radeke H.H., Stein J.M. (2008) Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324: 23–33 [DOI] [PubMed] [Google Scholar]
- Davie M.W., Lawson D.E., Emberson C., Barnes J.L., Roberts G.E., Barnes N.D. (1982) Vitamin D from skin: Contribution to vitamin D status compared with oral vitamin D in normal and anticonvulsant-treated subjects. Clin Sci 63: 461–472 [DOI] [PubMed] [Google Scholar]
- De Ley M., de Vos R., Hommes D.W., Stokkers P.C. (2007) Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst Rev Issue 4. Art. No.: CD005986. [DOI] [PubMed] [Google Scholar]
- Diaz G.D., Paraskeva C., Thomas M.G., Binderup L., Hague A. (2000) Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: Possible implications for prevention and therapy. Cancer Res 60: 2304–2312 [PubMed] [Google Scholar]
- Driscoll R.H., Jr, Meredith S.C., Sitrin M., Rosenberg I.H. (1982) Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology 83: 1252–1258 [PubMed] [Google Scholar]
- Feskanich D., Ma J., Fuchs C.S., Kirkner G.J., Hankinson S.E., Hollis B.W., et al. (2004) Plasma vitamin D metbolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 13: 1502–1508 [PubMed] [Google Scholar]
- Freedman D.M., Looker A.C., Chang S.C., Graubard B.I. (2007) Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 99: 1594–1602 [DOI] [PubMed] [Google Scholar]
- Food Standards Agency (2003) Safe Upper Levels for Vitamins and Minerals, Foods Standards Agency: London [Google Scholar]
- Froicu M., Weaver V., Wynn T.A., McDowell M.A., Welsh J.E., Cantorna M.T. (2003) A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17: 2386–2392 [DOI] [PubMed] [Google Scholar]
- Froicu M., Cantorna M.T. (2007) Vitamin D and the VDR are critical for control of the innate immune response to colonic injury. BMC Immunol 8: 5–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C.F., Garland F.C. (1980) Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9: 227–231 [DOI] [PubMed] [Google Scholar]
- Gibson P.R. (2004) Increased gut permeability in Crohn’s disease: Is TNF the link? Gut 53: 1724–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg P., Fleming M.C., Picard E.H. (1986) Multiple sclerosis: Decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 21: 193–200 [DOI] [PubMed] [Google Scholar]
- Gorham E.D., Garland C.F., Garland F.C., et al. (2007) Optimal vitamin D status for colorectal cancer prevention: A quantitative meta analysis. Am J Prev Med 32: 210–216 [DOI] [PubMed] [Google Scholar]
- Gorham E.D., Garland C.F., Garland F.C., et al. (2005) Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 97: 179–194 [DOI] [PubMed] [Google Scholar]
- Gregori S., Casorati M., Amuchastegui S., Smiroldo S., Davalli A.M., Adorini L. (2001) Regulatory T cells induced by 1a,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167: 1945–1953 [DOI] [PubMed] [Google Scholar]
- Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. (2000) Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun 270: 701–708 [DOI] [PubMed] [Google Scholar]
- Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. (2001) Dendritic cell modulation by 1alpha, 25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 22: 22–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries A.D., Brown R., Heatley R.V., Williams L.A., Woodhead S., Rhodes J. (1985) Vitamin D status in Crohn’s disease: Association with nutrition and disease activity. Gut 26: 1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Barger-Lux M.J. (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210 [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2004) Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79: 362–371 [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Hollis B.W., Wagner C.L. (2004) Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr 79: 717–726 [DOI] [PubMed] [Google Scholar]
- Jenab M., Bueno-de-Mesquita H.B., Ferrari P., van Duijnhoven F.J., Norat T., Pischon T., et al. (2010) Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: A nested case-control study. BMJ 340: b5500–b5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirapongsananuruk O., Melamed I., Leung D.Y. (2000) Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1 but not TH2 responses. J Allergy Clin Immunol 106: 981–985 [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. (2000) Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 192: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A.J., George B., Pulimood A.B., Seshadri M.S., Chacko A. (2009) 25(OH) Vitamin D level in Crohn’s disease association with sun exposure and disease activity. Indian J Med Res 130: 133–137 [PubMed] [Google Scholar]
- Kimball S.M., Ursell M.R., O’Connor P., Vieth R. (2007) Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr 86: 645–651 [DOI] [PubMed] [Google Scholar]
- Kong J., Zhang Z., Musch M., et al. (2008) Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216 [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274 [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Butcher E.C. (2002) Chemokines and the tissue-specific migration of lymphocytes. Immunity 16: 1–4 [DOI] [PubMed] [Google Scholar]
- Lappe J.M., Travers-Gustafson D., Davies K.M., Recker R.R., Heaney R.P. (2007) Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr 85: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Lemire J.M., Archer D.C., Beck L., Spiegelberg H.L. (1995) Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J Nutr 125: 1704S–1708S [DOI] [PubMed] [Google Scholar]
- Li Y.C., Kong J., Wei M., Chen Z.F., Liu S.Q., Cao L.P. (2002) 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. (2004) Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Mol Biol 89–90: 611–614 [DOI] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773 [DOI] [PubMed] [Google Scholar]
- Loftus E.V., Jr, Sandborn W.J. (2002) Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 31: 1–20 [DOI] [PubMed] [Google Scholar]
- Mahon B.D., Gordon S.A., Cruz J., Cosman F., Cantorna M.T. (2003) Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol 134: 128–132 [DOI] [PubMed] [Google Scholar]
- Mannon P.J., Fuss I.J., Mayer L., Elson C.O., Sandborn W.J., Present D., et al. (2004) Anti-interleukin-12 antibody for active Crohn’s disease. N Eng J Med 351: 2069–2079 [DOI] [PubMed] [Google Scholar]
- Manolagas S.C., Provvedini D.M., Murray E.J., Tsoukas C.D., Deftos L.J. (1986) The antiproliferative effect of calcitriol on human peripheral blood mononuclear cells. J Clin Endocrinol Metab 63: 394–400 [DOI] [PubMed] [Google Scholar]
- Marth T., Zeitz M., Ludviksson B.R., Strober W., Kelsall B.L. (1999) Extinction of IL-12 signalling promotes FAS-mediated apotosis of antigen-specific T-cells. J Immunol 162: 7233–7240 [PubMed] [Google Scholar]
- Martineau A.R., Wilkinson R.J., Wilkinson K.A., Newton S.M., Kampmann B., Hall B.M., et al. (2007) A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176: 208–213 [DOI] [PubMed] [Google Scholar]
- Meggouh F., Lointier P., Saez S. (1991) Sex steroid and 1,25 dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normal mucosa. Cancer Res 51: 1227–1233 [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. (2001) Interleukin- 10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765 [DOI] [PubMed] [Google Scholar]
- Moum B., Aadland E., Ekbom A., Vatn M.H. (1996) Seasonal variations in the onset of ulcerative colitis. Gut 38: 376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal, S., Na, S. and Rathnachalam, R. (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26: 662–687 [Epub 2005 Mar 29; Review]. [DOI] [PubMed] [Google Scholar]
- Nordvik I., Myhr K.M., Nyland H., Bjerve K.S. (2000) Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand 102: 143–149 [DOI] [PubMed] [Google Scholar]
- Peeters M., Geypens B., Claus D., Nevens H., Ghoos Y., Verbeke G., et al. (1997) Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology 113: 802–807 [DOI] [PubMed] [Google Scholar]
- Penna G., Adorini L. (2000) 1 Alpha, 25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation and survival of dendritic cells leading to impaired alloreactive T-cell activation. J Immunol 164: 2405–2411 [DOI] [PubMed] [Google Scholar]
- Piemonti P., Monti M., Sironi P., Fraticelli B.E., Leone E., Dal Cin P., et al. (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164: 4443–4451 [DOI] [PubMed] [Google Scholar]
- Probert C.S., Jayanthi V., Pinder D., Wicks A.C., Mayberry J.F. (1992) Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut 33: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert C.S., Mayberry J.F., Mann R. (1990) Inflammatory bowel disease in the rural Indian subcontinent: A survey of patients attending mission hospitals. Digestion 47: 42–46 [DOI] [PubMed] [Google Scholar]
- Provvedini D.M., Tsoukas C.D., Deftos L.J., Manolagas S.C. (1983) 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 221: 1181–1183 [DOI] [PubMed] [Google Scholar]
- Rigby W.F., Stacey T., Fanger M.W. (1984) Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest 74: 1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellu D.P. (1986) Seasonal variation in onset of exacerbations of ulcerative proctocolitis. J R Coll Surg Edinb 31: 158–160 [PubMed] [Google Scholar]
- Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., et al. (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8: 285–293 [DOI] [PubMed] [Google Scholar]
- Stamp T.C. (1975) Factors in human vitamin D nutrition and in the production and cure of classical rickets. Proc Nutr Soc 34: 119–130 [DOI] [PubMed] [Google Scholar]
- Stio M., Martinesi M., Bruni S., Treves C., Mathieu C., Verstuyf A., et al. (2007) The vitamin D analogue TX 527 blocks NF-B activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J Steroid Biochem Mol Biol 103: 51–60 [DOI] [PubMed] [Google Scholar]
- Stio M., Treves C., Celli A., Tarantino O., d’Albasio G., Bonanomi A.G. (2002) Synergistic inhibitory effect of cyclosporin A and vitamin D derivatives on T lymphocyte proliferation in active ulcerative colitis. Am J Gastroenterol 97: 679–689 [DOI] [PubMed] [Google Scholar]
- Stio M., Treves C., Martinesi M., Bonanomi A.G. (2005) Biochemical effects of KH 1060 and anti-TNF monoclonal antibody on human peripheral blood mononuclear cells. Int Immunopharmacol 5: 649–659 [DOI] [PubMed] [Google Scholar]
- van Etten E. (2007) Novel insights in the immune function of the vitamin D system: Synergism with interferon-beta. J Steroid Biochem Mol Biol 103: 546–551 [DOI] [PubMed] [Google Scholar]
- van Halteren A.G., van Etten E., de Jong E.C., Bouillon R., Roep B.O., Mathieu C. (2002) Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25-dihydroxyvitamin D(3). Diabetes 51: 2119–2125 [DOI] [PubMed] [Google Scholar]
- Vandewalle B., Adenis A., Hornez L., Revillion F., Lefebvre J. (1994) 1,25-dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues. Cancer Lett 86: 67–73 [DOI] [PubMed] [Google Scholar]
- Veldman C.M., Cantorna M.T., DeLuca H.F. (2000) Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374: 334–338 [DOI] [PubMed] [Google Scholar]
- Vieth R. (1999) Vitamin D supplementation, 25-hydroxyvitamin D levels and safety. Am J Clin Nutr 69: 842–856 [DOI] [PubMed] [Google Scholar]
- Vieth R. (2004) Why the optimal requirement for Vitamin D(3) is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol 89–90: 575–579 [DOI] [PubMed] [Google Scholar]
- Vieth R. (2006) Critique of the considerations for establishing the tolerable upper intake level for vitamin D: Critical need for revision upwards. J Nutr 136: 1117–1122 [DOI] [PubMed] [Google Scholar]
- Vieth R., Chan P.R., MacFarlane G.D. (2001) Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73: 288–294 [DOI] [PubMed] [Google Scholar]
- Vogelsang H., Ferenci P., Woloszczuk W., Resch H., Herold C., Frotz S., et al. (1989) Bone disease in vitamin D deficient patients with Crohn’s disease. Dig Dis Sci 34: 1094–1099 [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J., Kotchen J.M., Anderson G.L., Assaf A.R., Brunner R.L., O’Sullivan M.J., et al. (2006) Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354: 684–696 [DOI] [PubMed] [Google Scholar]
- Wang T.T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., et al. (2010) Direct and indirect induction by 1,25 dihydroxyvitamin D3 of the NOD2/CARD15-defnsin beta2 innate immune pathway defective in Crohn disease. J Biol Chem 285: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D.A. (1996) Oxford Textbook of Medicine, 3rd edition, Oxford University Press: Oxford, 3992–3998 [Google Scholar]
- Wu K., Feskanich D., Fuchs C.S., Willett W.C., Hollis B.W., Giovannucci E.L. (2007) A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 99: 1120–1129 [DOI] [PubMed] [Google Scholar]
- Yin L., Grandi N., Raum E., Haug U., Arndt V., Brenner H. (2009) Meta-analysis: Longitudinal studies of serum vitamin D and colorectal cancer risk. Alim Pharmacol Ther 30: 113–125 [DOI] [PubMed] [Google Scholar]
- Zeng L., Anderson F.H. (1996) Seasonal change in the exacerbations of Crohn’s disease. Scand J Gastroenterol 31: 79–82 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Mahon B.D., Froicu M., Cantorna M.T. (2005) Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 35: 217–224 [DOI] [PubMed] [Google Scholar]
- Zittermann A. (2006) Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 92: 39–48 [DOI] [PubMed] [Google Scholar]
- Zittermann A., Schleithoff S.S., Tenderich G., Berthold H.K., Körfre R., Stehle P. (2003) Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol 41: 105–112 [DOI] [PubMed] [Google Scholar]