Abstract
Background: Eosinophilic esophagitis (EoE) is a clinicopathologic disorder first described in 1978 which has gained significant recognition over the past 10 years. Numerous prevalence studies have been performed around the globe, both in pediatric and adult populations documenting a prevalence between 0.002% and 6.5%. The aim of this study is to assess the utility of routinely screening for EoE in patients with dysphagia.
Methods: A prospective, observational study in which adult patients with a complaint of esophageal dysphagia were enrolled.
Results: Of the 135 patients enrolled, 122 completed the study; 100 patients had nonobstructive dysphagia, while 22 patients had a luminal finding which could explain their dysphagia. The prevalence of EoE in the nonobstructive dysphagia group was 22% (95% CI: 13.9–30.1%); 32.7% of male patients with nonobstructive dysphagia were found to have EoE compared with 8.9% of females (p = 0.004). The mean age of nonobstructive patients found to have EoE was 37.8 years. White patients with nonobstructive dysphagia were found to have a 25.9% prevalence of EoE, compared with 0% of African Americans, 0% of Asians, and 14.3% of Hispanics. When comparing Whites with non-Whites, the prevalence of EoE was noted to be 25.9% versus 5.3%, respectively (p = 0.050).
Conclusions: EoE is a common cause of nonobstructive dysphagia. We believe that the high prevalence of EoE in patients with nonobstructive dysphagia supports the practice of routine biopsies to screen for the presence of abnormally high numbers of eosinophils in this subgroup.
Keywords: eosinophilic esophagitis, dysphagia, screening, esophageal biopsy
Background and aims
Eosinophilic esophagitis (EoE) is a clinicopathologic disorder first described in 1978 [Landres et al. 1978] and has gained significant recognition over the past 10 years [Sgouros et al. 2006]. In the adult population, EoE primarily affects middle-aged White males. The typical presentation in this age group is one of recurrent solid food dysphagia or food impaction [Desai et al. 2005]. EoE has been documented in association with allergic conditions such as asthma, eczema, food allergies, and seasonal aero-allergens. These conditions not only place patients at increased risk for developing EoE, but may also be responsible for its pathogenesis [Cristina et al. 2009; Spergel, 2007; Wang et al. 2007; Arora and Yamazaki, 2004; Fogg et al. 2003; Mishra et al. 2001; Kelly et al. 1995].
The current standard for diagnosing EoE is endoscopic visualization with multiple biopsies from the proximal and distal esophagus. Inspection of the esophageal mucosa may be normal, although abnormalities such as rings, linear furrowing, small-caliber esophagus, white plaques, erythema, edema, nodularity, and granularity are often seen [Parfitt et al. 2006]. The histopathologic criteria for EoE remains a subject of debate. Most endorse greater than 15–25 intraepithelial eosinophils (eos) per high-powered field (hpf) [Parfitt et al. 2006; Rothenberg, 2004; Rothenberg et al. 2001]. The most recent consensus report on EoE defines it as greater than 15 intraepithelial eos per hpf in the appropriate clinical setting, and in the absence of pathologic gastroesophageal reflux disease (GERD) [Furuta et al. 2007]. More specifically, in the setting of patients who have undergone a pH study to exclude GERD or in patients who are on proton-pump inhibitor (PPI) therapy at the time of biopsy.
The pathogenesis of EoE is not completely understood and is likely multifactorial. Evidence supports an allergic reaction with a resultant immunologic response [Bohm and Richter, 2008; Mishra et al. 2007, 2001; Spergel, 2007; Arora and Yamazaki, 2004]. GERD has been implicated as a cofactor by causing mucosal damage and loss of the epithelial tight junction, with increased permeability to potential allergens and inflammatory cells [Bohm and Richter, 2008; Spechler et al. 2007]. There also appears to be a genetic component with an identified familial pattern of inheritance [Collins et al. 2008; Blanchard et al. 2006].
Numerous prevalence studies have been performed around the globe, both in pediatric and adult populations, documenting a prevalence of between 0.002% and 6.5% [Prasad et al. 2009; Veerappam et al. 2009; Ronkainen et al. 2007; Cherian et al. 2006; Straumann and Simon, 2005; Noel et al. 2004]. A more recent study by Prasad and colleagues showed a prevalence of 15% in patients with a complaint of dysphagia [Prasad et al. 2007]. Many of these studies have also shown an increasing prevalence of EoE since its identification [Prasad et al. 2009; Ronkainen et al. 2007; Cherian et al. 2006; Straumann and Simon, 2005; Noel et al. 2004]. It is unclear whether or not there is a true increase in prevalence or an increased awareness of EoE, leading to increased numbers of diagnoses made.
The aim of our study is to assess the utility of routinely screening for EoE in patients presenting with dysphagia, as well as to assess the prevalence of EoE in this group.
Methods
This is a prospective observational study (ClinicalTrials.gov: NCT01028235) in which adult patients with a complaint of esophageal dysphagia were enrolled. Patients were recruited from the gastroenterology clinics at Wilford Hall Medical Center (Lackland AFB, TX) and Brooke Army Medical Center (Fort Sam Houston, TX). This study was approved by the institutional review board at both locations. If a chief complaint of dysphagia was encountered, or if a complaint of dysphagia was elicited in the review of systems, patients were considered for enrollment. Exclusion criteria included age <18, current pregnancy, coagulation disorders (iatrogenic or inherited), systemic or inhaled corticosteroids within the last 30 days, oropharyngeal dysphagia (based on physician judgment with reports of difficulty in initiation of swallowing, choking, cough, etc.), or a known history of EoE. Patients on warfarin were considered for enrollment if they were taking the medication for an indication in which temporary cessation was acceptable. If a patient was deemed a candidate for enrollment, they were consented utilizing a standardized consent form.
At the time of endoscopy, patients were assessed as having either obstructive or nonobstructive dysphagia based on the endoscopic view of the esophagus. This was determined by the operating endoscopist (fellow with attending supervision, or attending acting alone). Obstructive dysphagia was defined as the discovery of a distal esophageal ring, stricture, corrugations (defined as multiple large rings lining the esophagus, which cause multiple focal narrowings of the lumen), or mass. The patients that were found to have obstructive dysphagia were excluded from the primary analysis. All others were defined as having nonobstructive dysphagia.
Those with nonobstructive dysphagia underwent a standardized biopsy protocol, which included 4 duodenal biopsies, 4 gastric biopsies, and a total of 12 esophageal biopsies (4 distal, 4 mid, and 4 proximal). Duodenal and gastric biopsies were obtained to rule out a concomitant eosinophilic gastroenteritis. The locations of the esophageal biopsies were standardized. In order to account for variances in the length of the esophagus, biopsy locations were determined by landmark locations. Distal esophageal biopsies were taken at 1–2 cm above the gastroesophageal junction. Proximal esophageal biopsies were taken at 2–3 cm below the upper esophageal sphincter. Mid esophageal biopsies were taken at the midway point between the location of the distal and proximal biopsies. Those patients that were defined as having obstructive dysphagia underwent endoscopic therapy and/or biopsies at the discretion of the operating endoscopist.
Histopathologic criteria for EoE consisted of >15 eos per hpf. A single pathologist interpreted all of the samples. Three hematoxylin and eosin (H&E)-stained step sections were examined from each biopsy location (proximal, mid, distal) to evaluate for the presence of eos. The highest concentration of eos in any hpf for each specimen was utilized to assess whether the biopsy met the quantitative criteria. Pathology specimens were reported as either meeting diagnostic criteria or not. Specific numbers of eos per hpf were not consistently commented on. The presence or absence of micro-abscesses was also not specifically commented on.
Endoscopic findings and patient demographics were recorded prospectively. The age at which a patient was diagnosed with EoE was determined by date of birth and date of biopsy. In order to further study the nonobstructive dysphagia patients that were found to have esophageal biopsies consistent with EoE, a retrospective review of their initial consult visit was performed.
Results
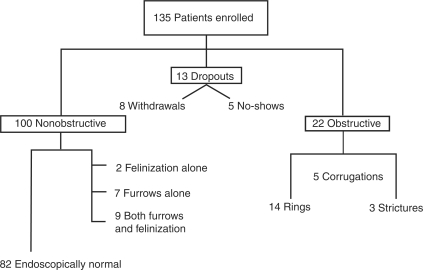
A total of 135 patients were enrolled between January 2007 and December 2009 (Figure 1): 71 patients were enrolled at Brooke Army Medical Center, while 64 patients were enrolled at Wilford Hall Medical Center. A total of 100 patients were noted to have nonobstructive dysphagia. Of the 100 patients, 82 were found to be endoscopically normal, 2 were found to have felinization, 7 were found to have linear furrowing, and 9 were found to have both felinzation and furrowing. A total of 13 patients were withdrawn from the study, 8 of which withdrew their consent on the day of the procedure, and 5 patients did not present for their procedure. A total of 22 patients were found to have obstructive dysphagia. Fourteen patients had a distal esophageal ring, three patients were noted to have a stricture, and five patients were noted to have corrugations.
Figure 1.
Breakdown of enrolled patients and endoscopic findings where applicable.
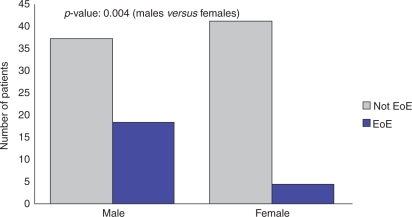
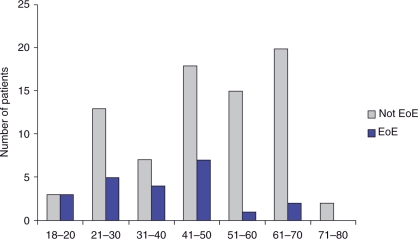
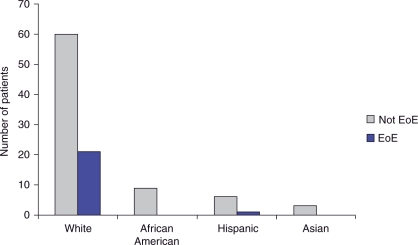
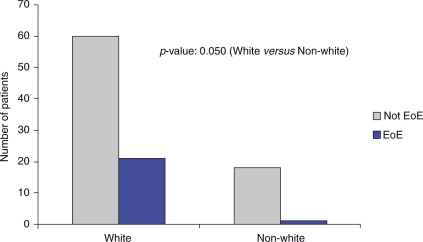
The prevalence of EoE in patients with nonobstructive dysphagia was 22% (95% CI: 13.9–30.1%). We found that 32.7% of male patients with nonobstructive dysphagia had EoE compared with 8.9% of females (p = 0.004) (Figure 2). The mean age of nonobstructive patients found to have EoE was 37.8 years (Figure 3). The majority of nonobstructive dysphagia patients found to have esophageal biopsies consistent with EoE were between the ages of 18 and 50 years old. White patients with nonobstructive dysphagia were found to have a 25.9% prevalence of EoE, compared with 0% of African Americans, 0% of Asians, and 14.3% of Hispanics (Figure 4). When comparing Whites with non-Whites, the prevalence of EoE was noted to be 25.9% versus 5.3%, respectively, with a p-value of 0.050 (Figure 5). Of the two patients noted to have felinization alone, both were found to have esophageal biopsies consistent with EoE. Of the seven patients noted to have furrowing alone, five (71.4%) were found to have esophageal biopsies consistent with EoE. Of the nine patients noted to have felinization and furrowing, seven (77%) were found to have esophageal biopsies consistent with EoE.
Figure 2.
Nonobstructive patients by sex. EoE, eosinophilic esophagitis.
Figure 3.
Nonobstructive patients by age. EoE, eosinophilic esophagitis.
Figure 4.
Nonobstructive patients by race. EoE, eosinophilic esophagitis.
Figure 5.
Non-obstructive patients, White versus non-White. EoE, eosinophilic esophagitis.
In the retrospective review of the initial consult visit (Table 1), for those patients with nonobstructive dysphagia (and biopsies consistent with EoE), it was found that 11 patients had a prior history of GERD. Nine of the patients with a history of GERD were on PPI therapy at the time of study enrollment and biopsy. Two patients had a remote history of GERD, and were not taking a PPI at the time of enrollment and biopsy. Of the 11 patients with GERD, 4 had a documented history of rhinitis, 2 had a history of eczema, and 1 had both rhinitis and eczema.
Table 1.
Background information on all 22 nonobstructive dysphagia patients with biopsies consistent with eosinophilic esophagitis (EoE).
| Age (years) | Sex | Race | Prior GERD | First Episode of Dysphagia | Meat Impaction | Allergies | Asthma | Eczema | On PPI |
|---|---|---|---|---|---|---|---|---|---|
| 38.9 | Female | White | No | No | No | Rhinitis | No | No | No |
| 48.8 | Male | White | Yes | No | No | Rhinitis | No | No | Yes |
| 33.3 | Male | White | No | Yes | Yes | Rhinitis | No | No | No |
| 29.2 | Male | White | Yes | No | No | Rhinitis | No | No | Yes |
| 39.1 | Male | White | No | No | No | No | No | No | Yes |
| 44.6 | Female | Hispanic | Yes | Yes | Yes | Rhinitis | No | Yes | Yes |
| 54.5 | Male | White | Yes | No | No | Rhinitis | No | No | Yes |
| 27.8 | Male | White | Yes | No | No | Rhinitis | No | No | Yes |
| 24.5 | Male | White | No | No | No | No | No | No | Yes |
| 19.3 | Male | White | No | No | No | No | No | No | Yes |
| 21.9 | Female | White | No | No | No | No | No | No | No |
| 25.4 | Male | White | No | No | No | No | No | No | No |
| 43.5 | Male | White | No | No | No | Rhinitis | Yes | No | No |
| 46 | Female | White | Yes | No | No | No | No | No | No |
| 47.8 | Male | White | Yes | No | No | Rhinitis | No | Yes | Yes |
| 19.8 | Male | White | No | Yes | Yes | No | No | No | No |
| 19.2 | Male | White | No | No | No | Rhinitis | No | No | No |
| 65.6 | Male | White | Yes | No | No | No | No | No | No |
| 46.9 | Male | White | Yes | No | No | No | No | No | Yes |
| 62.8 | Male | White | Yes | No | No | No | No | No | Yes |
| 31.2 | Male | White | No | No | No | Rhinitis | No | No | Yes |
| 42.5 | Male | White | Yes | No | No | No | No | Yes | Yes |
GERD, gastroesophageal reflux disease; PPI, proton-pump inhibitor.
A total of 11 patients did not have a documented history of GERD. Four of these 11 patients were on PPI therapy at the time of enrollment and biopsy. An extensive review of the chart found that PPI therapy was started for the symptom of dysphagia and not for that of GERD. One of the 11 patients had a documented history of rhinitis. Another patient had a documented history of asthma.
Three patients out of 22 presented after their initial episode of dysphagia. These patients had presentations of meat impaction. The remaining 19 patients had a recurrent history of dysphagia, 4 of which had a meat impaction at some point during the duration of their symptoms.
EoE was also found in patients with obstructive dysphagia. Of the 14 patients noted to have distal a distal esophageal ring, 2 (14.3%) were found to have esophageal biopsies consistent with EoE. Of the three patients noted to have an esophageal stricture, none (0%) were found to have esophageal biopsies consistent with EoE. Of the five patients noted to have corrugations, four (80%) were found to have esophageal biopsies consistent with EoE.
The prevalence of EoE in all patients with dysphagia (nonobstructive and obstructive) was approximately 23% (95% CI: 14.6–28.4%). We found that 33.3% of male patients with dysphagia had EoE, compared with 6.7% of females (p < 0.002). The average age of patients found to have EoE (all patients with dysphagia) was noted to be 36.8 years. White patients with dysphagia had a prevalence of EoE of 26.2%, compared with 0% of African Americans, 0% of Asians, and 7.7% of Hispanics. When comparing Whites with non-Whites, a prevalence of 26.2% and 3.6% was noted, respectively (p = 0.010).
Discussion and conclusions
Our study reports the highest prevalence of EoE of any study to date at 22% (22/100) in patients with nonobstructive dysphagia and a prevalence of nearly 23% (28/122) in all patients who completed the study. We propose several explanations for this difference. The strict definition of EoE (15 eos per hpf in the absence of pathologic GERD) was not specifically addressed by this study. A total of 13 patients in the nonobstructive group that were found to have biopsies consistent with EoE were noted, after retrospective review, to have been prescribed PPI therapy prior to enrollment and biopsy. We realize that even though these patients were prescribed PPI therapy, there is a chance that they may not have been taking them on a consistent basis, or even at all in the timeframe surrounding the biopsies. The other nine patients were not documented to be on PPI therapy, however pH testing was not undertaken to exclude GERD, and a trial of PPI with repeat biopsy was not performed. This is a major limitation of this study. This could falsely elevate the prevalence of EoE in our study, however the primary goal of this study was to assess the utility of routinely screening for EoE in patients with dysphagia. Once biopsies consistent with EoE are found, patients could undergo pH testing, repeat biopsy on PPI therapy, or go straight to treatment if the diagnosis is confirmed.
We also recognize that our threshold of 15 eos per hpf is lower than the 20 eos per hpf utilized in the study by Prasad and colleagues [Prasad et al. 2007]. Clearly we expected this lower threshold to improve our overall sensitivity in the identification of EoE, although an unintended reduction in our specificity may also have been introduced. In addition, the mean age of our patients was lower than in the study by Prasad and colleagues. As EoE is generally a disease of young patients, it is conceivable that more patients with EoE would be found from a younger population base. As both centers are military hospitals, a referral bias of young men may well exist. Moreover, young soldiers and airmen in training are typically allotted shorter meal times thereby eliminating a coping mechanism for chronic dysphagia (eating slowly), perhaps prompting a referral into one of our clinics. We tended to see a higher rate of food impaction in this subgroup. Young patients are largely the population base of a military medical center, which would stand in stark contrast to a community-based setting where the majority of patients seeking medical care are a bit older. It is important to be aware of this as the overall prevalence is likely going to be less in a community practice.
In the retrospective chart review, it was noted that 86% (19/22) of nonobstructive patients with biopsies consistent with EoE presented with recurrent dysphagia, some over many years. This is especially important when looking at the prevalence in patients 50–60 years of age, as they probably had symptoms well before this, further giving support to EoE primarily being a disease of the young. It may be a simple explanation of delayed diagnosis or the unwillingness of a younger patient to have their symptoms evaluated medically.
Our study validated the demographic findings noted in previous studies. Males were four times more likely to be diagnosed with EoE. The mean age of diagnosis was in the fourth decade of life, although cases were found up to the seventh decade. When comparing prevalence rates between races, we found a significantly higher rate in Whites. No African American patients were found to have EoE in our study. This is in stark contrast to the study by Veerappan and colleagues in which 10/107 African American patients who underwent endoscopy were found to have EoE [Veerappan et al. 2009]. Of note, only 2 of these 10 patients underwent endoscopy for the evaluation of dysphagia while the remaining 8 African American patients underwent endoscopy for other reasons. By narrowing our enrollment to patients with dysphagia, we significantly reduced our sample size, thereby decreasing the chance of finding a case of EoE in this ethnic group. Given that 80% of the African American patients found to have EoE in the study by Veerappan and colleagues underwent endoscopy for reasons other than dysphagia, this could suggest a variance in the predominant presenting symptoms depending on ethnicity. In our study, no Asians were found to have EoE. This also can be explained by our low enrollment in this ethnic group.
In our study, a history of asthma, eczema, or allergies had a correlation of up to 50% (11/22) in patients with nonobstructive dysphagia. However, our study was not powered to fully evaluate this data. A history of meat impaction is one of the typical symptoms of EoE, however this was only documented in 13.6% (3/22) in patients with nonobstructive dysphagia.
Endoscopic findings alone do not ensure the possibility of underlying EoE. In our study population, 10% (8/82) had a normal appearing endoscopy and histologic evidence of EoE. Fourteen of 18 (78%) had features of felinization, furrows, or a combination of both, along with histologic evidence of EoE. In patients with obstructive features such as strictures, rings, or corrugations, the prevalence of EoE was 27% (6/22) although in patients with corrugations, 80% (4/5) had histologic evidence of EoE. In the study by Veerappan and colleagues, corrugations had a 52% sensitivity and a 94% specificity, while furrows had a 48% sensitivity and a 95% specificity [Veerappan et al. 2009]. Our data suggests that there may be a higher sensitivity and specificity of the aforementioned endoscopic findings when coupled with a clinical history of dysphagia. However, our study was not powered specifically for this data analysis.
Our study is the first to perform multiple biopsies throughout the upper gastrointestinal tract, in order to ensure the exclusion of an underlying systemic eosinophilic condition. Routinely performing biopsies of the stomach and duodenum would not be recommended in patients with esophageal complaints alone (no cases of eosinophilic gastroenteritis were found in our study) (Table 2). Biopsies of the stomach and duodenum to look for eosinophilic gastroenteritis should be reserved for patients who express symptoms consistent with the syndrome.
Table 2.
Demographics of all patients diagnosed with eosinophilic esophagitis (EoE), their endoscopic findings, and location of biopsies consistent with EoE.
| Race | Age | Gender | Endoscopic Finding | Duodenal | Gastric | Distal | Mid | Proximal |
|---|---|---|---|---|---|---|---|---|
| White | 18.7 | Male | Corrugations | − | − | + | + | + |
| White | 38.9 | Female | Normal | − | − | + | − | − |
| White | 38.6 | Male | Ring | − | − | + | + | + |
| White | 40.5 | Male | Corrugations | − | − | + | + | + |
| White | 34.6 | Male | Corrugations | − | − | + | − | − |
| White | 48.8 | Male | Furrows | − | − | + | − | − |
| White | 33.3 | Male | Felinization/Furrows | − | − | + | + | + |
| White | 29.2 | Male | Linear Furrowing | − | − | + | + | + |
| White | 39.1 | Male | Normal | − | − | + | + | − |
| Hispanic | 44.6 | Female | Normal | − | − | − | + | + |
| White | 54.5 | Male | Normal | − | − | + | − | − |
| White | 27.8 | Male | Normal | − | − | + | − | − |
| White | 24.5 | Male | Felinization/Furrows | − | − | + | + | + |
| White | 19.3 | Male | Felinization/Furrows | − | − | + | + | + |
| White | 21.9 | Female | Furrows | − | − | + | + | + |
| White | 25.4 | Male | Felinization/Furrowing | − | − | + | − | − |
| White | 43.5 | Male | Normal | − | − | + | + | + |
| White | 46 | Female | Furrows | − | − | + | + | + |
| White | 47.8 | Male | Felinization | − | − | + | − | − |
| White | 19.8 | Male | Furrows | − | − | + | + | + |
| White | 33.8 | Male | Corrugations | − | − | + | − | − |
| White | 19.2 | Male | Felinization/Furrows | − | − | + | + | + |
| White | 65.6 | Male | Felinization/Furrows | − | − | + | + | + |
| White | 46.9 | Male | Felinization | − | − | + | + | + |
| White | 32.2 | Male | Ring/Felinization/Furrows | − | − | + | + | + |
| White | 62.75 | Male | Normal | − | − | − | + | + |
| White | 31.2 | Male | Felinziation/Furrows | − | − | − | + | + |
| White | 42.5 | Male | Normal | − | − | + | + | + |
In summary, the prevalence of EoE is high in patients with nonobstructive dysphagia. Importantly, 10% of patients noted to have an endoscopically normal esophagus met histopathologic criteria for EoE, and would not have been discovered on the basis of endoscopic appearance alone. Classic findings of EoE, while common in the EoE group, were absent in a sufficient number of subjects to lead the authors to recommend that biopsies be taken routinely as a part of the evaluation for nonobstructive dysphagia, particularly in White males.
Acknowledgments
Thanks are due to Rhonda Naus, PA Brooke Army Medical Center Gastroenterology Clinic, for her help.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Arora A.S., Yamazaki K. (2004) Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 2: 523–523 [DOI] [PubMed] [Google Scholar]
- Blanchard C., Wang N., Stringer K.F., Mishra A., Fulkerson P.C., Albonia J.P., et al. (2006) Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116: 536–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm M., Richter J.E. (2008) Treatment of eosinophilic esophagitis: Overview, current limitations, and future direction. Am J Gastroenterol 103: 2635–2644 [DOI] [PubMed] [Google Scholar]
- Cherian S., Smith N.M., Forbes D.A. (2006) Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child 91: 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.H., Blanchard C., Abonia J.P., Kirby C., Akers R., Wang N., et al. (2008) Clinical, pathologic, and molecular characterization of familial eosinophilic esophagitis compared with sporadic cases. Clin Gastroenterol Hepatol 6: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina A., Krishna M., Buchner A., Ghabril M.S., Talley N., DeVault K.R., et al. (2009) Seasonal distribution in newly diagnosed cased of eosinophilic esophagitis in adults. Gastroenterology 104: 828–833 [DOI] [PubMed] [Google Scholar]
- Desai T.K., Stecevic V., Chang C.H., Goldstein N.S., Badizadegan K., Furuta G.T. (2005) Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc 61: 795–801 [DOI] [PubMed] [Google Scholar]
- Fogg M.I., Ruchelli E., Spergel J.M. (2003) Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 112: 796–797 [DOI] [PubMed] [Google Scholar]
- Furuta G.T., Liacouras C.A., Collins M.H., Gupta S.K., Justinich C., Putnam P.E., et al. (2007) Eosinophilic esophagitis in children and adults: A systemic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133: 1342–1363 [DOI] [PubMed] [Google Scholar]
- Kelly K.J., Lazenby A.J., Rowe P.C., Yardley J.H., Perman J.A., Sampson H.A. (1995) Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with amino acid-based formula. Gastroenterology 109: 1503–1512 [DOI] [PubMed] [Google Scholar]
- Landres R.T., Kuster G.G., Strum W.B. (1978) Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 74: 1298–1301 [PubMed] [Google Scholar]
- Mishra A., Hogan S.P., Brandt E.B., Rothenberg M.E. (2001) An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Schlotman J., Wang M., Rothenberg M.E. (2007) Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol 81: 916–924 [DOI] [PubMed] [Google Scholar]
- Noel R.J., Putnam P.E., Rothenberg M.E. (2004) Eosinophilic esophagitis. N Engl J Med 351: 940–941 [DOI] [PubMed] [Google Scholar]
- Parfitt J.R., Gregor J.C., Suskin N.G., Jawa H.A., Driman D.K. (2006) Eosinophilic esophagitis in adults: Distinguishing features from gastroesophageal reflux disease: A study of 41 patients. Mod Pathol 19: 90–96 [DOI] [PubMed] [Google Scholar]
- Prasad G.A., Alexander J.A., Schleck C.D., Zinsmeister A.R., Smyrk T.C., Elias R.M., et al. (2009) Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastrenterol Hepatol 7: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G.A., Talley N.J., Romero Y., Arora A.S., Kryzer L.A., Smyrk T.C., et al. (2007) Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: A prospective study. Am J Gastroenterol 102: 2627–2632 [DOI] [PubMed] [Google Scholar]
- Ronkainen J., Talley N.J., Aro P., Storskrubb T., Johannson S.E., Lind T., et al. (2007) Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 56: 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M.E. (2004) Eosinophilic gastrointestinal disorders. J Allergy Clin Immunol 13: 11–28 [DOI] [PubMed] [Google Scholar]
- Rothenberg M.E., Mishra A., Collins M.H., Putnam P.E. (2001) Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 108: 891–894 [DOI] [PubMed] [Google Scholar]
- Sgouros S.N., Bergele C., Mantides A. (2006) Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol 18: 211–217 [DOI] [PubMed] [Google Scholar]
- Spechler S.J., Genta R.M., Souza R.F. (2007) Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 120: 1301–1306 [DOI] [PubMed] [Google Scholar]
- Spergel J.M. (2007) Eosinophilic esophagitis in adults and children: Evidence for a food allergy component in many patients. Curr Opin Allergy Immunol 7: 274–278 [DOI] [PubMed] [Google Scholar]
- Straumann A., Simon H. (2005) Eosinophilic esophagitis: Escalating epidemiology? J Allergy Clin Immunol 115: 418–419 [DOI] [PubMed] [Google Scholar]
- Veerappan G.R., Perry J.L., Duncan T.J., Baker T.P., Maydonovitch C., Lake J.M., et al. (2009) Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: A prospective study. Clin Gastroenterol Hepatol 7: 420–426 [DOI] [PubMed] [Google Scholar]
- Wang F.Y., Gupta S.K., Fitzgerald J.F. (2007) Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol 41: 451–453 [DOI] [PubMed] [Google Scholar]