Abstract
K/BxN mice develop a spontaneous destructive arthritis driven by T cell dependent anti-glucose-6-phosphate isomerase (GPI) antibody production. In this study, a modified version of the K/BxN model, the KRN-cell transfer model (KRN-CTM), was established to determine the contribution of Th17 cells in the development of chronic arthritis. The transfer of naive KRN T cells into B6.TCR.Cα−/−H-2b/g7 T cell deficient mice induced arthritis by day 10 of transfer. Arthritis progressively developed for a period of up to 14 days following T cell transfer, thereafter the disease severity declined, but did not resolve. Both IL-17A and IFNγ were detected in the recovered T cells from the popliteal lymph nodes and ankles. The transfer of KRN Th17 polarized KRN CD4+ T cells expressing IL-17A and IFNγ induced arthritis in all B6.TCR.Cα−/−H-2b/g7 mice however the transfer of Th1 polarized KRN CD4+ T cells expressing IFNγ alone induced disease in only 2/3 of the mice and disease induction was delayed compared to Th17 transfers. Th17 polarized KRN/T-bet−/− cells induced arthritis in all mice and surprisingly, IFNγ was produced demonstrating that T-bet expression is not critical for arthritis induction, regardless of the cytokine expression. Neutralization of IFNγ in KRN Th17 transfers resulted in earlier onset of disease while the neutralization of IL-17A delayed disease development. Consistent with K/BxN mice, naive KRN T cell transfers and Th17 polarized KRN/T-bet−/− transfers induced anti-GPI IgG1 dominant responses while KRN Th17 cells induced high levels of IgG2b. These data demonstrate that Th17 cells can participate in the production of autoantibodies that can induce arthritis.
1. Introduction
Rheumatoid arthritis (RA) is a debilitating autoimmune disease of the synovial joints characterized by inflammation and ultimate destruction of the joints. The K/BxN murine model of spontaneous arthritis shares similarities with the human disease in that it is a progressive disease leading to synoviocyte proliferation, pannus formation, and cartilage and bone destruction with anarchic remodeling of the joints [1–3]. K/BxN mice are a cross between KRN TCR transgenic (Tg) and NOD mice and 100% of the progeny develop chronic arthritis. The KRN transgenic TCR is specific for bovine RNase (42–56)/I-Ak and the self-derived peptide glucose-6-phosphate isomerase (GPI)/I-Ag7. In K/BxN mice, autoreactive KRN T cells escape negative selection in the thymus [4] and are activated in the periphery by GPI where they provide help to GPI-reactive B cells [5, 6]. The resulting arthritigenic autoantibodies are necessary and sufficient for arthritis to ensue. The transfer of serum containing anti-GPI antibodies into most strains of mice results in disease [7–9]. These serum transfer studies have elucidated the pathogenesis of the disease and the requirement for anti-GPI antibody deposition in the joint resulting in the recruitment of inflammatory mediators into the joint.
RA has often been considered to be a Th1-depedent disease. However, there is conflicting data concerning the role of IFNγ. Studies exist demonstrating that IFNγ can both increase and decrease disease severity [10–12]. The Th17 subset is found to be important in the development of autoimmune diseases such as EAE, colitis, and psoriasis [13–16]. The detection of IL-17 in the synovial tissues of RA patients [17–20] and the discovery of Th17 cells has caused a re-examination of the corresponding animal models to determine the role of IL-17 in the pathogenesis of arthritis. It has now been shown that Th17 cells are critical for pathogenesis in several models of arthritis [21–29]; however, there are a few examples of arthritis models where Th17 cells are not apparently involved. IL-17−/− mice are susceptible to the proteoglycan-induced arthritis (PGIA) model of RA [30, 31]. A decrease in disease severity is observed in IFNγ−/− PGIA treated mice supporting a Th1-type response [30, 32] and not a Th17-type response. Glucose 6-phosphate isomerase (GPI)-specific CD4+ T cells from DBA/1 mice immunized with GPI differentiated in vivo into Th1 and Th17 cells but not Th2 cells, suggesting a role for both Th1 and Th17 cells in arthritis [33]. In the K/BxN arthritis model, it has been shown that Th17 cells can promote arthritis when co-injected with the autoantibodies [34]. Recently, a fascinating relationship has been reported showing that the K/BxN model requires IL-17 production in the lamina propria, and that filamentous bacteria in the gut can drive this Il-17 production [35]. Thus, Th17 cells are critical in the K/BxN arthritis model, but their precise role in B cell help to anti-GPI B cells had not been directly examined. Kuchroo and colleagues have shown that Th17 cells can act as effective B cell helpers for an anti-MOG response [36].
To ascertain the role of Th17 cells in B cell help in the K/BxN arthritis model we utilized our recently developed KRN-cell transfer model (KRN-CTM) of chronic arthritis in which the timing of arthritis induction and the type of T cells could be controlled [37]. In our model, KRN T cells were transferred into the T cell deficient B6.TCR.Cα−/−.H-2b/g7 recipients and we were able to analyze the role of different T helper subsets on arthritis induction. Here we demonstrated that in the KRN-CTM naive T cells or Th17 cells from KRN or KRN/T-bet−/− mice could induce arthritis and autoantibody production, whereas KRN Th1 cells were much less efficient. These findings demonstrate that Th17 cells can act as T helper cells in the production of arthritogenic autoantibodies.
2. Materials and Methods
2.1. Mice
KRN TCR Tg mice on a C57BL/6 background and K/BxN mice have been described [4]. The B6.G7 and BALB/c mouse strains were purchased from The Jackson Laboratory (JAX, Bar Harbor, ME). B6.TCR.Cα−/− mice used in these studies have been previously described [38] and were purchased from JAX. B6.TCR.Cα−/− were bred with B6.G7 mice to create B6.TCR.Cα−/−H-2b/g7 mice. These mice were T cell deficient and expressed H-2b/g7 allowing them to present GPI peptide. T-bet−/− (Tbx21−/−) mice were purchased from JAX and were crossed with KRN TCR Tg mice. All mice were bred and housed under specific pathogen-free conditions in the animal facility at the Washington University Medical Center (St. Louis, MO). Studies were performed in accordance with National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
2.2. Arthritis assessment
The two rear ankles of B6.TCR.Cα−/−H-2b/g7 mice were measured starting at day 0 of T cell transfer. Measurement of ankle thickness was made above the footpad, axially across the ankle joint by using a Kafer Dial Thickness Gauge J15 (Long Island Indicator Service Inc.,Hauppauge, NY). Ankle thickness was rounded to the nearest 0.1 mm. Mice were also given a clinical score of disease where 0 represents no inflamed paws, 1 represents 1 inflamed paw, etc. For histopathology, the rear ankles were fixed in 10% Neutral Buffered Formalin (NBF) for 24 hours at 20°C, followed by decalcification in Immunocal™ (Decal Chemical Corporation, Tallman NY) for 7 days at 20°C. Decalcified joints were then paraffin-embedded with medial aspect down for longitudinal view, sectioned twice (4 µm each), and stained with hematoxylin & eosin (H&E) for general histopathologic evaluation or toluidine blue for specific assessment of cartilage changes. The tissues were then examined by a veterinary pathologist (TPL).
2.3. Adoptive T cell transfer
Naive KRN T cells were isolated from the spleen by using CD4(L3T4) MicroBeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. By passing the T cells through a second LS column (Miltenyi), they were purified to >98% T cells. The T cells were washed extensively with 1X HBSS and resuspended in 1X HBSS at a concentration of 3.5×106 cells/ml. B6.TCR.Cα−/−H-2b/g7 mice were anesthetized with a mixture of Ketamine/xylazine and 100 µl of the cell suspension was injected i.v.
2.4. Th differentiation
Naive KRN T cells were isolated from the spleen as described. KRN T cells were cultured in IMDM supplemented with 10% fetal calf serum, 0.5% gentamicin, 1% glutamax, 1% HEPES, 1% NEAA, 1% NaPyr, and 1% 2-ME at a ratio of 1:5 with irradiated B6.G7 splenic APC along with 1.7 mM G7m peptide (GKKVATFVHAGYG). G7m was previously shown to stimulate KRN T cells with 10–100-fold increased sensitivity compared with GPI(281–293) [5]. Cells were cultured under Th1 conditions [5 U/ml recombinant murine IL-12 (Peprotech Inc., Rocky Hill, NJ), and 10% 11B11 supernatant containing anti-IL-4] or Th17 conditions [6 ng/ml recombinant human TGF-β1(Peprotech), 40 ng/ml recombinant murine IL-6 (Peprotech), 10 µg/ml hamster anti-murine IFNγ monoclonal antibody (kindly provided by R. Schreiber, Washington University), 10% Tosh supernatant containing anti-IL-12 and anti-IL-4] for 3 weeks. During weeks 2 and 3, 20 ng/ml recombinant mouse IL-23 (eBioscience, San Diego, CA) was also included. Cultures were restimulated each week with irradiated B6.G7 splenic APC, G7m peptide, and the appropriate Th conditions [39–41]. For the adoptive T cell transfer of Th differentiated cells, the cells were washed extensively with 1X HBSS and resuspended in 1X HBSS. 10×106 Th cells were injected i.v. into B6.TCR.Cα−/−H-2b/g7 mice.
2.5. Ankle analysis
Ankles were harvested from mice and the skin removed. Ankle tissue was clipped away from the bones then digested for 60 min at 37°C in 20 ml of HBSS containing 0.55 Wuensch units collagenase (Liberase Blendzyme 3; Roche, Indianapolis, IN) and 10 µg/ml DNase I (Sigma-Aldrich, St. Louis, MO), with vortexing every 10 min. Cells were washed twice in RPMI 1640 plus 10% FCS, filtered, and resuspended in RPMI 1640 plus 10% FCS.
2.6. Flow cytometry
Spleen, popliteal lymph nodes, or polarized Th1 or Th17 cells (1×106) were first stained with LIVE/DEAD fixable dead cell stain, near IR fluorescent reactive dye (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The cells were then surface stained according to standard protocols. The following antibodies were used: biotin-conjugated rat anti-mouse V β6 (previously shown to represent >60% of the TCR in this model) [42] (BD Pharminogen, San Diego, CA), Streptavidin-Phycoerythrin-Cy7 (BD Pharmingen) R3-34-FITC Rat IgG1 κ isotype (BD Pharmingen), R35-95-APC Rat IgG2a κ isotype (BD Pharmingen), R3-34-PE Rat IgG1 κ isotype (BD Pharmingen). All samples were analyzed on a FACSCanto™ (BD Biosciences, San Diego, CA) using FACSDiva™ software (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.7. Intracellular cytokine staining
For intracellular cytokine staining, cells from the popliteal lymph node or ankles were stimulated for 4 h with 50 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich, St. Louis, MO) and 1 µg/ml ionomycin (Calbiochem, San Diego, CA). For the last 2 h, 10 µg/ml of Brefeldin A (Sigma) was added to inhibit the export of cytokines. Cells were surface stained for 30 min at 4°C with LIVE/DEAD fixable dead cell stain (Invitrogen) according to manufacturer’s instructions and anti-Vβ6 antibodies. For intracellular staining, T cells were fixed and permeablilized with BD Cytofix fixation buffer (BD Biosciences) according to the manufacturer’s instructions and stained with Alexa Fluor® 488 anti-mouse IL-17 (BioLegend, San Diego, CA), APC anti-mouse IFNγ (BioLegend), and PE anti- mouse IL-4 (BioLegend). Samples were gated for live/Vβ6+ T cells. Quadrant gates were drawn on unstained popliteal lymph node samples. All samples were analyzed on a FACSCanto™ (BD Biosciences, San Diego, CA) using FACSDiva™ software (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.8. Anti-GPI ELISA
Mice were bled once a week starting day 0 of T cell transfer. Sera were stored at −20°C before analysis. Sera were plated at an initial dilution of 1:100 and diluted serially 1:5 in Immulon II plates (Fisher Scientific, Pittsburgh, PA) coated with 5 µg/ml recombinant GPI-histidine. Donkey anti-rabbit total Ig-horseradish peroxidase (HRP) (Jackson ImmunoResearch, West Grove, PA), goat anti-mouse IgM-HRP, IgG1-HRP, IgG2b-HRP, IgG2c-HRP, or IgG3-HRP (Southern Biotechnology Associates, Birmingham, AL) were used as secondary antibodies. Serum antibody was detected using ABTS substrate (2,2-azino-di-[3-ethylbenzthiazoline sulfonate] diammonium salt) (Roche Molecular Biochemicals, Indianapolis, IN). Absorbance was measured at 414 nm. The serum titer was defined as the reciprocal of the last dilution which gave an OD>3X higher than that of the background.
2.9. Antibody depletion
The hybridoma 412-79.9 was kindly provided by L. A. Herzenberg (Stanford University). This hybridoma produces an anti-IgG1 antibody that reacts with Igh-C[b] haplotype. The mAb was coupled to Cyanogen bromide (CNBr) Sepharose 4B beads (Sigma) according to the manufacture’s instructions and serum from Th17 induced arthritic mice was applied to the column to deplete IgG1. After depletion the serum was dialyzed against PBS, pH 7.4 before use. Depletion was monitored by ELISA assay.
2.10. Statistical analysis
Statistical significance was determined by using the Mann-Whitney nonparometric test or the Kaplan-Meir survival curve using GraphPad Prizm (GraphPad Software, La Jolla, CA).
3. Results
3.1. Th17 and Th1 cells are found in the K/BxN arthritis model
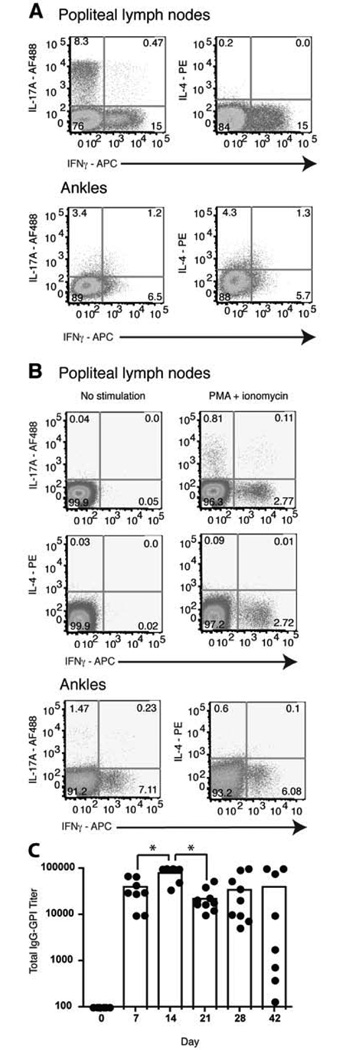
To determine the role of T helper subsets in the K/BxN arthritis model, we analyzed what Th subsets were found in the K/BxN mice. Previous work demonstrated that ankle swelling in K/BxN mice became measurable at age 4 weeks and all mice expressed full disease by day 35 [43]. Popliteal lymph nodes from K/BxN mice aged day 21, 28, 35, or 55 were harvested and stimulated for 4 h in the presence of PMA + ionomycin. Intracellular cytokine staining showed that both IL-17A and IFNγ were detected while there was little IL-4 (Fig. 1a), as has been reported [34]. The cytokine profiles were similar for diseased mice in each age group. There were 6–27% of the T cells producing IL-17A, 10–22% producing IFNγ, and <1% producing IL-4 (Table I). IL-17A, IFNγ and IL-4 were also present in the ankles (Fig. 1a). Thus, the presence of T helper cells in the draining lymph nodes and at the site of inflammation suggests a role for both Th17 and Th1 cells in the K/BxN arthritis model.
Figure 1.
Expression of IL-17 and IFNγ in the popliteal lymph nodes and ankles. a, Representative dot plots of five K/BxN mice per group aged day 21, 28, and 35 (n = 15 mice). Popliteal lymph nodes and ankles were stimulated for 4 h with PMA + ionomycin. b, On day 0, 3.5×105 Vβ6+/CD4+ KRN T cells were injected i.v. into B6.TCR.Cα−/−H-2b/g7 mice (popliteal lymph nodes n = 10 mice, ankles n = 5 mice). Popliteal lymph nodes and ankles were examined on day 28 for cytokine expression. T cells were gated on TCR Vβ6+ live cells and stained for intracellular IL-17, IFNγ and IL-4. Gates were drawn on unstained cell samples from popliteal lymph nodes. c, Total IgG-GPI specific titers as measured by ELISA. Each point represents the serum antibody titer to GPI for an individual mouse and the bars represent the means. Results are representative of 9 mice (Day 0 is significantly different from all other days, p < 0.001. *, p < 0.05).
Table 1.
Cytokine expression in popliteal lymph nodes of K/BxN mice.
| Mouse | IL-17 | IFNγ | IL-17/IFNγ | IL-4 |
|---|---|---|---|---|
| 1 | 15.2 | 19.1 | 1.33 | 0.42 |
| 2 | 10.8 | 17.7 | 0.88 | 0.98 |
| 3 | 9.28 | 22 | 0.76 | 0.83 |
| 4 | 27.1 | 14.1 | 1.89 | 0.28 |
| 5 | 6.4 | 20.9 | 0.58 | 0.37 |
| 6 | 8.92 | 13.2 | 0.67 | 0.41 |
| 7 | 9.55 | 10.2 | 0.27 | 2.4 |
| 8 | 4.63 | 20.7 | 0.18 | 0.14 |
| 9 | 8.3 | 15 | 0.47 | 0.2 |
Percentage of Vβ6+ T cells from the popliteal lymph nodes that are positive for the indicated cytokines.
3.2. Naive KRN T cells induce arthritis in T cell deficient mice
To study the role of different Th subsets in the development of arthritis, we developed a cell transfer model, referred to as KRN-CTM [37], based upon the established approach of transferring autoreactive T cells into lymphopenic hosts [44, 45]. We used the T cell deficient mice B6.TCR.Cα−/− H-2b/g7 which permitted the GPI autoantigen to be presented by I-Ag7, and the host to be histocompatibile with the transferred KRN T cells (H-2b). Purified naive KRN T cells were transferred i.v. into the recipient strain B6.TCR.Cα−/−H-2b/g7 and the development of arthritis monitored by clinical score, rear paw thickness, and histopathology. The arthritis which developed was nearly identical to that described for the K/BxN mice. The disease initiated by day 7–10, continued to increase in severity through day 24 and chronic arthritis was maintained throughout the life of the animal [37]. At day 28, popliteal lymph nodes and ankles were harvested. Recovered T cells were stimulated for 4 h in the presence of PMA + ionomycin then assayed for cytokine production. Both IL-17A (0.5–0.8%) and IFNγ (2.9–4.3%) were present in the draining lymph nodes and ankles (Fig. 1b) of all naive T cell transfer mice and there was little IL-4 expression. The presence of IL-17A and IFNγ suggests that the transferred naive T cells polarized into Th17 or Th1 cells, similar to what was observed in the K/BxN mice (Fig. 1a).
The development of arthritis in the K/BxN mouse model is dependent on antibodies directed against the ubiquitously-expressed protein GPI. Therefore, we assayed for total anti-GPI IgG by ELISA. Anti-GPI IgG expression preceded the measurable increase in ankle swelling in all recipients receiving naive KRN T cells (Fig. 1c). Furthermore, an increase in antibody titer paralleled the increase in ankle thickness, and total anti-GPI remained high for > 7 weeks, or until the animal was sacrificed. Thus, like in K/BxN arthritis, naive KRN T cell induced arthritis produced anti-GPI IgG.
3.3. Th17 and Th1 polarized cells induced arthritis
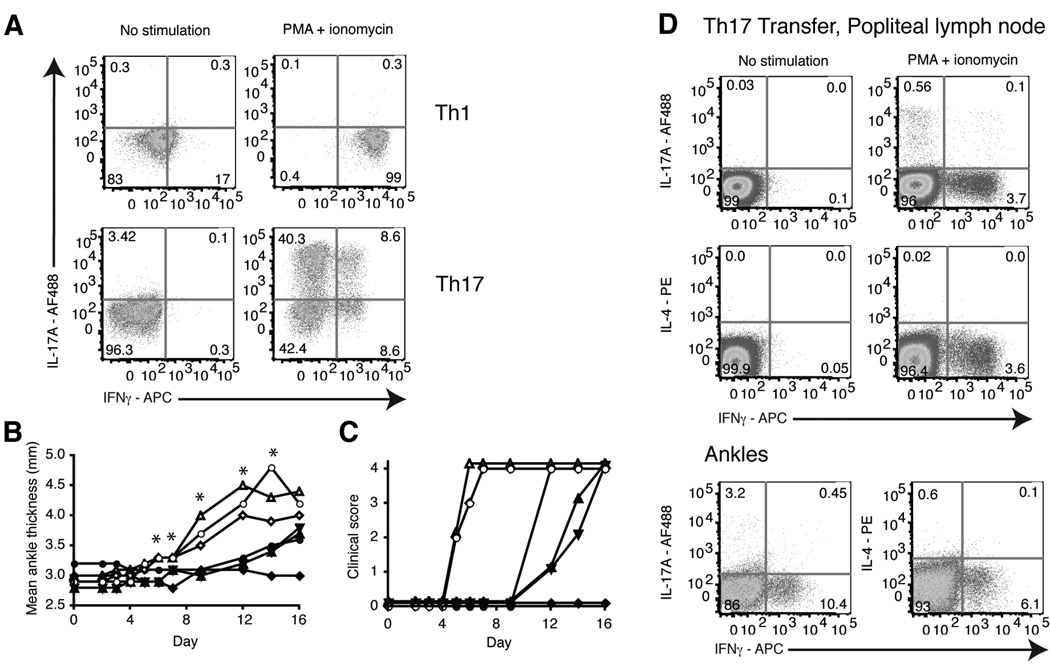
The presence of IL-17A and IFNγ in the K/BxN and KRN-CTM models led us to investigate whether Th1 or Th17 cells alone could induce arthritis. We purified naive CD4+ T cells from spleens of KRN mice and stimulated them in vitro with G7m peptide (a strongly stimulating mimic of the GPI epitope) [5] and B6.G7 splenocytes along with IL-12 and anti-IL-4 to trigger Th1 differentiation or TGFβ, IL-6, anti-IL-4, anti-IL-12, and anti-IFNγ to trigger Th17 differentiation. After polarizing the cells for 3 weeks, we stimulated the cells for 4 h with PMA + ionomycin and assessed their expression of IL-17A, IFNγ, (Fig. 2a) and IL-4 (data not shown) by intracellular cytokine staining to evaluate the polarization. KRN T cell cultures primed in Th1-inducing conditions generated T cells that exclusively produced IFNγ. T cells cultured with Th17-inducing conditions generated cells producing IL-17A and a small population of IFNγ and IL-17A/IFNγ producing cells, similar to what was observed by others [41, 46, 47]. There was no IL-4 detected. Cytokine bead array analysis (CBA) of these cultures confirmed that little IFNγ was produced in the Th17 cultures and that higher levels of IL-17A were produced compared to IFNγ in the Th17 cultures (data not shown).
Figure 2.
Th17 and Th1 polarized KRN T cells transferred into B6.TCR.Cα−/−H-2b/g7 mice induced arthritis. Naive KRN T cells were cultured for 3 weeks under Th17 or Th1 polarizing conditions using B6.G7 splenocytes as antigen presenting cells. a, Polarized cells were stimulated for 4 h with PMA + ionomycin. T cells were gated on TCR Vβ6+ live cells and stained for intracellular IL-17A and IFNγ. b, 10×106 Th17 or Th1 polarized KRN T cells were transferred i.v. into B6.TCR.Cα−/−H-2b/g7 mice. The thickness of both rear ankles was measured as an indication of arthritis. Results are representative of at least 10 mice per group (*p < 0.05). c, Mice were examined for disease severity and given a clinical score of disease. Open symbols, Th17 cells. Filled symbols, Th1 cells. Each symbol represents one mouse. Data from three independent experiments are shown (n = 10 mice per group). Clinical score was significantly different using the Kaplan-Meir survival curves, p < 0.05 with a confidence level of 95%. d, On day 28, popliteal lymph nodes and ankles were harvested and cells stimulated for 4 h with PMA + ionomycin. T cells were gated on live Vβ6+ cells and stained for intracellular IL-17A, IFNγ and IL-4. Results are representative of 10 mice.
To determine whether Th1 or Th17 cells alone could induce disease, we transferred the Th1 or Th17 polarized cells into B6.TCR.Cα−/−H-2b/g7 mice. Transfer of Th17 cells induced disease in all of the recipient mice. In contrast, Th1 cells induced a delayed disease in only 2/3 of the mice (Fig. 2b and c). The Th17 induced arthritis initiated between days 5 and 9, and the severity continued to increase up to day 14 and was maintained for at least 28 days, or until the animal was sacrificed. Histologic assessment of hind paws 28 days post Th17 cell transfer revealed a severe, destructive polyarthritis. There were high numbers of pleomorphic inflammatory cells entering the paws via the synovium, with infiltration into the joint space and extraarticular soft tissues. In addition, there was moderate to severe damage to articular cartilage with loss of chondrocytes and their matrix as well as numerous areas of focal to expansive ulceration. Lastly, there was moderate to severe osteoclast-mediated bone resorption affecting the distal tibia and numerous metatarsal and phalangeal bones, with correlative fibrovascular stromal (pannus) invasion of their medullary space (data not shown and [37]). Thus, in vitro polarized Th17 KRN T cells were able to induce arthritis, similar to that observed in KRN-CTM.
At day 28, popliteal lymph nodes and ankles were harvested. The samples were stimulated for 4 h in the presence of PMA + ionomycin and assayed for cytokine production (Fig. 2d). In the Th17 transferred mice, both IL-17A and IFNγ were present in the draining lymph nodes and ankles. IL-17A and IFNγ were also produced in the draining lymph nodes of mice transferred with Th1 cells (data not shown). It has been previously reported that restimulation of Th17 cells with antigen can induce IFNγ production in an EAE model [48]. These findings indicate that Th17 cells are able to induce arthritis in the KRN-CTM, but neither the Th17 nor Th1 phenotypes are completely stable in vivo.
3.4. In vivo neutralization of IFNγ exacerbates Th17 induced arthritis
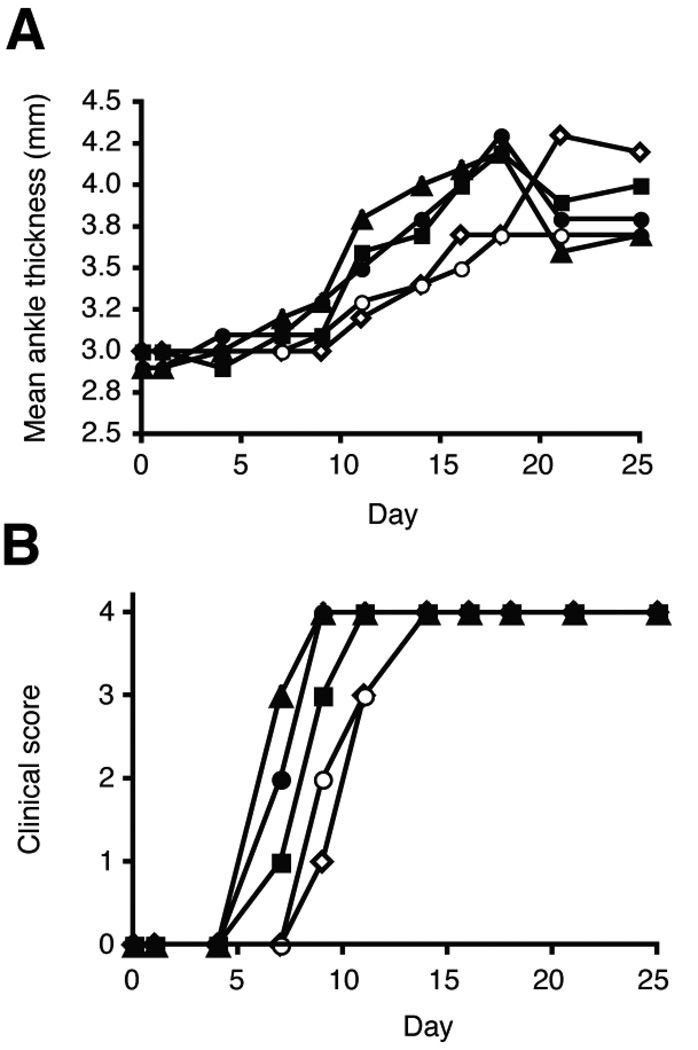
The presence of IFNγ producing cells in the Th17 T cell transfers raised the possibility that IFNγ was involved in the development of the arthritogenic antibodies. To address this issue, Th17 polarized cells were transferred into B6.TCR.Cα−/−H-2b/g7 mice that were injected with neutralizing anti-IFNγ or control antibody (both kindly provided by Robert Schreiber) at day 0 and arthritis development was monitored. The mice that received anti-IFNγ developed arthritis slightly earlier than those receiving Th17 cells alone and the disease was more severe (Fig. 3a and b). Disease development, as measured by clinical score, was found to be statistically different between the IFNγ and control treated groups using the Kaplan-Meir curve (Fig. 3b). Thus, in the KRN-CTM, as has been observed by others, IFNγ is acting as an inhibitory cytokine. Overall, these findings indicated that IFNγ was not contributing to the initiation of disease induced by transfer of Th17 cells.
Figure 3.
Neutralizing anti-IFNγ antibody enhances Th17 induced arthritis. On day 0 of Th17 cell transfer mice were injected with 250 µg anti-IFNγ or control PIP antibody. Mice were injected every 10 days with antibody. a, The thickness of both rear ankles was measured as an induction of arthritis. b, Mice were examined for disease severity and given a clinical score of disease (n = 9 mice per group). Open symbols, control PIP antibody. Filled symbols, anti-IFNγ antibody. Each symbol represents one mouse. Clinical score was significantly different using the Kaplan-Meir survival curves, p < 0.05 with a confidence level of 95%.
Anti-IL-17A treatment delays onset of arthritis induction by Th17 cells
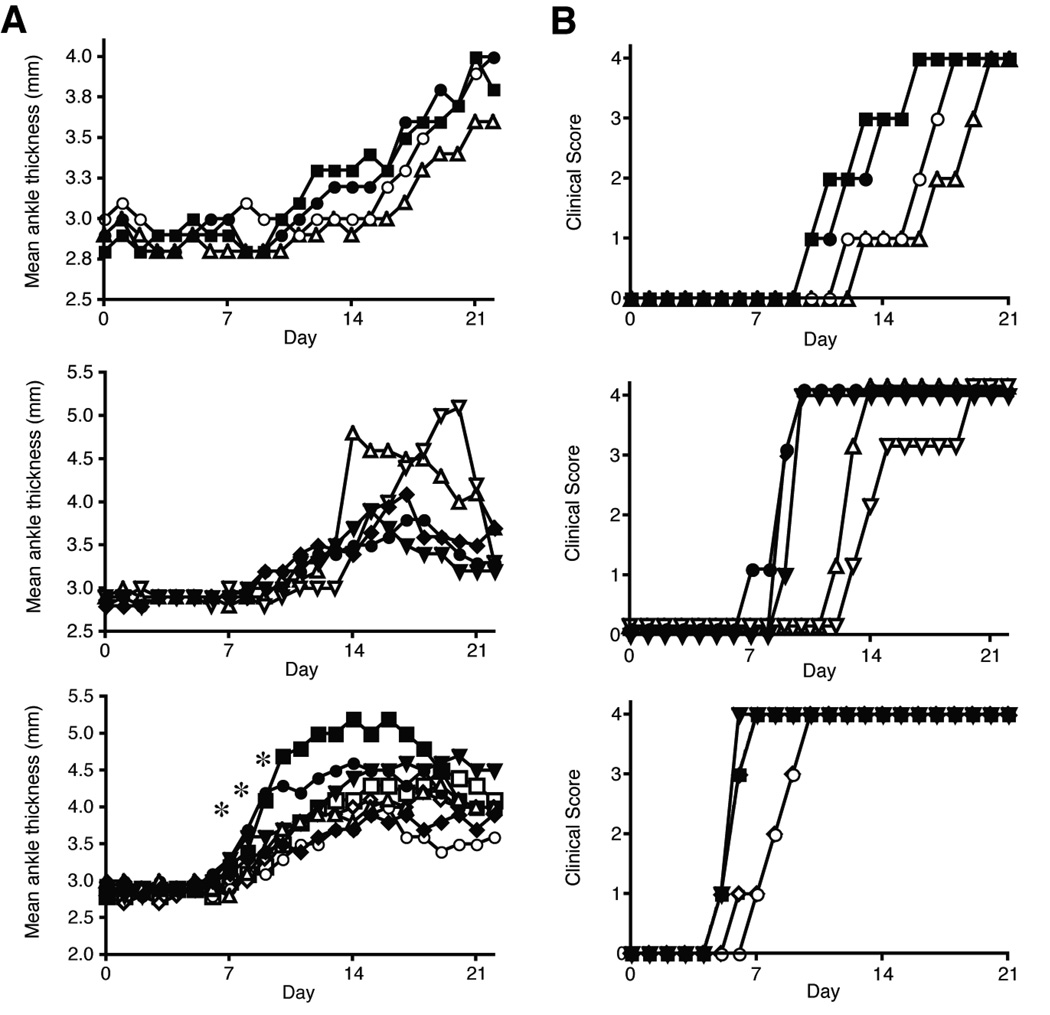
To assess the role of the Th17 cell signature cytokine IL-17A in the development of arthritis, Th17 polarized cells were transferred into B6.TCR.Cα−/−H-2b/g7 mice that were injected with 500 µg anti-IL-17A or control antibody. The mice were subsequently injected every three days with 250 µg of anti-IL-17A or control antibody. In three separate experiments, in vivo neutralization of IL-17A delayed disease onset by 2–5 days as measured by clinical score (Fig. 4b), which was statistically significant using the Kaplan-Meir curve. Eventually all of the mice did develop arthritis (Fig. 4a and b). Wu et al. reported in K/BxN mice that anti-IL-17 neutralization blocked the spontaneous arthritis induction [35]. Our ability to observe less of an inhibitory effect of anti-IL-17 may reflect differences in the spontaneous K/BxN and our KRN-CTM models, and the robust and synchronized induction in the KRN-CTM model. The results in this study suggest that IL-17A has a significant early but redundant role in the development of Th17 induced KRN-CTM arthritis.
Figure 4.
Neutralizing anti-IL-17A antibody delayed Th17 induced arthritis. On day 0 of Th17 cell transfer mice were injected with 500 µg anti-IL-17A or control antibody. Mice were injected every 3 days with 250 µg antibody. a, The thickness of both rear ankles was measured as an induction of arthritis. b, Mice were examined for disease severity and given a clinical score of disease. Open symbols, control antibody. Filled symbols, anti-Il-17A antibody. Each symbol represents one mouse. Results are representative of 8 mice per group. Clinical score was significantly different using the Kaplan-Meir survival curves, p < 0.05 with a confidence level of 95%. * p < 0.05.
3.5. Role of T-bet in the differentiation of KRN T cells and arthritis induction
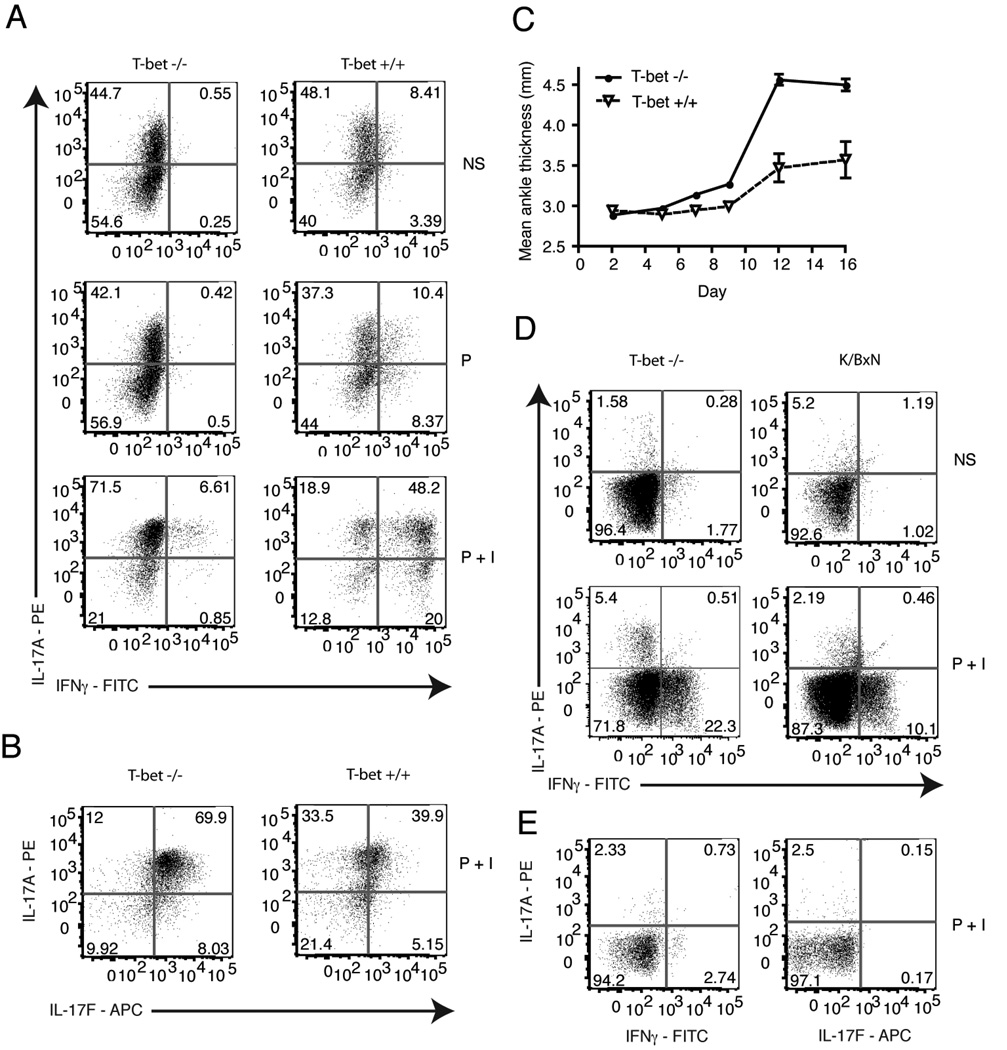
In vivo, there is emerging evidence of the plasticity of the Th17 cells, consistent with our finding of IFNγ producing cells in the Th17 cell transfer recipients [49, 50]. The T-box transcription factor T-bet drives the expression of IFNγ in Th1 cells. To better determine the contribution of IFNγ and Th1 cells in arthritis induction in our model, KRN/T-bet−/− mice were generated. Naive KRN/T-bet−/− T cells cultured in Th17 inducing conditions generated Th17 cells that produced more IL-17A and less IFNγ than KRN/T-bet+/+ T cells (Fig. 5a). Interestingly, when the Th17 cultured cells were stimulated with PMA alone there was less IFNγ detected than in the presence of PMA + ionomycin. This suggests that PMA + ionomycin is such a potent stimulant that it may not be a fair comparison to what occurs in vivo to the natural GPI stimulant. The Th17 cultures also express IL-17F (Fig. 5b), another Th17 associated cytokine. There was no IL-21 or IL-22 detected in any of the Th17 cultures (data not shown).
Figure 5.
IFNγ is suppressed in Th17 polarized T cells from KRN/T-bet−/− mice in vitro but not in vivo. Naive KRN/T-bet−/− T cells were cultured for 3 weeks under Th17 polarizing conditions using B6.G7 splenocytes as antigen presenting cells. a, Polarized cells were stimulated for 4 h with PMA or PMA + ionomycin. T cells were gated on TCR Vβ6+ live cells and stained for IL-17A and IFNγ, or b, IL-17A and IL-17F. c, On day 0, 10×106 KRN/T-bet−/− or KRN (T-bet+/+) Th17 polarized cells were injected i.v. into B6.TCR.Cα−/−H-2b/g7 mice and the thickness of both rear ankles was measured as a indication of arthritis (n= 9 KRN/T-bet−/− mice and 3 KRN mice). Results are representative of two independent experiments. Open symbols, KRN/T-bet+/+. Filled symbols, KRN/T-bet−/−. d, On day 16 popliteal lymph nodes were harvested and cells stimulated for 4 h with PMA + ionomycin. T cells were gated on live Vβ6+ cells and stained for intracellular IL-17A and IFNγ. Results are representative of 13 mice. e, On day 1 popliteal lymph nodes were harvested and cells stimulated for 4 h with PMA + ionomycin. T cells were gated on live Vβ6+ cells and stained for intracellular IL-17A, IFNγ, and IL-17F. Results are representative of one mouse. NS, no stimulation. P, PMA. P+I, PMA + ionomycin.
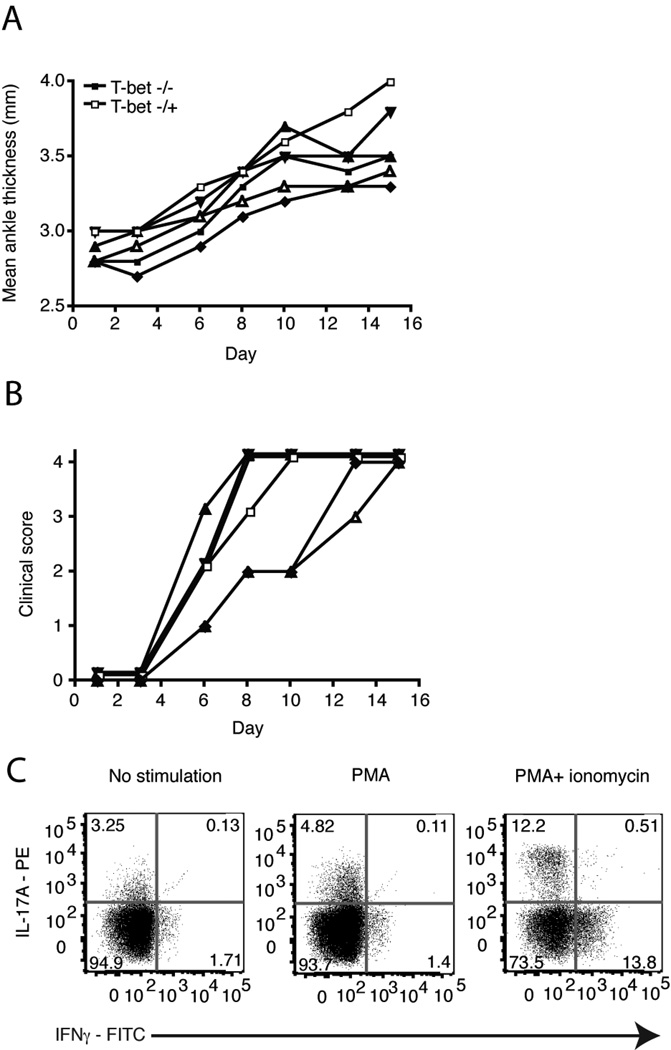
The transfer of KRN/T-bet−/− Th17 cells induced arthritis in B6.TCR.Cα−/−H-2b/g7 mice (Fig. 5c). On day 16 popliteal lymph nodes were harvested and stimulated for 4 h in the presence of PMA or PMA + ionomycin and cytokine production was determined. Surprisingly, recovered KRN/T-bet−/− Th17 cells produced not only IL-17A but IFNγ despite the lack of T-bet. IFNγ was detected in transferred cells as early as day 1 post transfer (Fig. 5e). The transfer of naive KRN/T-bet−/− T cells induced arthritis (Fig. 6a and b) and IL-17A and IFNγ were also detected after T cell recovery (Fig. 6c). Thus knocking-out T-bet expression did not inhibit arthritis induction. Also, the production of IFNγ in this model is T-bet independent.
Figure 6.
Naive T cells from KRN/T-bet−/− mice induce arthritis. On day 0, 1×106 Vβ6+/CD4+ KRN/T-bet−/− or KRN/T-bet−/+ T cells were injected i.v. into B6.TCR.Cα−/−H-2b/g7 mice. a, The thickness of both rear ankles was measured as an indication of arthritis. b, Mice were examined for disease severity and given a clinical score of disease. Results are representative of two independent experiments. Open symbol, KRN/T-bet−/+. Filled symbols, KRN/T-bet−/−. Each symbol represents one mouse. c, On day 16 popliteal lymph nodes were harvested and cells stimulated for 4 h with PMA + ionomycin. T cells were gated on live Vβ6+ cells and stained for intracellular IL-17A, IFNγ, and IL-17F. Results are representative of 11 mice.
3.6. Th17 cells direct autoantibody responses
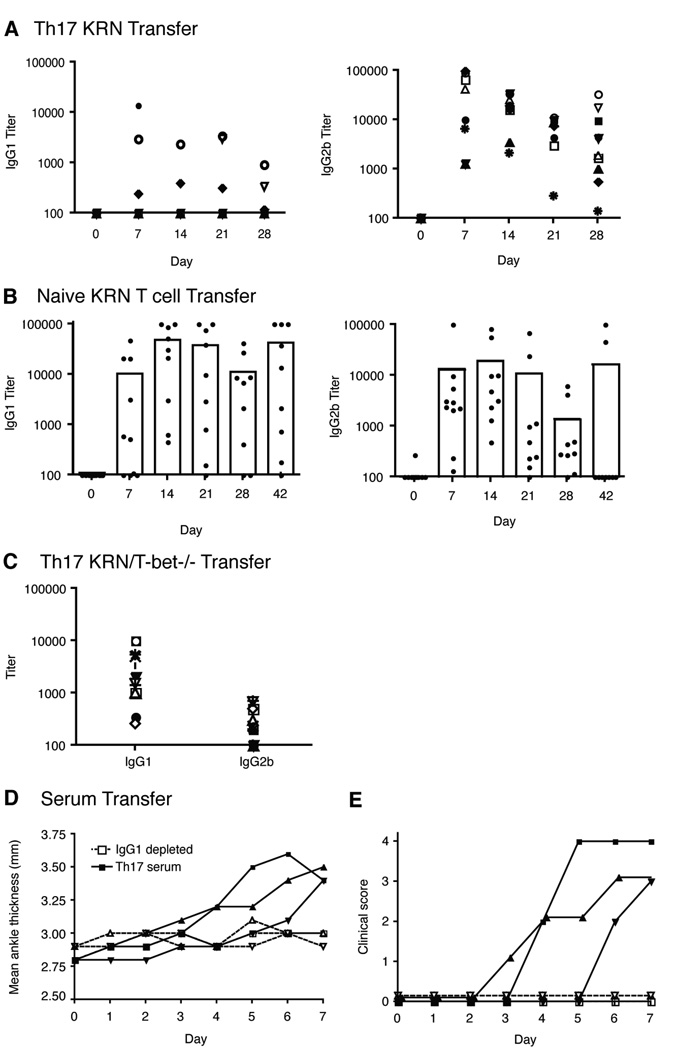
It has been previously shown in the K/BxN mouse model, that anti-GPI IgG1 was the dominant isotype and essential for arthritis induction [4, 51]. In general Th1-type responses are IgG2a dominant whereas Th2-type responses are IgG1 dominant. Mitsdoerffer et al. have shown that Th17 cells are able to promote class switching to IgG1, IgG2a, IgG2b, and IgG3. Therefore, we were interested in determining the isotype usage in Th17 induced anti-GPI responses. Surprisingly, there were high levels of IgG2b anti-GPI antibodies in mice where disease was induced by KRN Th17 cell transfer, and much less IgG1 (Fig. 7a). In 6 of 10 mice there was little to no IgG1 expression (Fig. 7a), even when the disease was at its most severe stage. In all 10 mice, IgG2b was the dominant isotype at all time points tested. It is intriguing that despite having the capacity to induce class switch for all of the IgGs, the KRN Th17 cells predominantly produced IgG2b. Mice treated with or without IFNγ neutralizing antibody had similar levels of antibody expression (data not shown). GPI specific IgM was detected in all mice at day 7 before there was a noticeable change in ankle thickness (data not shown). IgG2c was also present at moderate levels and no IgG3 was detected (data not shown).
Figure 7.
IgG2b is the dominant anti-GPI isotype in mice transferred with Th17 polarized KRN T cells while IgG1 is dominant in mice transferred with naive T cells and KRN/T-bet−/− Th17 cells. Serum titers of individual isotypes of anti-GPI were measured every 7 days by ELISA using isotype-specific secondary antibodies. a, Antibody titers of 10 mice transferred with 9.5×106 Th17 polarized KRN T cells. Each symbol represents one mouse. b, Antibody titers of 9 mice transferred with 3.5×105 naive KRN T cells. c, Antibody titers of 13 mice transferred with 9.5×106 Th17 polarized T cells from KRN/T-bet−/− mice on day 14 post transfer. d, The thickness of both rear ankles was measured as an induction of arthritis in Balb/c mice transferred with Th17 serum or Th17 serum depleted of IgG1. e, Mice were examined for disease severity and given a clinical score of disease. Results are representative of 5 mice per group.
The pattern of anti-GPI expression in KRN-CTM when naive T cells were transferred was similar to what was previously reported for K/BxN mice [4, 51]. When naive KRN T cells were transferred into B6.TCR.Cα−/−H-2b/g7 mice, arthritis developed and IgG1 was dominant in all mice, but there was also substantial expression of IgG2b (Fig. 7b). Like naive T cells transfers, the transfer of Th17 KRN/T-bet−/− induced a dominant IgG1 response (Fig. 7c). These data suggest that the dominant isotype is dependent on the type of T cell that induced KRN arthritis.
To determine if GPI-specific IgG2b isotype alone could induce arthritis we separated the various isotypes from K/BxN serum by fractioning on a protein A-sepharose column [52]. Injection of 500 µg of purified IgG2b on day 0 or 500 µg on day 0 and day 1 did not induce arthritis in BALB/c mice (data not shown). However, injection of purified GPI-specific IgG1 did induce disease (data not shown). To address whether IgG1 was absolutely required for arthritis induction we depleted recovered serum from KRN Th17 induced arthritis of IgG1. BALB/c mice were injected with 500 µg of IgG1 depleted serum on day 0, 1, and 2. IgG1 depleted serum did not induce arthritis (Fig. 7 d and e), supporting the finding of Maccioni et al. [51]. Therefore, the dominance of IgG2b in arthritic mice induced by KRN Th17 cells appears not to be involved in arthritis induction. The simplest explanation for the lack of detection of IgG1 anti-GPI antibodies in the serum of Th17 cell recipients is that there are low levels of IgG1 produced and all of it is present in the joint and/or bound to FcR bearing cells.
4. Discussion
To study the role of T helper subsets in the development of RA, we used the KRN-CTM, a chronic yet synchronized version of the K/BxN mouse which relies upon transfer of autoreactive T cells into lymphopenic host. Through this approach we demonstrated that either naive T cells or Th17 cells could induce chronic arthritis and autoantibody production whereas Th1 cells were much less efficient. When the transferred naive T cells were recovered on day 28 from the popliteal lymph node and ankles, the presence of IL-17A and IFNγ suggested that they polarized into Th17 and Th1 cells, similar to what was observed in K/BxN mice. Transfer of Th17 cells induced disease in all of the recipient mice. In contrast, Th1 cells induced a delayed disease in only 2/3 of the mice. Neutralization of IFNγ resulted in earlier onset of arthritis while IL-17A inhibition delayed disease onset. Also, T-bet expression is not crucial for arthritis induction. Overall these findings indicate that IFNγ did not contribute to the initiation of the disease while IL-17A had a significant role in Th17 induced KRN-CTM arthritis. Furthermore, we demonstrated that Th17 cells direct autoantibody responses.
We hypothesized that at least one KRN T helper subsets would be sufficient for arthritis induction. Our data indicate that Th17 cells alone were indeed able to induce arthritis in the KRN-CTM, with disease developing between days 5–9 after transfer and progressing to a point by day 28 which was histologically consistent with the KRN-CTM after naive T cell transfer. GPI-specific autoantibodies were detected as early as day 7, thereby suggesting that the transferred Th17 cells provided the initial switch factors necessary for B cell help and autoantibody production. In KRN-CTM neither Th17 nor Th1 phenotypes were completely stable in vivo. After transfer the host’s environment influenced the cells to express other cytokines demonstrating that in vitro cultured cells have a tremendous amount of plasticity once injected in vivo [53]. This inherent plasticity of the polarized cells made it impossible to draw conclusions from the transferred cells at later stages of the disease in KRN Th17 transferred arthritis [49, 50]. The inability to polarize KRN T cells to strictly IL-17A-producing cells has been observed by others in different model systems [41, 46, 47]. It is still unclear if T cells producing both IL-17A and IFNγ are truly Th17 cells or if they have not polarized completely to one subset.
Historically IFNγ is viewed as a proinflammatory factor. In the current study, we initiated IFNγ inhibitory studies to verify whether IL-17A or IFNγ was the mediator of arthritis induction. In the presence of anti-IFNγ Th17 cells induced disease, thereby demonstrating that IL-17A mediates arthritis induction. Surprisingly arthritis developed earlier when anti-IFNγ was combined with Th17 cell transfers. This suggests that in KRN-CTM IFNγ acts as an inhibitory cytokine; thus, IFNγ may have a protective role in K/BxN arthritis rather than a pathogenic role. However, Th1 cell transfer did induce disease which seems contradictory to the fact that blocking IFNγ production caused disease to develop earlier. Perhaps with time the production of other cytokines such as IL-17A in Th1 cell transfers allowed limited disease to develop. It has also been reported that DBA/1 IFNγ receptor knock-out mice develop CIA [54, 55]. Furthermore, Chu et al. observed that IFNγ can play an anti-inflammatory role. They found that C57BL/6 mice were resistant to CIA by IFNγ-mediated suppression of IL-17 [29]. In a two-year prospective study of RA patients, those patients that had higher levels of IFNγ were protected against progressive joint damage [19]. These observations suggest that IFNγ plays a beneficial role during the development of arthritis, perhaps by regulating IL-17 signaling. Harrington et al. demonstrated that IFNγ may be inhibiting IL-23R expression on Th17 cells thus preventing their expansion [56]. Also, IFNγ has been reported to inhibit osteclastogenesis by interfering with receptor activator of nuclear factor NF-κB ligand (RANKL) signaling [57] but the mechanisms for IFNγ-mediated suppression of Th17 cells are not fully understood.
When polarized KRN Th17 cells were transferred along with neutralizing anti-IL-17A, disease onset was delayed. In CIA [24] and EAE [58–60], treatment with anti-IL-17 after disease onset reduced disease severity. Even at late stages of disease anti-IL-17 reversed disease progression. This partial protection observed in our model as well as in other models indicates that other factors produced by Th17 cells such as IL-17F, IL-6, IL-21, IL-22, and IL-23 may be important for disease induction. In particular, IL-17F is highly homologous to IL-17A and binds to the same receptor [61]. Currently there are no commercially available neutralizing antibodies specific for IL-17F. Another Th17 factor, IL-6, was observed to play a role in a model of chronic inflammatory bowel disease (IBD). Neutralization of both IL-17 and IL-6 significantly lowered the disease score but neutralizing IL-6 alone or IL-17 alone did not significantly change the disease [15]. In this IBD model, IL-23 is necessary. Multiple studies have demonstrated a role for IL-23 in autoimmune diseases [13, 62]. IL-23 has been shown to promote IL-17 expression [15, 63, 64] and IL-6 [15]. This raises the possibility that a combination treatment of anti-IL-17A with anti-IL-6 and/or anti-IL-23 may result in significant improvement of Th17 induced arthritis. Future studies will be required to distinguish the role of other Th17 factors on disease induction.
In order to delineate the contribution of IFNγ in arthritis induction we generated KRN/T-bet−/− mice. We hypothesized that these mice would not be capable of producing significant levels of IFNγ and that the induction of disease would be attributed to IL-17A expression. In the absence of T-bet, both KRN/T-bet−/− naive T cells and Th17 cells induced arthritis. However, upon recovery of the adoptively transferred cells they were found to be expressing high levels of IFNγ. Guo et al. also observed IFNγ expression in adoptively transferred naive T-bet−/− T cells after challenge and recovery [65]. At the time of transfer, KRN/T-bet−/− Th17 cells were not expressing IFNγ but as early as day 1 post transfer IFNγ producing cells were detected after recovery. Not only do these KRN/T-bet−/− mice demonstrate that T-bet expression is not critical for arthritis induction, but that there is an unknown mechanism that drives IFNγ production in CD4+ T cells.
Bai et al. have reported that IL-17, and not IFNγ, was key to autoantibody responses in an experimental murine autoimmune myasthenia gravis model [66]. The authors noted that IL-17 elicited higher antibody responses to the autoantigen acetylcholine receptor (AChR). More specifically, autoreactive Th17 cells promoted IgG2b antibody responses. Th17 development depends on both TGFβ and IL-6. Interestingly, TGFβ favors class switching of IgG2b [67, 68]. Also, IL-6 is a well-known B cell stimulatory factor [69]. These observations support our current findings and suggest that KRN Th17 can provide B cell help and promote IgG2b antibody switching. Interestingly, both KRN naive T cell and KRN/T-bet−/− Th17 transfer induced a dominant IgG1 response. The three transfer methods studied in this paper all induce arthritis, and the recovered cells all produce IL-17A and IFNγ. We speculate that the level of the individual cytokines and timing of their expression may play a role in class-switching and determine which isotypes are dominant. In a previous study Maccioni et al. showed that IgG1 was the dominant isotype [51]. They also noted that the dominant isotype varied with age. Younger mice expressed more IgG2b and IgG2c but when the mice became older IgG1 had increased to become the dominant isotype. Our attempts to induce arthritis with purified IgG2b and serum depleted of IgG1 were not successful which was surprising since several mice with Th17 induced arthritis had no detectable IgG1. We hypothesize that those mice that tested negative for IgG1, had IgG1 present in the joints but not in the circulation. Further studies will need to be performed to determine the significance of Th17 induced IgG2b class switch.
In conclusion, by using a novel chronic arthritis transfer model of RA (KRN-CTM) we have highlighted the importance of Th17 cells in arthritis development. We demonstrated that T-bet expression is not required for Th17 induced arthritis and that these Th17 cells express IFNγ. We found that IFNγ inhibition actually exacerbates arthritis development, whereas blocking IL-17A inhibits reduces disease. Thus, Th17 cells provide B cell help and antibody switching thereby indicating that Th17 cells play a key role in induction of disease.
Acknowledgement
We thank Robert Schreiber for providing the anti-IFNγ monoclonal antibody and Len Herzenberg for the anti-IgG1b hybridoma cell line. This work was supported by the National Institutes of Health Grants AI31238 and AI24157 and the Washington University/Pfizer BioMedical Agreement.
References
- 1.Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:133–147. doi: 10.1007/BF00832003. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 3.Weissmann G. Pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2004;10:S26–S31. doi: 10.1097/01.rhu.0000130687.75646.44. [DOI] [PubMed] [Google Scholar]
- 4.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 5.Basu D, Horvath S, Matsumoto I, Fremont DH, Allen PM. Molecular basis for recognition of an arthritic peptide and a foreign epitope on distinct MHC molecules by a single TCR. J Immunol. 2000;164:5788–5796. doi: 10.4049/jimmunol.164.11.5788. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto I, Lee DM, Goldbach-Mansky R, Sumida T, Hitchon CA, Schur PH, et al. Low prevalence of antibodies to glucose-6-phosphate isomerase in patients with rheumatoid arthritis and a spectrum of other chronic autoimmune disorders. Arthritis Rheum. 2003;48:944–954. doi: 10.1002/art.10898. [DOI] [PubMed] [Google Scholar]
- 7.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto I, Stab A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 9.Scatizzi JC, Bickel E, Hutcheson J, Haines GK, 3rd, Perlman H. Bim deficiency leads to exacerbation and prolongation of joint inflammation in experimental arthritis. Arthritis Rheum. 2006;54:3182–3193. doi: 10.1002/art.22133. [DOI] [PubMed] [Google Scholar]
- 10.Boissier MC, Chiocchia G, Bessis N, Hajnal J, Garotta G, Nicoletti F, et al. Biphasic effect of interferon-gamma in murine collagen-induced arthritis. Eur J Immunol. 1995;25:1184–1190. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima H, Takamori H, Hiyama Y, Tsukada W. The effect of treatment with interferon-gamma on type II collagen-induced arthritis. Clin Exp Immunol. 1990;81:441–445. doi: 10.1111/j.1365-2249.1990.tb05353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams RO, Williams DG, Feldmann M, Maini RN. Increased limb involvement in murine collagen-induced arthritis following treatment with anti-interferon-gamma. Clin Exp Immunol. 1993;92:323–327. doi: 10.1111/j.1365-2249.1993.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 17.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- 18.Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Mol Cells. 2005;19:180–184. [PubMed] [Google Scholar]
- 19.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 20.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 21.Guedez YB, Whittington KB, Clayton JL, Joosten LA, van de Loo FA, van den Berg WB, et al. Genetic ablation of interferon-gamma up-regulates interleukin-1beta expression and enables the elicitation of collagen-induced arthritis in a nonsusceptible mouse strain. Arthritis Rheum. 2001;44:2413–2424. doi: 10.1002/1529-0131(200110)44:10<2413::aid-art406>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 23.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 25.Latham KA, Whittington KB, Zhou R, Qian Z, Rosloniec EF. Ex vivo characterization of the autoimmune T cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic joints. J Immunol. 2005;174:3978–3985. doi: 10.4049/jimmunol.174.7.3978. [DOI] [PubMed] [Google Scholar]
- 26.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubberts E, Schwarzenberger P, Huang W, Schurr JR, Peschon JJ, van den Berg WB, et al. Requirement of IL-17 receptor signaling in radiation-resistant cells in the joint for full progression of destructive synovitis. J Immunol. 2005;175:3360–3368. doi: 10.4049/jimmunol.175.5.3360. [DOI] [PubMed] [Google Scholar]
- 28.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 31.Doodes PD, Cao Y, Hamel KM, Wang Y, Farkas B, Iwakura Y, et al. Development of proteoglycan-induced arthritis is independent of IL-17. J Immunol. 2008;181:329–337. doi: 10.4049/jimmunol.181.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnegan A, Grusby MJ, Kaplan CD, O'Neill SK, Eibel H, Koreny T, et al. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:3345–3352. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- 33.Iwanami K, Matsumoto I, Edmonds JP, Inoue A, Mihara M, Ohsugi Y, et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs JP, Wu HJ, Benoist C, Mathis D. IL-17-producing T cells can augment autoantibody-induced arthritis. Proc Natl Acad Sci U S A. 2009;106:21789–21794. doi: 10.1073/pnas.0912152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu HJ, Ivanov, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaBranche TP, Hickman-Brecks C, Meyer DM, Storer CE, Jesson M, Shelvin KM, et al. Characterization of the KRN cell transfer model of rheumatoid arthritis (KRN-CTM), a chronic yet synchronized version of the K/BxN mouse. Am J Pathol. 2010;177:1388–1396. doi: 10.2353/ajpath.2010.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 39.Lohoff M, Duncan GS, Ferrick D, Mittrucker HW, Bischof S, Prechtl S, et al. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J Exp Med. 2000;192:325–336. doi: 10.1084/jem.192.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Shih FF, Mandik-Nayak L, Wipke BT, Allen PM. Massive thymic deletion results in systemic autoimmunity through elimination of CD4+ CD25+ T regulatory cells. J Exp Med. 2004;199:323–335. doi: 10.1084/jem.20031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandik-Nayak L, Wipke BT, Shih FF, Unanue ER, Allen PM. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. PNAS. 2002;99:14368–14373. doi: 10.1073/pnas.182549099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, et al. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webb A, Johnson A, Fortunato M, Platt A, Crabbe T, Christie MI, et al. Evidence for PI-3K-dependent migration of Th17-polarized cells in response to CCR2 and CCR6 agonists. J Leukoc Biol. 2008;84:1202–1212. doi: 10.1189/jlb.0408234. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 51.Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ey PL, Prowse SJ, Jenkin CR. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 53.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 55.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 56.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;11:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 57.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 58.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 Cytokine Family. Vitam Horm. 2006;74:255–282. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- 62.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 63.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 64.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Guo S, Cobb D, Smeltz RB. T-bet inhibits the in vivo differentiation of parasite-specific CD4+ Th17 cells in a T cell-intrinsic manner. J Immunol. 2009;182:6179–6186. doi: 10.4049/jimmunol.0803821. [DOI] [PubMed] [Google Scholar]
- 66.Bai Y, Liu R, Huang D, La Cava A, Tang YY, Iwakura Y, et al. CCL2 recruitment of IL-6-producing CD11b+ monocytes to the draining lymph nodes during the initiation of Th17-dependent B cell-mediated autoimmunity. Eur J Immunol. 2008;38:1877–1888. doi: 10.1002/eji.200737973. [DOI] [PubMed] [Google Scholar]
- 67.Lebman DA, Edmiston JS. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes and Infection. 1999;1:1297–1304. doi: 10.1016/s1286-4579(99)00254-3. [DOI] [PubMed] [Google Scholar]
- 68.McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR, Mond JJ, et al. Transforming Growth Factor B1 Selectivity Stimulates Immunoglobulin G2b Secretion by Lipopolysaccharide-activted Murine B Cells. J Exp Med. 1993;177:1031–1037. doi: 10.1084/jem.177.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Inv. 1987;5:343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]