Abstract
Helicobacter pylori causes a lifelong infection and provides a model of bacterial adaptation and persistent colonization. Adenosine is an anti-inflammatory mediator that limits tissue damage during inflammation. We studied the role of adenosine in the T-cell-mediated regulation of gastritis and bacterial persistence. After 4h of activation, human T helper (Th) cells increased A2A adenosine receptor (A2AAR) mRNA level (sevenfold). A2AAR was the predominant subtype expressed in resting and stimulated gastric or peripheral Th cells. Stimulation with ATL313, an A2AAR agonist, increased cyclic AMP (cAMP) accumulation and reduced interleukin-2 (IL-2) production by 20–50%. ATL313 also attenuated tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) production, which was inhibited by an A2AAR antagonist. Infection of IL-10-deficient mice with H. pylori is cleared spontaneously due to the marked inflammation. Administration of ATL313 during infection reduced gastritis and pro-inflammatory cytokine responses while bacterial load increased. In contrast, infection of A2AAR-deficient mice enhanced gastritis. Thus, A2AAR limits the pro-inflammatory effects of Th cells and favor chronic Helicobacter infection.
INTRODUCTION
Helicobacter pylori infects more than half of the world ’s population and persists for the life of its host. 1 H. pylori infection is associated with chronic antral gastritis, which is characterized by a mucosal infiltration of polymorphonuclear and mononuclear leukocytes. 2 Evidence for a pathogenic role of H. pylori infection in gastroduodenal ulcer comes from clinical investigations showing that the cure of H. pylori infection accelerates ulcer healing and prevents ulcer relapse. 3,4 Besides ulcer disease, H. pylori also causes gastric lymphoma or carcinoma. 5 Although immunization of animals can be effective, it is unclear why the immune system is unable to eliminate the infection spontaneously.
Data show that subpopulations of CD4+ T lymphocytes play distinct roles in mediating and regulating H. pylori-induced gastritis. 6–9 For example, adoptive transfer of CD4+ CD45RBhi effector T cells from naive donors into immunodeficient recipients causes severe gastritis in H. pylori-infected recipients, whereas co-transfer of CD4+ CD45RBlo regulatory T cells (Treg) protects against gastritis. 10 Several studies have suggested that CD4+/CD25+/Foxp3+ Treg control gastric inflammation and contribute to the persistence of H. pylori infections. 11–13 The anti-inflammatory activity mediated by these Treg may reflect cell contact and/or the production of soluble anti-inflammatory mediators, including transforming growth factor-β, interleukin (IL)-10, IL-35 or adenosine. 12,14,15
Adenosine is a purine nucleoside that accumulates in inflamed or hypoxic tissues largely because of CD39 (nucleoside triphosphate dephosphorylase) mediating the dephosphorylation of ATP to ADP then to 5′-AMP. Subsequently, CD73 (5′-ectonucleotidase) catalyzes the terminal reaction to convert 5′-AMP into adenosine. 16 The responses controlled by adenosine are mediated by four G-protein-coupled receptors (A1, A2A, A2B, and A3). Activation of A2A adenosine receptors (A2AAR) on T cells produces a series of responses that have been categorized as anti-inflammatory. 16 For example, extracellular adenosine targets the A2AAR to trigger cyclic AMP (cAMP) accumulation and inhibit activation-induced CD25 expression. 17
Adenosine analogs limit collateral damage associated with inflammation, including colitis 18 and Clostridium difficile toxin-induced diarrhea. 19 Lappas e t al.17 reported that A2AAR induction inhibits interferon-γ (IFN-γ) production in murine CD4+ T cells. Furthermore, activation of A2AARs attenuate gastric mucosal inflammation in rats. 20 Other reports suggest that Treg cells exert their suppressive action through the production of 14,15,21 and/or response to adenosine. 22,23 These observations suggest that A2AAR play a critical role in mucosal immune regulation due to their novel effects on T-cell cytokine production. 22
Currently, little is known about the role of A2AAR in human mucosal Th cell function or its role in controlling the host response to infection with Helicobacter spp. In this study, we examined the expression of ARs on human Th cells from the blood or gastric tissue and evaluated the role of A2AAR in regulating gastritis and bacterial burden.
RESULTS
Human Th cell activation induces A2AAR mRNA expression
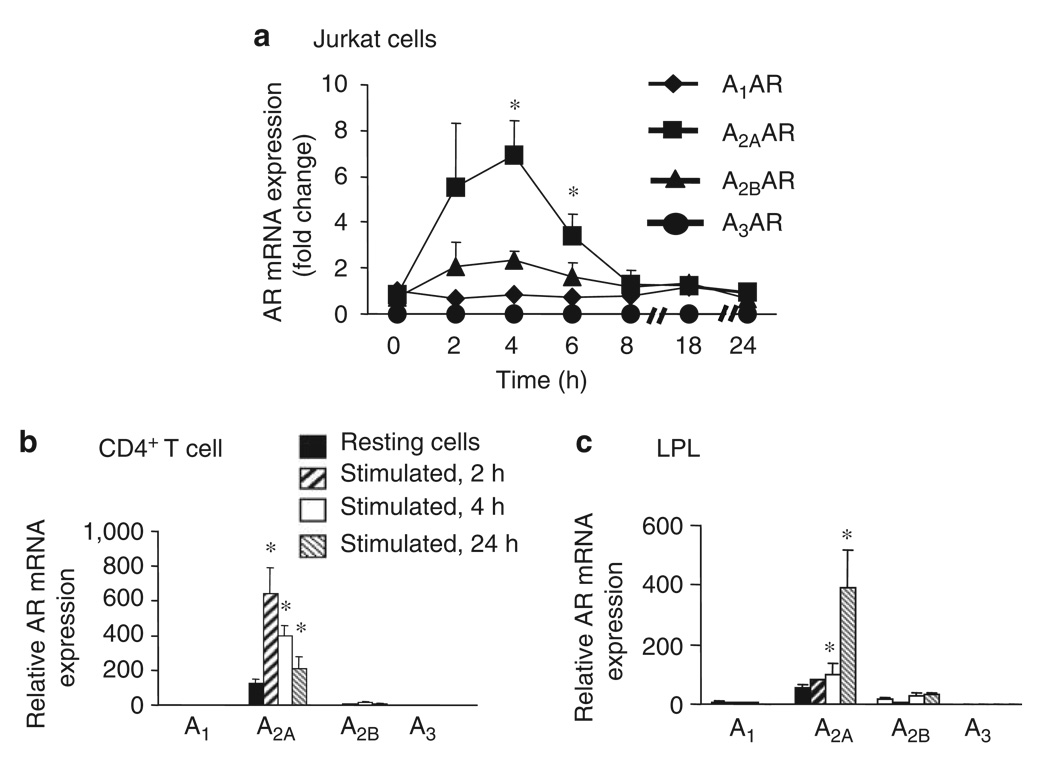
Evidence suggests that the activation of mouse CD4+ Th cells significantly upregulates A2AAR expression. 17 To translate these observations to humans, we examined the expression of ARs in human Th cells incubated on immobilized anti-CD3/CD28 monoclonal antibody (mAb) or Phorbal myristate acetate (PMA)/ionomycin for different lengths of time. The activation of Jurkat T cells or CD4+ Th cells by signaling through the T cell receptor (TCR) or alternatively by PMA/ionomycin resulted in a rapid and transient increase in the expression of A2AAR mRNA, as determined by quantitative reverse transcriptase-PCR (RT-PCR). The induction of A2AAR transcripts in Jurkat T cells peaked between 2 and 4h of incubation with PMA/ionomycin resulting in an approximate sevenfold increase in transcript compared with that in unstimulated T cells at that time point, and returned to baseline after 18h (Figure 1a). When CD4+ Th cells were used, the amount of A2AAR transcript accumulation again peaked after 2h of activation, with a fivefold increase over resting unstimulated level. A2AAR mRNA levels in this cell population also returned to baseline after 24h of activation (Figure 2b). To directly characterize AR expression in gastric Th cells, we isolated lamina propria lymphocytes (LPL) from human gastric biopsy specimens of uninfected subjects. LPL activation resulted in a similar augmentation of A2AAR transcripts (Figure 1c), although with different kinetics of induction. Although low levels of A2BAR mRNA were also detected in both peripheral Th cells and LPL T cells, no significant induction of this transcript was observed.
Figure 1.
A2AAR is the predominant isoform expressed in T cells. AR expression was assessed in (a) Jurkat cells and reported as the fold change in mRNA in stimulated T cells relative to unstimulated T cells at the indicated time points. Subsequently, the expression of AR in (b) purified human CD4+ Th cells or preparations of (c) gastric lamina propria lymphocytes (LPL) was assessed and reported as the increase in mRNA in stimulated T cells relative to unstimulated T cells at the resting time point. In each case, T cells were activated with PMA + inomycin (50 + 500 ng ml−1, respectively) at 37 ° C for varying lengths of time, and A1AR, A2AAR, A2BAR and A3AR mRNA expressions were measured by real-time RT-PCR. Data shown are the mean ± s.e.m. from three independent experiments. * Significant difference (P < 0.05) between unstimulated and stimulated cells.
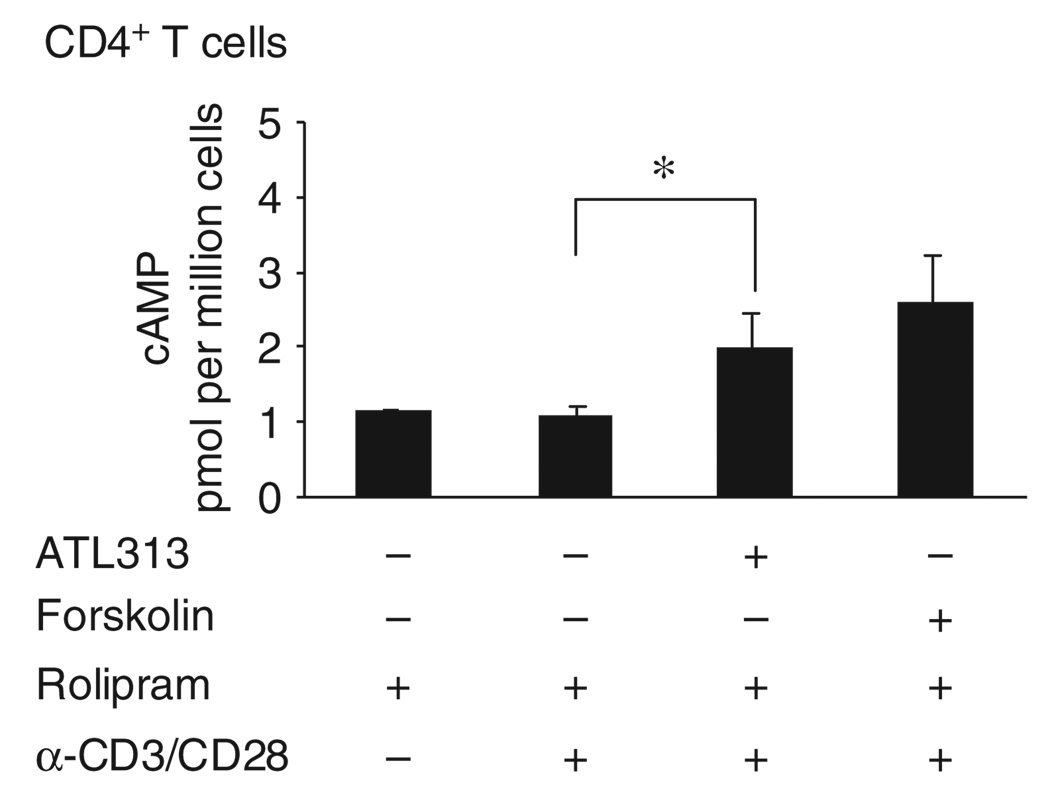
Figure 2.
A selective A2AAR agonist, ATL313, triggers cAMP accumulation in helper T cells. Purified human CD4+ Th cells were activated for 2h and treated for 10 min with 1 U ml −1 ADA in the presence of vehicle or 1 µM rolipram±1,000 nm ATL313 or forskolin, a positive control for cAMP accumulation. Intracellular cAMP accumulation was measured using a chemiluminescent immunoassay system. Data shown are the mean±s.e.m. from three independent experiments performed in triplicate. * Significant difference (P < 0.05) between the ATL313-treated and ATL313-untreated cells.
To show the function of A2AAR receptor in human Th cells, the efficacy of the selective A2AAR agonist, ATL313, to stimulate cAMP accumulation was tested. We found a significant increase in cAMP level after 1,000 nm of agonist treatment in both Jurkat (data not shown) and CD4+ Th cells over agonist untreated cells (Figure 2).
A2AAR activation by adenosine analogs inhibits IL-2 production
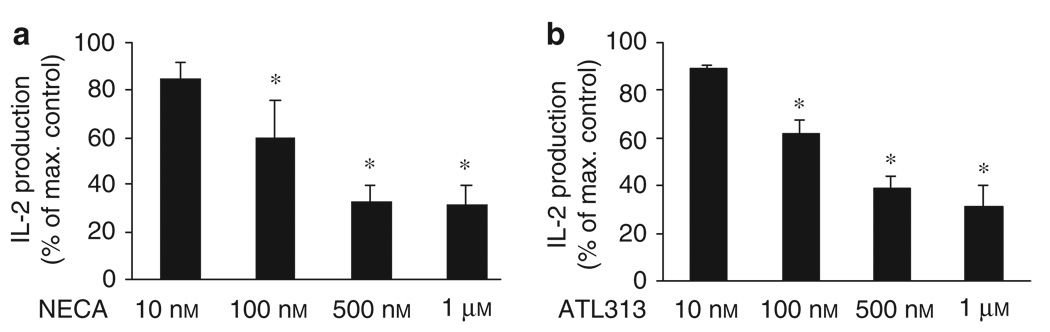
Next, we tested the effect of ATL313 treatment on IL-2 production in activated human CD4+ Th cells. The activation of CD4+ Th cells in the presence of various adenosine analogs resulted in a dose-dependent inhibition of TCR-mediated production of both IL-2 protein (Figures 3 and 4a) and mRNA (Figure 4b). 5′-N-Ethylcarboxamidoadenosine (NECA), a nonselective agonist, also inhibited IL-2 production (Figures 3a and 4a). In addition to inhibiting activation-induced IL-2 production, treatment with 100 nm of ATL313 attenuated the TCR-induced CD25 expression (data not shown).
Figure 3.
Suppression of IL-2 expression by CD4+ Th cells is mediated through the A2A adenosine receptor. Purified human CD4+ T cells were activated in plates coated with 5 µg ml−1 anti-CD3/CD28 mAb with 1U ml −1 ADA or after the addition of the nonselective A2AAR agonist, (a) NECA, or a selective A2AAR agonist, (b) ATL313, and IL-2 protein was measured by ELISA in supernatants collected after 24h and expressed as the production relative to stimulated Th cells in the absence of either NECA or ATL313. Data shown are the mean ± s.e.m. from three or four independent experiments performed in triplicate. * Significant difference (P < 0.05) between the NECA- or ATL313-treated and -untreated cells.
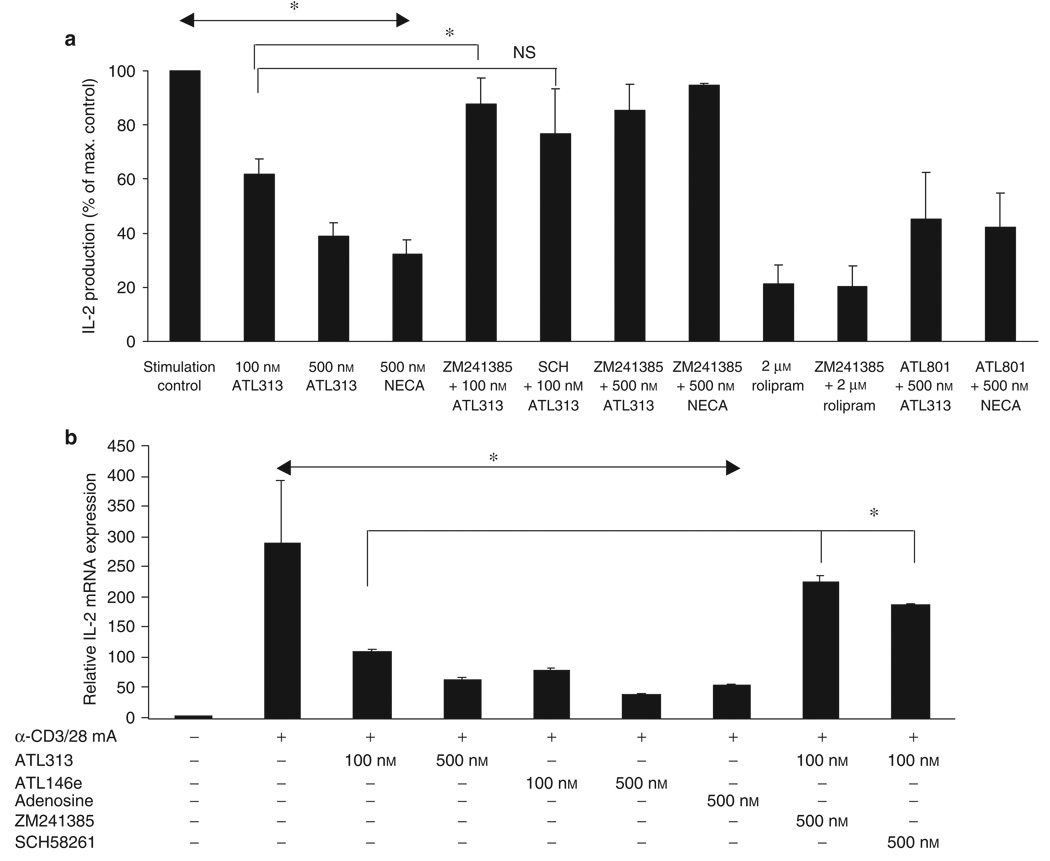
Figure 4.
Inhibition of IL-2 production by ATL313. (a) Purified human CD4+ T cells were incubated on immobilized anti-CD3/CD28 mAb with 1 U ml−1 ADA (Stimulation Control) or with adenosine analogs (ATL313, NECA) in the presence or absence of A2A antagonists (ZM241385 or SCH58261 = SCH) or ATL801, an A2B antagonist. Rolipram, a phosphodiesterase inhibitor, was used to allow cAMP to accumulate. Supernatants were collected after 24 h, and IL-2 concentrations were determined by ELISA and expressed as described in Figure 3. ELISA data shown are the mean±s.e.m. from three or four independent experiments performed in triplicate. (b) CD4+ T cells were cultured as described in panel a, but in this case, another A2A agonist, ATL146e, was also used. Cells were collected after 24h before IL-2 mRNA was measured by quantitative RT-PCR. Activated control refers to CD4+ T lymphocytes activated in the absence of any drugs. mRNA data shown are from a single experiment performed in triplicate, representative of three independent experiments. * Significant difference (P < 0.05) of the treatment groups compared with the “ stimulation control. ”
A selective A2AAR antagonist inhibits the effect of ATL313 on IL-2 production
To further confirm that adenosine acts through A2AAR, CD4+ Th cells were activated in the presence of ATL313 and 500 nm of two different A2AAR-selective antagonists ZM241385 or SCH58261. This treatment resulted in the almost complete reversal of the suppressive activity (Figure 4). Treatment of ZM241385 was more effective (P = 0.02) reversing the suppression than that of SCH58261. ZM241385 alone did not induce any appreciable change in IL-2 production during the of time assay (data not shown). When an A2BAR antagonist, ATL801, was used, there was no inhibition of the agonist supporting the notion that these agonists exert an inhibitory effect predominantly through the A2AAR and not through A2BAR (Figure 4a).
ATL313 triggers intracellular cAMP accumulation by initiating signaling through the Gs-coupled A2AAR (Figure 2). Similarly, a type IV phosphodiesterase inhibitor, rolipram, elevates intracellular cAMP level 24 (without engaging ARs) and suppresses IL-2 production (Figure 4a). Human CD4+ Th cells were activated in the presence of 2 µm rolipram with or without treatment of 500 nM of the selective A2AAR antagonist, ZM241385. A2AAR antagonist did not reverse the inhibition of IL-2 production by rolipram, as it did in ATL313-treated CD4+ Th cells (Figure 4a).
Effect of A2AAR agonist on other pro-inflammatory cytokines
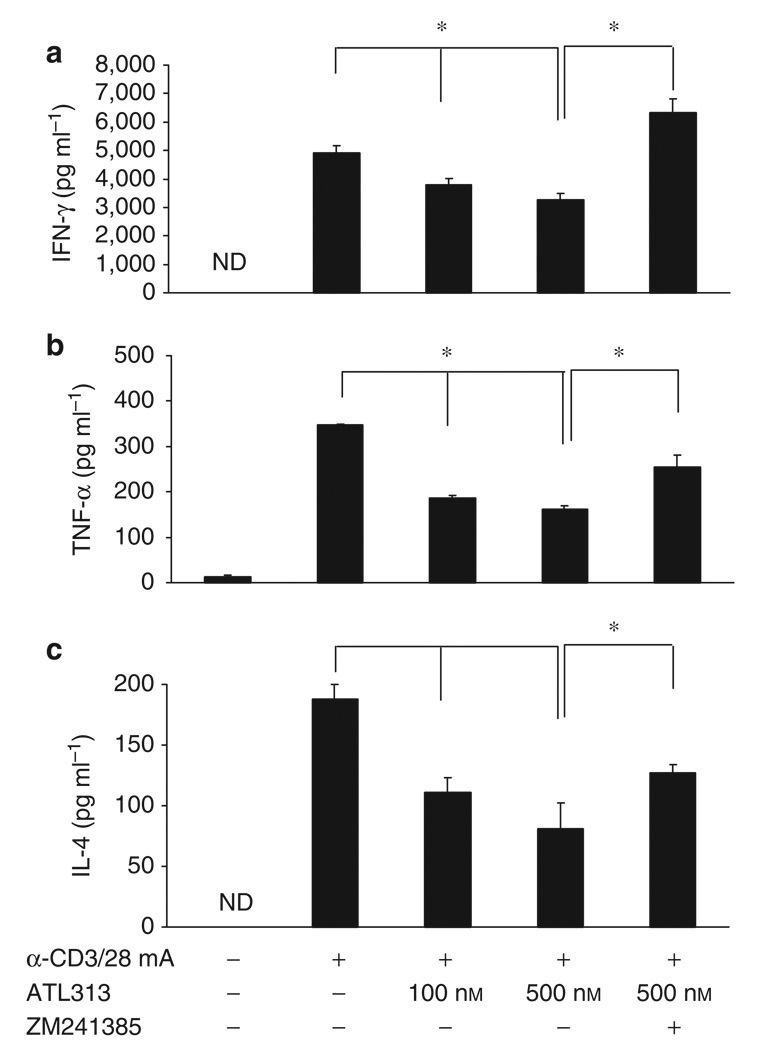
Naganuma et al. 22 showed that ATL313 effectively suppresses cytokine responses in mice, including IFN-γ. 22 Here, we confirmed that an adenosine agonist inhibits anti-CD3/CD28-induced cytokine (IFN-γ, IL-4, and TNF-α) production in human CD4+ T cells (Figure 5).
Figure 5.
Suppression of pro-inflammatory cytokines by A2AAR agonists. CD4+ T cells were stimulated and supernatants were collected after 24 h. Subsequently, (a) IFN-γ, (b) TNF-α, and (c) IL-4 were measured using a mutiplex bead array. The effects of the A2AAR agonist (ATL313) were also compared in the presence of an A2AAR antagonist (ZM241385). Data shown are from a single experiment performed in triplicate, representative of three independent experiments. ND = not detectable. * Significant difference (P < 0.05) of the treatment groups compared with the “ stimulation control.
A2AAR agonist inhibited IL-2 and IFN-γ protein in activated gastric T cells
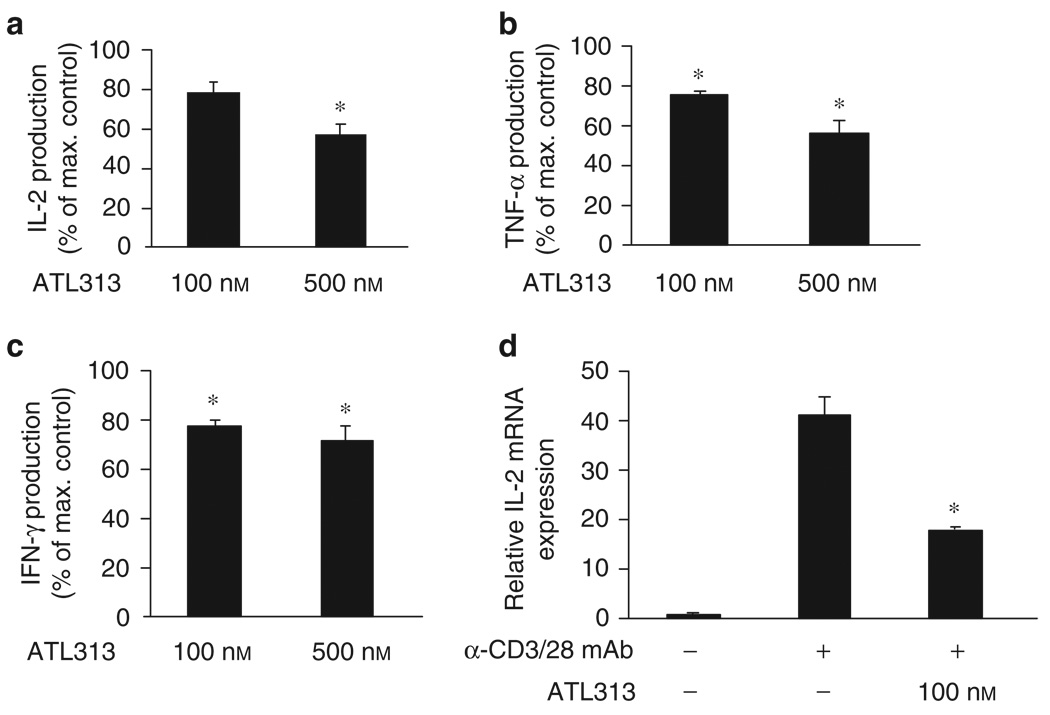
Although the effect of A2AAR agonists on murine Th cells is well known, there is no information on the sensitivity of mucosal Th cells to this stimulation. In Figure 1c, LPL derived from gastric biopsy specimens were shown to express high levels of A2AAR. Although not entirely pure, a significant number of the cells in these preparations were CD4+ Th cells. 8,25 We found that activation of A2AAR with ATL313 also inhibited pro-inflammatory cytokines, including IL-2, IFN-γ, and TNF-α (Figure 6), in LPL, which was consistent with its effects on CD4+ Th cells from peripheral blood (Figure 5). These observations further suggest that A2AAR play a role in controlling the pro-inflammatory cytokines in the gastric mucosa.
Figure 6.
Suppression of pro-inflammatory cytokine production by gastric T cells is partially mediated through the A2A adenosine receptor. LPL isolated from human gastric biopsy specimens were incubated on immobilized anti-CD3/CD28 mAb with 1 U ml−1 ADA or in the presence of the A2AAR agonist, ATL313. Supernatants were collected after 24h, and (a-c) IL-2, IFN-γ, and TNF-α proteins were measured using a mutiplex bead array and expressed as the production relative to stimulated Th cells in the absence of ATL313. Cytokine protein data shown are the mean±s.e.m. from three independent experiments performed in triplicate. IL-2 mRNA was extracted from T cells (from panels a-c) and assayed by real-time RT-PCR (d). mRNA data shown are from a single experiment performed in triplicate, representative of three independent experiments. * Significant difference (P <0.05) between the ATL313- treated and -untreated cells.
A2AAR-deficient mice have more Helicobacter felis-induced gastritis
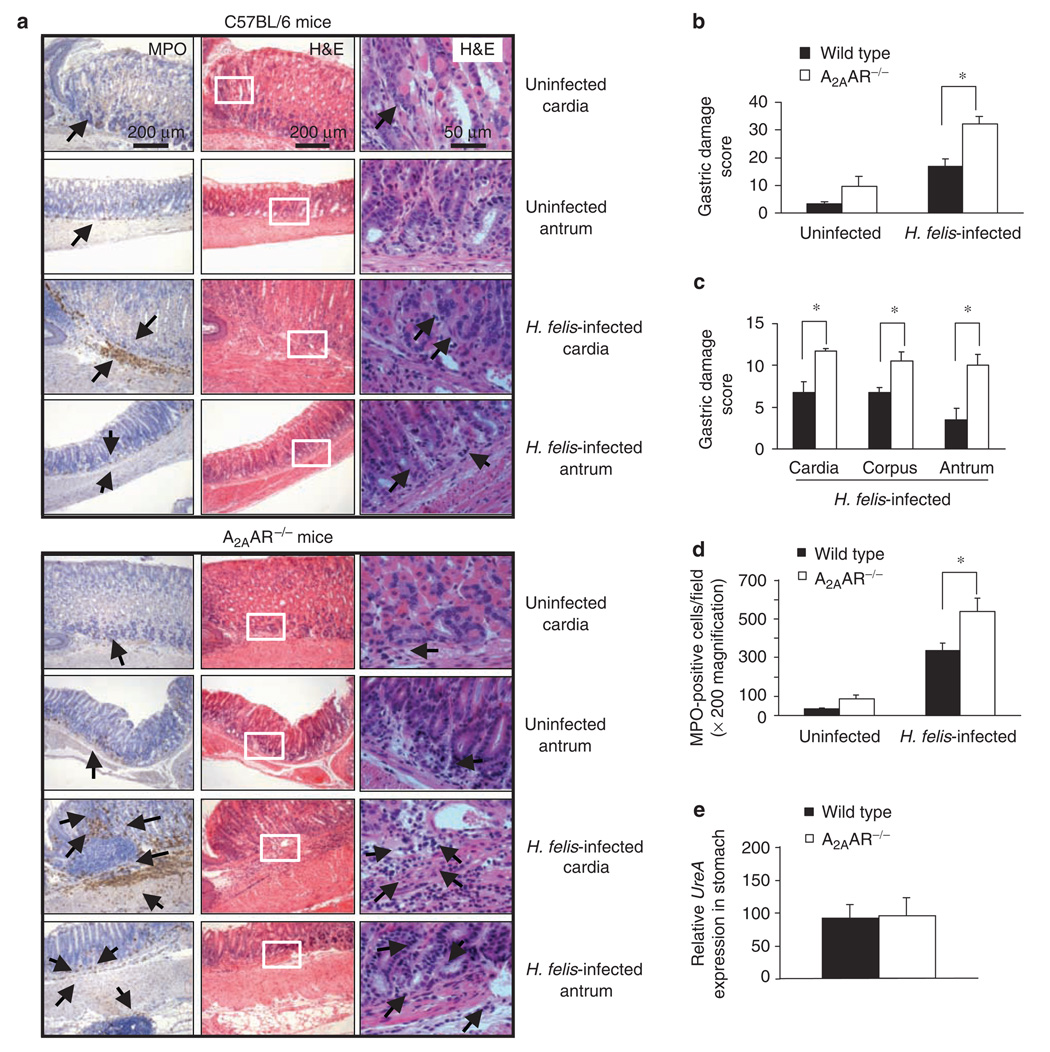
H. pylori infection in humans is studied in animal models directly or using the related organism Helicobacter felis. 26 H. felis colonizes rodent gastric mucosa effectively and results in the classic sequence of histological changes seen in human infection, such as chronic gastritis, atrophy, metaplasia, dysplasia, and adenocarcinomas. 27 Infection with H. pylori results in a relatively mild chronic colonization and hypertrophy, but these mice do not appear to consistently develop dysplasia and carcinoma. Thus, wild-type and A2AAR-deficient mice were infected with H. felis or H. pylori, and the effects of the mutation in the AR was compared in both species of bacteria. At 4 weeks after infection with H. felis, the stomachs of A2AAR-deficient mice were significantly more inflamed compared with tissue from infected wild-type mice. Figure 7a show the gastric mucosa at the cardia–corpus or corpus–antral junction of a representative mouse with relatively few mucosal lymphocytes. In contrast, a marked increase in inflammation occurred in A2AAR−/− mice 4 weeks after infection, with large numbers of mononuclear cells (MNCs) infiltrating the gastric lamina propria and submucosa. A hyperplastic thickening of the gastric wall with extensive granulocytic (myeloperoxidase, (MPO)-positive cells) infiltrates between the glands can be observed. The corpus of the stomach of the infected A2AAR−/− mice was also significantly inflamed. Figure 7b-d compares the quantification of the gastritis and infiltrating MPO-positive cells in A2AAR−/− mice and H. felis-treated control mice. In addition, gastritis was more evident in uninfected mice lacking A2AAR. Together, these data suggest that A2AAR act in an anti-inflammatory role to control gastritis in vivo. Although these mice developed more gastritis, it did not have a significant effect on colonization (Figure 7e). Similar findings were also observed when A2AAR−/− mice were infected with H. pylori, but overall inflammation was less (data not shown).
Figure 7.
Gastritis is more severe in Helicobacter felis-infected A2AAR-deficient mice. Mice were infected by gavage with 1 × 108 CFU of H.felis per inoculation every other day for three separate inoculations and euthanized 4 weeks later. Gastric tissue was processed for histology and H. felis colonization was assessed. (a) Myeloperoxidase (MPO) (left panel) and H & Estained (two right panels with inset from center panel shown at higher magnification in the outer right panel) of gastric sections from representative uninfected or infected C57BL/6 (upper panels), and uninfected or infected A2AAR −/− (lower panels) mice. Arrows indicate MPO-expressing cells or MNC (original magnification of × 100). Only a few scattered MNC-and MPO-positive granulocytes can be seen in the submucosa and lamina propria with no abnormal thickening of the gastric wall of uninfected control mice. Severe gastritis with dense MNC infiltration and diffuse MPO-positive granulocytes in the submucosa and mucosa of the infected A2AAR−/− mice in both the cardia and antral regions. MNCs aggregate between the glands are spanning the entire width of the mucosa in some cases. A concomitant thickening of the gastric wall as well as gastric of glandular atrophy can be observed. (b) Overall gastritis score in uninfected and infected wild-type and A2AAR−/− mice. (c) Regional gastritis scores in infected wild-type and A2AAR−/− mice. (d) Quantitative expression of MPO-positive cells in uninfected and infected, control, and A2AAR−/− mice expressed per ×200 field. (e) H. felis colonization in gastric tissue analyzed by the presence of H. felis-specific UreA gene. Data are mean±s.e.m. from pooled data including two identical experiments with four mice per group. * Significant difference (P < 0.05) between the wild-type and A2AAR−/− mice.
A2AAR agonists reduce inflammation, but favor H. pylori persistence in IL-10 deficient mice
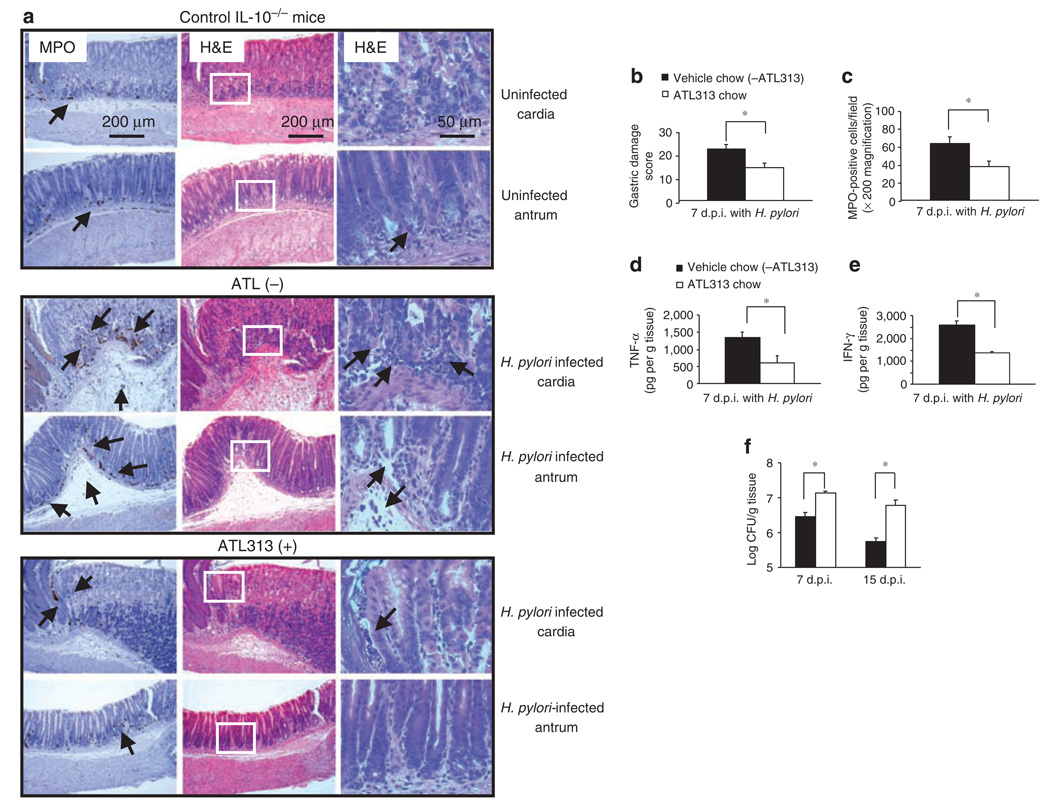
As the inflammation induced by H. pylori in mice is relatively modest, investigators have challenged IL-10-deficient mice to enhance inflammation, which also leads to the spontaneous clearance of H. pylori infection. 28,29 To examine the effect of adenosine and its receptors on persistent infection, IL-10-deficient mice infected with H. pylori were fed chow containing ATL313. By 7 days after infection, both the gastritis score and the number of infiltrating MPO-positive granulocytes were lower in the adenosine agonist-treated mice (Figure 8b and c). When gastric mucosal TNF-α and IFN-γ production was tested, the adenosine agonist also significantly attenuated their production (Figure 8d and e) in the tissue site. Interestingly, suppression of inflammation by adenosine inversely affected H. pylori colonization in the stomach (Figure 8f) and resulted in significantly increased bacterial colonization. These data suggest that the inhibition of gastritis in IL-10-deficient mice favors persistent colonization with H. pylori.
Figure 8.
Oral administration of an A2AAR agonist in Helicobacter pylori-infected IL-10−/− mice reduces gastric inflammation but increases bacterial load. IL-10-deficient mice were infected as described in Figure 7. All mice were given either vehicle control chow (ATL313(−), n = 4) or ATL313 chow (ATL313(+), n = 4) for 1 week before infection and continued until killing. Mice were killed 7(7d.p.i.) or 15 days (15d.p.i.) later, and gastric tissue (each longitudinal section) was processed for histology, MPO staining, mucosal cytokine expression, and H. pylori colonization by colony count. (a) Myeloperoxidase (MPO) (left panel) and H&E stained (two right panels with inset from center panel shown at higher magnification in the outer right panel) of gastric sections from representative uninfected IL-10−/− (upper panels), and infected IL-10−/− without (middle panels) or with ATL313 chow-treated mice (lower panels). Arrows indicate MPO-expressing cells (original magnification of × 100). Some scattered mononuclear cells (MNCs) and MPO-positive granulocytes can be seen in the submucosa and lamina propria, with no abnormal thickening of the gastric wall of uninfected control IL-10-deficient mice. Severe gastritis with dense MNC infiltration and diffuse MPO-positive granulocytes in the submucosa and mucosa of the untreated infected mice in both cardia and antral regions. A concomitant hyperplasia with widespread thickening of the gastric wall as well as of gastric atrophy can be observed. ATL313-treated mice are almost normal with few inflammatory cells infiltration. (b) Histological scoring of gastritis, (c) quantitative expression of MPO-positive cells expressed per field, and (d) mucosal TNF-α, and (e), IFN-γ production in gastric tissue of in H. pylori-infected IL-10-deficient mice without or with ATL313 treatment. (f) H. pylori colonization in gastric tissue measured by colony count on days 7 and 15 after infection with same treatment as mentioned earlier. Data are mean±s.e.m. from one experiment with four mice per group that was carried out at least twice with similar results. * Significant difference (P < 0.05) between the untreated and treated mice.
DISCUSSION
As it is of interest to learn how to clear chronic infections that contribute to disease, it becomes essential to understand the factors that regulate the host response in a manner that protects the host from inflammation and may also favor a more commensal relationship with the persistent colonization of some organisms. This study shows that human Th cells express A2AAR, and this receptor is induced after activation. 30 Importantly, this pattern of expression was comparable in preparations of gastric Th cells, and adenosine receptor agonists appear to attenuate the host response in a manner that may favor the commensal relationship.
Lappas et al. 17 reported that A2AAR induction inhibited IFN-γ production in murine CD4+ Th cells. Similarly, Naganuma et al. 22 reported that A2AAR played a critical role in the inhibition of several pro-inflammatory cytokines that contributed to T-cell-mediated colitis. This study, using human Th cells, isolated from the blood or gastric biopsy specimens, shows that A2AAR could efficiently suppress IL-2 production (Figures 3 and 4) and other pro-inflammatory cytokines (Figures 5 and 6). Thus, adenosine generated in the inflamed stomach can target T cells and may attenuate the host response.
In general, T cells in the stomach are biased mostly toward the Th1 or Th17 phenotype, as marked by their ability to generate IFN-γ and IL-17, 31 but little IL-4. 7,8,32 Regardless of the evident gastritis associated with Helicobacter infection, infection persists for life. The failure to clear the infection may lead to a compensatory induction of Th cells with regulatory function to protect the gastric mucosa. Indeed, there are now several reports that Th cells resembling Treg are present in the gastric mucosa during infection in humans and mice. 11,33–35 Moreover, Rad et al. 13 have provided compelling data in a mouse model that these Treg may favor the development of a more commensal relationship between the host and the organism. The Treg cells may produce sufficient levels of IL-10, TGF-β, or adenosine to attenuate host responses rather than to prevent gastritis totally. We observed that treatment of activated LPL with an A2AAR receptor agonist inhibited pro-inflammatory cytokines. In addition, using A2AAR-deficent mice in the H. felis infection model, we observed increased gastric inflammation and confirmed the importance of A2AAR in regulating inflammatory responses in vivo, and thus extend this principle to mucosal tissues. In addition, treatment of H. pylori-infected IL-10-deficient mice with an A2AAR agonist resulted in a significant reduction in the gastric inflammation as well as in a significant increase in bacterial colonization. Although these data in vivo support the hypothesis, we cannot state whether the effect was mediated solely by Th cells and/or any other inflammatory targets that are known to express the A2AAR.
A wide variety of immune cells other than Th are found to express A2AAR, including neutrophils, monocytes, macrophages, platelets, and mast cells. 36 Other literature suggests myeloid cells, such as neutrophils, can be inhibited in the gastric mucosa as a result of A2AAR engagement. 20 Recently, we reported that gastric Th that express higher levels of CD39 and CD73 may act to suppress inflammatory responses during gastric infection through the production of adenosine. 22,23 Several reports now suggest that adenosine may be one anti-inflammatory mediator produced by Treg, 14,21,22 which could contribute to the restraint on the host response that favors persistence.
As observed in IL-10-deficient mice, marked inflammatory responses are sufficient to clear Helicobacter spp. infection. 29,37 Moreover, the data reported here suggest that the absence of A2AAR in mice led to mild gastritis before infection, inferring that endogenous adenosine cannot mediate anti-inflammatory action without its specific receptor. Furthermore, the infection of A2AAR−/− mice led to greater inflammation when compared with that of wild-type mice. These data are consistent with the fact that activation of A2AAR by a specific agonist attenuates gastritis in rats. 20 It is noteworthy that the IL-10 knockout mice could decrease bacterial colonization, whereas the A2AAR-deficient mouse had no difference. This may reflect the higher degree of gastritis in the IL-10-deficient mice or the possibility that some of the specific host responses enhanced in these mice are not found in the A2AAR-deficient animals.
We studied the effect of H. felis infection in A2AAR KO mice for 4 weeks, and at this time point, there was an increase in inflammation with large numbers of MNCs infiltrating the gastric lamina propria and submucosa. In other models in which adenosine responses are impaired, CD73-deficient mice were shown to have a reduced H. felis colonization 60 days after infection. However, at this time point, there were no changes in the gastric inflammatory responses compared with infected, wild-type mice. 12 These observations suggest that the decrease in bacterial load may be associated with an attenuation in inflammation. Perhaps, the transient increase in gastritis may wane and favor clearance without any adverse effects on the development of gastric cancer. These issues remain to be studied.
In summary, activating A2AAR by adenosine agonists in Th cells can limit over exuberant inflammation during gastric infection with Helicobacter spp. The A2AAR provides a mechanism for limiting the pro-inflammatory effects of Th cells, thereby reducing inflammation in the gastric mucosa and impairing immunity to Helicobacter infection, which may favor persistence. Therefore, adenosine can be added to the list of mediators that have the potential to control gastritis in response to a local infection with Helicobacter spp., and in so doing, regulate colonization.
METHODS
Reagents and antibodies
Adenosine deaminase (ADA) was purchased from Roche (Mannheim, Germany). Phorbol myristate acetate (PMA), ionomycin, NECA, adenosine, and rolipram were purchased from Sigma-Aldrich (St Louis, MO). 4-{3-[6-Amino-9-(5-cyclopropylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl] prop-2-ynyl}piperidine-1-carboxylic acid methyl ester (ATL313) was gift from Adenosine Therapeutics LLC (Charlottesville, VA). 4-(2-[7-Amino-2-[2-furyl][1,2,4]triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl)phenol (ZM241385) and 2-(2-furanyl)-7-(2-phenylethyl)-7-H-pyrazolo[4,3-e] [1,2,4]triazolo[1,5-c]pyrimidin-5-amine (SCH58261) were purchased from Tocris (Ellisville, MO). Anti-human CD3 mAb (clone OKT3) from eBioscience (San Diego, CA), and anti-human CD28 mAb (Clone CD28.2) was from BD Pharmingen (San Diego, CA).
Cell lines
Human Jurkat T cell line (ATCC Clone E6-1, Manassas, VA) was maintained in RPMI 1640 (HyClone Laboratories, Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum, 2 mm L-glutamine, 0.1 mM nonessential amino acid solution, 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer, 100 U ml−1 penicillin, 100 µg of streptomycin, 1 mm sodium pyruvate, and 55 µm 2-marcaptoethanol (2-ME) at 37 °C under 5% CO2.
Isolation of PBMC and purification of CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) were obtained from peripheral blood or buffy coat preparation derived from healthy donors (Virginia Blood Bank Richmond, VA), using Ficoll/Hypaque (Amersham, Uppsala, Sweden) density centrifugation and stored in liquid nitrogen until further use. PBMCs were first depleted of monocytes using CD14 magnetic beads. CD4+ T cells were then purified from CD14-negative PBMCs using CD4 magnetic-activated cell sorting beads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Isolation of LPL from gastric biopsy samples
Biopsy specimens of the gastric antrum were obtained from consenting subjects undergoing gastroesophogeal duodenoscopy for various clinical indications, as approved by our respective instructional review broads at the University of Virginia. All subjects were uninfected and were free of gastritis. Gastric T cells were isolated using a modified technique. 8,25 Briefly, the biopsy specimens were collected into sterile collection medium (calcium- and magnesium-free Hank’s balanced salt solution with 5% fetal calf serum and penicillin plus streptomycin). The biopsy specimens were stored at 4 °C for up to 18h before processing, this having been shown earlier not to alter T-cell function. The biopsy specimens were rinsed with aqueous betadine and immediately rinsed four times in collection medium containing dithiothreitol (1 mm) and EDTA (1 mm) (Sigma, St Louis, MO). The specimens were agitated for 1h at 37 °C to detach intraepithelial lymphocytes and epithelial cells, removed, placed in complete RPMI 1640 medium (10% fetal calf serum, and penicillin/streptomycin), and washed three times with this medium. Subsequently, lamina propria T cells were liberated by treatment with collagenase (30 U ml−1) in complete RPMI 1640 medium. The resulting cell suspensions were washed, and the viability of the MNCs was determined by trypan blue exclusion. The cells were not used if viability did not exceed 90%.
T-cell treatment with adenosine agonists
Purified CD4+ T cells or gastric T cells (2 × 105 cells/well) were stimulated with 5 µg ml−1 immobilized anti-CD3 mAb and 5 µg ml−1 anti-CD28 mAb in the presence or absence of various concentration of ATL313, NECA, or adenosine. In some experiments, 500 nm of ZM241385 or SCH58261 (A2AAR antagonists) or ATL801 (an A2BAR antagonist) were added to the cultures. All co-culture assays are carried out in the presence of 1 U ml−1 ADA.
Measurement of intracellular cAMP
Purified CD4+ T cells were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 100 U ml−1 penicillin, 100 µg of streptomycin. Either immediately after purification or after incubation on immobilized anti-CD3 mAb for varying lengths of time, cells were incubated at 37 ° C for 10 min with 1 µm rolipram and 1 U ml−1 ADA in the presence or absence of ATL313. Rolipram, a type IV phosphodiesterase inhibitor, was used to stabilize cAMP production, as it inhibits the natural phosphodiesterase breakdown of cAMP to 5′-AMP. 24 cAMP accumulation in response to 1 µm of rolipram in the absence of ATL313 did not change in cells as a result of TCR activation (data not shown).
Forskolin (Sigma) was used as a positive control to induce cAMP production in cells. Cells were then lysed, and intracellular cAMP levels were measured using the chemiluminescent immunoassay system for the quantification of cAMP from mammalian cells, cAMP-Screen System, according to the manufacturer ’ s protocol (Applied Biosystems, Foster City, CA).
Cytokines assays
Supernatants were collected for cytokine assays and cells were harvested as a source of mRNA. IL-2 concentrations in supernatants of CD4+ T-cell or gastric T-cell cultures were measured by human IL-2 ELISA kit according to the manufacturer ’s protocol (BD Biosciences, San Diego, CA). IL-2 levels were determined using a standard curve calibrated against recombinant human IL-2 standard supplied by the manufacturer. TNF-α and IFN-γ levels were measured using a mutiplex bead array (Upstate, Temecula, CA) and analyzed with the Bio-Plex work-station and associated software (Bio-Rad, Hercules, CA). All cytokine concentrations were measured using a standard curve and expressed as picogram per milliliter and/or presented as the percentage of their activated controls, that is, percentage of maximum control. Percentile data were used to normalize the variability issues among individual human PBMCs and also antibody stimulation because of lot variation.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from cells using the QIAamp Blood Mini Kit (Qiagen, Valencia, CA), and was reverse transcribed to yield cDNA using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Real-time RT-PCR was performed in a SmartCycler (Cepheid, Sunnyvale, CA), using primer and dual-labeled probe (Applied Biosystem) for IL-2 and adenosine receptors (A1, A2A, A2B, and A3). Real-time PCR reactions were carried out in a total volume of 25 µl containing 1 µl of cDNA, 12.5 µl of Universal PCR Master Mix (Applied Biosystems), 300–900 nM of each forward and reverse primer, and 200 nM of each probe. Duplicate PCRs were performed for each sample, and the average threshold cycle number was determined using the Opticon software. Normalized levels of each mRNA were determined using the formula 2(Rt − Et), where Rt is the threshold cycle for the reference gene (18S rRNA) and Et the threshold cycle for the experimental gene (ΔΔCT method).38 Data are expressed as arbitrary units relative to an appropriate unstimulated control.
Mice
C57BL/6 mice and IL-10-deficient (IL-10−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) whereas A2AAR-deficient mice (A2AAR−/−) mice inbred onto the C57BL/6 background 17 were maintained in a conventional animal care facility at the University of Virginia (Charlottesville, VA). All procedures were approved by the Animal Care and Use Committee at the University of Virginia.
Helicobacter growth and challenge, and A2AAR agonist treatment of mice
H. pylori is the pathogen of humans, but H. felis infection of mice more closely mimics the histological sequences of H. pylori infection in humans. Therefore, we have compared the effects of adenosine receptor stimulation using both microbes in two different models. H. pylori Sydney strain (SS2000) or H. felis strain (ATCC 51211) were cultured on trypticase soy agar (TSA) II agar plates containing 5% sheep blood (BBL, BD, Becton, Dickinson Laboratory, Sparks, MD) in a microaerophilic condition as described earlier. The bacteria were then harvested and inoculated into Brucella broth (BD Biosciences, Sparks, MD) supplemented with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT) as described elsewhere. 39,40 To establish a primary H. pylori infection, mice were inoculated intragastrically through a 20-G feeding needle with a 200-µl suspension of H. pylori containing 1 × 108 CFU of the bacteria on 3 consecutive days and were killed different weeks after inoculation. In some experiments, mice receiving H. pylori or H. felis were fed chow containing 1.875 mg kg−1 of ATL313 or vehicle chow containing dimethylsulfoxide control 1 week before their infection. ATL313 chow was continued until the experiments are completed, and all mice were fed with fresh chow, which was changed every 4–5 days interval to maintain its drug effect. At 4–6 weeks after challenge, mice were killed, and gastric tissue was processed for H. pylori culture, histology, and immunohistochemistry as described below.
Assessment of bacterial colonization
For a quantitative measurement of H. pylori bacteria, longitudinal segments of gastric tissue were homogenized in 0.5 ml of Brucella broth supplemented with 10% fetal calf serum, and replicate serial 10-fold dilutions were plated on Helicobacter-selective blood agar plates to determine bacterial colonization as described earlier. 41 The plates were incubated at 37 °C under microaerobic conditions (10% CO2, 5% O2, and 85% N2), quantified 5–7 days later, and reported as the number of CFU per gram.
Gastric tissue DNA extraction and assessment of H. felis colonization
Stomach DNA from infected wild-type mice was prepared using Maxwell 16 Tissue DNA Purification Kit (Promega, Madison, WI) exactly as per the manufacturer’ s instructions. Gastric colonization of H. felis was assessed by quantifying a H. felis-specific UreA gene relative to host tissue housekeeping gene, as described earlier. 12,13 The amount of H. felis UreA DNA was expressed relative to mouse gastric GAPDH (glyceral-dehyde-3-phosphate dehydrogenase), as determined by the comparative ΔΔCT method mentioned above. Primer sequences for H. felis UreA, 5′-TCGATCGCGCAAAAGCTT-3′ and 5′-CGCACCGTTCCAGAT-3′. Primers sequences for GAPDH, 5′-GCTAAGCAGTTGGTGCA-3′ and 5′-TCACCACCATGGAGAAGGC-3′.
Preparation of gastric homogenates for TNF-α and IFN-γ measurement
One longitudinal strip of stomach mucosa (40–50 mg) was homogenized for 30 s with a polytron homogenizer (PT 1200, Kinematica AG, Littau, Switzerland) in 0.5 ml of ice-cold phosphate buffer (pH 7.4) with Complete, Mini (Protease Inhibitor Cocktail Tablets; Roche). After homogenization, all tissues weight was expressed as 25 mg ml−1 concentration and sonicated. Aliquots of homogenate supernatants were obtained by centrifugation and kept at −70 ° C until use. Total protein was measured by Bradford ’ s method. Concentration of TNF-α and IFN-γ cytokine in the supernatant of mucosal homogenates was determined by ELISA (BD Biosciences, CA) as mentioned above.
Gastric tissue analyses and histological evaluation
For histopathology, longitudinal segments, including the antrum and corpus plus proximal duodenum, were fixed in Bouin ’ s fixative solution (Ricca Chemical, Arlington, TX) for 24 h, washed twice with 70% ethanol and embedded in paraffin, cut into 3- to 5-µm sections, and stained with hematoxylin and eosin. For immunohistochemistry, similar 3-µm gastric sections were stained with polyclonal anti-MPO antibody (Novus Biochemicals, Littleton, CO), and tissue-bound peroxidase activity was visualized with DAB (3,3′-diaminobenzidine). Hematoxylin was used for nuclear counter staining. The number of MPO-positive cells were counted in 10 different fields at an original magnification of ×200 and expressed per field. Gastric inflammation was assessed using a modified scoring system, as described earlier by Ismail et al. 42 Briefly, two sections were collected from each stomach, and each region of the stomach (forestomach or cardia, corpus, and antrum) was assessed individually for three parameters: (1) thickening, (2) infiltration of polymorphonuclear cells and (3) infiltration of MNCs. Severity was graded based on the absence (0) or presence (1) of each parameter, with polymorphonuclear infiltration further examined (absence or presence) for focal, diffuse, or abscess involvement. Similarly, MNC infiltration was examined for focal, diffuse, or aggregate involvement in the lamina propria. A total score was calculated by summing the score values for each region of the stomach for one section. Results are reported as total damage scores±s.e.m.
Statistical analysis
Results are expressed as mean±s.e.m. Data were compared by Student ’s t-test (unpaired), and results were considered significant if P-values were < 0.05.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants DK50980, AI069880, and AI70491 (to PBE) and AI075526 (to R Guerrant in support of ATL reagent development). Support from the Immunology and Cell Isolation Core, the Molecular Biology Core, as well as from the Morphology/Imaging Core of the UVA Digestive Health Research Center (DK 56703) is gratefully acknowledged.
Footnotes
DISCLOSURE
Drs Figler, Linden, and Rieger hold shares in Adenosine Therapeutics LLC.
REFERENCES
- 1.Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin. Microbiol. Rev. 2006;19:597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon MF. Pathophysiology of Helicobacter pylori infection. Scand. J. Gastroenterol. 1994;29:7–10. [PubMed] [Google Scholar]
- 3.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 4.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Miller MJS. Carcinogenesis, apoptosis and cell proliferation. Br. Med. Bull. 1998;54:151–162. doi: 10.1093/oxfordjournals.bmb.a011665. [DOI] [PubMed] [Google Scholar]
- 6.Karttunen R, et al. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin. Exp. Immunol. 1990;82:485–488. doi: 10.1111/j.1365-2249.1990.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Elios MM, et al. T helper 1 effector cells specific for Helicobacter pylori in gastric antrum of patients with peptic ulcer disease. J. Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 8.Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 9.Ahlstedt I, et al. Role of local cytokines in increased gastric expression of the secretory component in Helicobacter pylori infection. Infect. Immun. 1999;67:4921–4925. doi: 10.1128/iai.67.9.4921-4925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 2001;166:7456–7461. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren A, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 2005;73:523–531. doi: 10.1128/IAI.73.1.523-531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam MS, et al. CD73 is expressed by human regulatory Th cells and suppresses pro-inflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rad R, et al. CD25+/Foxp3+ T cells regulate gastric infl ammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobie JJ, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 16.Sitkovsky MV, et al. Physiological control of immune response and infl ammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 17.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 18.Mabley J, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur. J. Pharmacol. 2003;466:323–329. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 19.Chaves IC, et al. Effect of a novel A2A adenosine receptor agonist (ATL 313) on Clostridium difficile toxin induced murine ileal enteritis. Infect. Immun. 2006;74:2606–2612. doi: 10.1128/IAI.74.5.2606-2612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odashima M, et al. Selective adenosine A receptor agonist, ATL-146e, attenuates stress-induced gastric lesions in rats. J. Gastroenterol. Hepatol. 2005;20:275–280. doi: 10.1111/j.1440-1746.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- 21.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 22.Naganuma M, et al. Cutting edge: critical role for adenosine A2A receptors in the T cell mediated regulation of colitis. J. Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 23.Zarek PE, et al. A2A receptor signaling promotes peripheral tolerance by inducing T cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seybold J, et al. Induction of phosphodiesterases 3B, 4A4, 4D1, 4D2, and 4D3 in Jurkat T-cells and in human peripheral blood T-lymphocytes by 8-bromo-cAMP and Gs-coupled receptor agonists. Potential role in beta2-adrenoreceptor desensitization. J. Biol. Chem. 1998;273:20575–20588. doi: 10.1074/jbc.273.32.20575. [DOI] [PubMed] [Google Scholar]
- 25.Haeberle HA, et al. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, et al. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–1952. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 28.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J. Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 29.Berg DJ, Lynch NA, Lynch RG, Lauricella DM. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am. J. Pathol. 1998;152:1377–1386. [PMC free article] [PubMed] [Google Scholar]
- 30.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- 31.Luzza F, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 32.Karttunen R, Karttunen T, Ekre H-PT, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goll R, et al. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12:185–192. doi: 10.1111/j.1523-5378.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 34.Enarsson K, et al. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin. Immunol. 2006;121:358–368. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Stromberg E, et al. Down-regulation of epithelial IL-8 responses in Helicobacter pylori-infected duodenal ulcer patients depends on host factors, rather than bacterial factors. Clin. Exp. Immunol. 2005;140:117–125. doi: 10.1111/j.1365-2249.2005.02736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linden J. New insights into the regulation of inflammation by adenosine. J. Clin. Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail HF, Zhang J, Lynch RG, Berg DJ. Complement in clearance of H. pylori infection in IL-10 knock out mice. Infect. Immun. 2003;71:7140–7148. doi: 10.1128/IAI.71.12.7140-7148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Thompson LJ, et al. Chronic Helicobacter pylori infection with Sydney strain 1 and a newly identified mouse-adapted strain (Sydney strain 2000) in C57BL/6 and BALB/c mice. Infect. Immun. 2004;72:4668–4679. doi: 10.1128/IAI.72.8.4668-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding SZ, et al. Helicobacter pylori and H2O2 increases AP endonuclease-1/redox factor-1 expression in human gastric epithelial cells. Gastroenterology. 2004;127:845–858. doi: 10.1053/j.gastro.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Pappo J, et al. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail HF, Zhang J, Lynch RG, Wang Y, Berg DJ. Role for complement in development of Helicobacter-induced gastritis in interleukin-10-deficient mice. Infect. Immun. 2003;71:7140–7148. doi: 10.1128/IAI.71.12.7140-7148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]