Abstract
Work over the past two decades revealed a previously unexpected role for striatal cholinergic interneurons in the context of basal ganglia function. The recognition that these interneurons are essential in synaptic plasticity and motor learning represents a significant step ahead in deciphering how the striatum processes cortical inputs, and why pathological circumstances cause motor dysfunction. Loss of the reciprocal modulation between dopaminergic inputs and the intrinsic cholinergic innervation within the striatum appears to be the trigger for pathophysiological changes occurring in basal ganglia disorders. Accordingly, there is now compelling evidence showing profound changes in cholinergic markers in these disorders, in particular Parkinson's disease and dystonia. Based on converging experimental and clinical evidence, we provide an overview of the role of striatal cholinergic transmission in physiological and pathological conditions, in the context of the pathogenesis of movement disorders.
Keywords: acetylcholine, striatum, interneuron, Parkinson's disease, dystonia, movement disorders
Introduction
The basal ganglia include different interconnected subcortical nuclei that are involved in serving critical motivation, motor planning, and procedural learning function (Graybiel et al., 1994; Yin and Knowlton, 2006; Nicola, 2007; Kreitzer and Malenka, 2008). The striatum represents the main input nucleus of the basal ganglia. It receives excitatory afferents from the cortex and thalamus, and is densely innervated by midbrain dopamine neurons (Bolam et al., 2000; Kreitzer and Malenka, 2008).
The large majority of striatal neurons are GABAergic. Most of these GABAergic neurons are represented by medium spiny projection neurons (MSNs; Izzo et al., 1987). At least three types of GABAergic interneurons have been identified, according to their electrophysiological and neurochemical properties. GABAergic interneurons may colocalize with the calcium-binding proteins parvalbumin or calretinin, or neuropeptide Y, somatostatin, and NADPH diaphorase (Kawaguchi, 1993; Tepper and Bolam, 2004). Accordingly, they have been classified, respectively, as fast-spiking (FS) neurons, persistent and low-threshold spike (PLTS) neurons, or low-threshold spike (LTS) neurons (Kawaguchi et al., 1989; Tepper and Bolam, 2004). A recent study has characterized an additional group of GABAergic interneurons, expressing tyrosine hydroxylase (TH+), which have been electrophysiologically classified into four distinct types (Tepper et al., 2010). Indeed, the existence of TH+ neurons in the striatum of rodents and primates had been reported since the late 1980s (for review, see Ibáñez-Sandoval et al., 2010).
In addition to the numerically prevailing population of GABAergic neurons, the striatum also contains a small percentage of interneurons which provide this area with one of the highest acetylcholine (ACh) levels in the brain (Graybiel, 1990; Mesulam et al., 1992; Contant et al., 1996). These are the large aspiny cholinergic interneurons (ChIs) characterized by dense local axonal arborizations, and by tonic firing activity (Bolam et al., 1984; Wilson et al., 1990; Kawaguchi, 1993; Aosaki et al., 1995; Bennett and Wilson, 1998; Bennett et al., 2000; Zhou et al., 2002).
It has long been known that striatal ChIs play a central role in the basal ganglia circuitry both in the control of voluntary movements and in the pathophysiology of movement disorders, such as Parkinson's disease (PD), and dystonia (Pisani et al., 2003a, 2007; Aosaki et al., 2010). Indeed, anticholinergic drugs have long been a first choice therapy for PD and dystonia (Duvoisin, 1967; Jankovic, 2006). Here, in light of the most recent findings, we will review the role of ChIs in striatal function and in the pathogenesis of basal ganglia disorders.
Morphological and electrophysiological properties of cholinergic interneurons
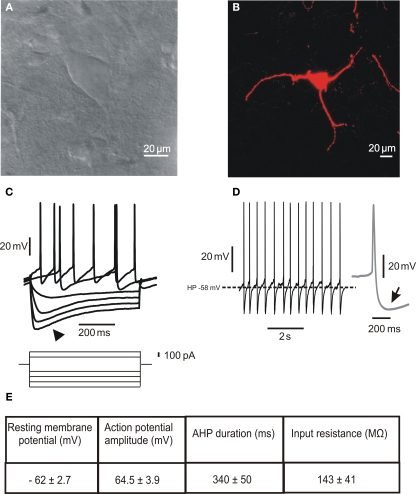
Large aspiny ChIs represent less than 2% of the entire striatal neuronal population. Their neurochemical identification is due to the expression of ChAT, the biosynthetic enzyme for ACh. Morphologically (Figures 1A,B), they are characterized by a large polygonal soma (Ø 20–50 μm), widespread dendritic and axonal fields (Bolam et al., 1984; Smith and Bolam, 1990; Wilson et al., 1990), and a preferential distribution in the matrix area flanking the patches border (van Vulpen and van der Kooy, 1998). These features suggest that ChIs may integrate synaptic inputs over relatively large regions, and act as an associative interneuron in the striatum (Kawaguchi et al., 1995; Miura et al., 2007).
Figure 1.
Morphological and electrophysiological properties of striatal cholinergic interneurons. (A) Infrared differential interference contrast image of a cholinergic interneuron in a striatal slice showing the peculiar polygonal shape and large somatic size of this neuronal subtype. (B) Confocal microscope image of a biocytin-loaded cholinergic interneuron. The cell was loaded with 2% biocytin by means of the recording electrode during an electrophysiological experiment. Note the absence of spines along the dendrites. (C) Representative current-clamp recording of the I–V relationship. The arrowhead indicates the prominent Ih evoked by hyperpolarizing current injection. (D) Spontaneous firing activity of a cholinergic interneuron. The inset on the right (gray) shows a single action potential, followed by a prominent AHP (arrow). (E) Table summarizing the main electrophysiological properties characterizing striatal cholinergic interneurons. Data are presented as mean ± SEM; n = 5.
In vitro electrophysiological recordings have described the peculiar membrane properties of ChIs, that distinguish these neurons from all other striatal neuronal subtypes (Figures 1C–E). These include a relatively depolarized resting membrane potential, long-lasting action potential, high input resistance, prominent afterhyperpolarization (AHP) current, and hyperpolarization-activated cation current (Ih; Bolam et al., 1984; Wilson et al., 1990; Kawaguchi, 1993; Aosaki et al., 1995; Bennett and Wilson, 1998; Bennett et al., 2000; Zhou et al., 2002).
These cells are autonomously active, showing a range of spontaneous tonic firing patterns, from irregular single spiking to rhythmic bursting, even in the absence of synaptic input, suggesting that they are intrinsic in origin (Bennett and Wilson, 1999; Bennett et al., 2000; Goldberg and Wilson, 2005; Wilson, 2005; Wilson and Goldberg, 2006; Goldberg et al., 2009). The prevalence of a spiking pattern in any single neuron was shown to be dependent on the underlying Ca2+-activated K+ conductances. In particular, single spiking depends on a medium-duration AHP (mAHP) current generated by rapid SK currents, which are associated with high-voltage-activated (HVA) CaV2.2 Ca2+ channels. On the other hand, periodic bursting is driven by a delayed and slowly decaying AHP (sAHP) current, associated with CaV1 Ca2+ channels (Bennett et al., 2000; Goldberg and Wilson, 2005; Wilson and Goldberg, 2006). The specific association between HVA Ca2+ channel subtypes and the K+ currents underlying the mAHP and sAHP currents is generated by the dynamics of Ca2+ redistribution among cytoplasmic binding sites with different binding kinetics (Goldberg et al., 2009).
Striatal ChIs are recipients of a prominent glutamatergic drive from both the cortex and the centromedian and parafascicular (Cm–Pf) thalamic nuclei (Lapper and Bolam, 1992; Sidibe and Smith, 1999; Thomas et al., 2000), as well as of an extensive dopaminergic innervation from the substantia nigra pars compacta (Olson et al., 1972; Lavoie et al., 1989; Dimova et al., 1993; Smith and Villalba, 2008).
The predominant effect of dopamine on ChIs is mediated by activation of D2-like D2 receptors (Figure 2), which inhibit striatal ACh efflux (DeBoer et al., 1996), by reducing both autonomous action potential firing and synaptic inputs to ChIs. The former effect is achieved by enhancing the slow inactivation of voltage-dependent Na+ channels (Maurice et al., 2004) and by modulating Ih current (Deng et al., 2007). The reduction of synaptic inputs is achieved through inhibition of HVA Ca2+ channel (Yan and Surmeier, 1996; Pisani et al., 2000).
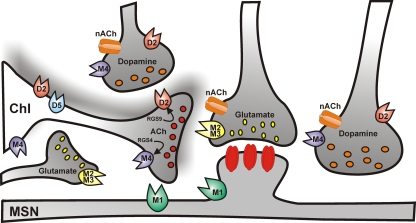
Figure 2.
Cholinergic control of striatal medium spiny neuron activity. Simplified cartoon of the striatal circuitry reporting the distribution of muscarinic and nicotinic receptors. Cholinergic receptors regulate the activity of medium spiny neurons both at the postsynaptic level, and presynaptically, by modulating glutamate, dopamine, and acetylcholine neurotransmission.
In addition, striatal ChIs express D1-like D5 subtype receptors (Figure 2; Bergson et al., 1995; Yan and Surmeier, 1997), which are mainly somatodendritic and depolarize the cell by promoting the non-selective opening of cation channels and the closure of K+ channels, thus, in turn, enhancing ACh release (Damsma et al., 1990; Imperato et al., 1993; DeBoer and Abercrombie, 1996; Aosaki et al., 1998; Pisani et al., 2000).
An additional level of control of striatal ACh release is represented by M2/M4 muscarinic autoreceptors (Figure 2). Autoreceptor activation reduces ACh release by closing CaV2 Ca2+ channels which mediate exocytosis, and by increasing opening of Kir3 potassium channels, which hyperpolarize terminals and further reduce Ca2+ channel opening (Yan and Surmeier, 1996; Calabresi et al., 1998b).
Furthermore, ChIs receive extrinsic excitatory serotonergic (Lavoie et al., 1989; Bonsi et al., 2007) and noradrenergic afferents (Pazos et al., 1985; Pisani et al., 2003b), and an intrinsic inhibitory GABAergic innervation from both MSNs and FS interneurons (Bolam et al., 1986; Martone et al., 1992; Aosaki et al., 2010).
Postsynaptic potentials evoked by electrical stimulation of fibers innervating ChIs are mediated by activation of ionotropic NMDA, AMPA, and GABAA receptors. Upon complete inhibition of both the glutamatergic and GABAergic synaptic components, a slow inhibitory synaptic potential is unmasked, which is mediated by a K+ conductance activated by M2-like receptors (Calabresi et al., 1998b).
The activity of striatal ChIs is therefore highly regulated, through a complex interaction between intrinsic properties and the neuromodulatory control exerted by several transmitters.
Origin of the pause response in ChI tonic firing activity
Striatal ChIs exhibit a variety of spontaneous firing patterns also during in vivo recordings (Wilson et al., 1990; Reynolds et al., 2004). Indeed, these neurons correspond to the tonically active neurons (TANs) recorded in vivo from the primate striatum, which respond with a pause in their ongoing firing activity to reward-related stimuli (Apicella et al., 1991, 1998; Aosaki et al., 1994; ). Several mechanisms are likely to contribute to this pause response, through the modulation of both intrinsic and synaptic properties of ChIs. It has been suggested that these pauses in firing may be due to AHP currents intrinsically generated via Ih transient deactivation following cortical excitatory synaptic inputs (Reynolds et al., 2004; Oswald et al., 2009). Of interest, Ih is regulated by dopamine (Deng et al., 2007). In fact, it is known that synaptic inputs arising both from the dopaminergic nigrostriatal system and from thalamic nuclei involved in sensorimotor integration modulate the responsiveness of these neurons to reward-related stimuli (Aosaki et al., 1994; Matsumoto et al., 2001). High-frequency stimulation (HFS) of the substantia nigra during in vivo recordings increases the AHP (Reynolds et al., 2004). Similarly, in neurons exhibiting regular firing in vitro exogenous application of dopamine causes a prolongation of a depolarization-induced pause and an increase in the duration of sAHP (Deng et al., 2007). Recent in vitro experimental evidence shed further light on the origin of the pause response in striatal ChIs (Ding et al., 2010). This report showed that high-frequency thalamic stimulation elicits an initial burst followed by a pause in the firing activity of ChIs. Both D2 dopamine and nicotinic ACh (nACh) receptors were shown to be involved in this response. These data suggest that the biphasic response to thalamic stimulation might be driven by the initial excitation of ChIs, which induces ACh release and activation of presynaptic nACh receptors located on dopaminergic terminals (Figure 2); hence the stimulation of dopamine release and D2 receptor activation, which prolongs the AHP by inhibiting Ih and Na+ channel currents (Aosaki et al., 2010).
Striatal ChIs have been shown, both in vivo and in vitro, to undergo long-term plastic changes of synaptic efficacy, which might lastingly influence the pattern of firing activity (Suzuki et al., 2001b; Bonsi et al., 2004; Reynolds et al., 2004; Fino et al., 2008). In slice preparations HFS of glutamatergic afferent fibers induces a long-term potentiation (LTP) of both the AMPA-mediated excitatory and GABAergic inhibitory postsynaptic potentials, which is dependent on D5 receptor activation, and on a critical level of intracellular Ca2+ rise through CaV1 channels (Suzuki et al., 2001b; Bonsi et al., 2004). Interestingly, intracellular recordings of ChIs from striatal slices of rats that have learned a rewarded, externally cued sensorimotor task show an increase in spontaneous GABAA-mediated synaptic activity with respect to untrained animals (Bonsi et al., 2003), further suggesting a role for GABAergic transmission in the generation of the pause response. More recently, spike-timing-dependent plasticity (STDP) protocols were shown to induce bidirectional long-term plasticity in ChIs (Fino et al., 2008). STDP–LTP was mainly presynaptic and involved NMDA-receptor activation, while long-term depression (STDP–LTD) had a postsynaptic origin and involved metabotropic glutamate receptors.
Thus, it is plausible that long-term changes of both glutamatergic and GABAergic synaptic potential amplitude are also involved in the generation of the firing activity pattern (Aosaki et al., 2010).
The pattern of spiking and pauses of ChIs is able to filter the striatal output, by directly and indirectly influencing MSN activity (Phelps et al., 1985; Izzo and Bolam, 1988; Chang and Kita, 1992; Wang et al., 2006; Pakhotin and Bracci, 2007; Bonsi et al., 2008). There is experimental evidence indicating that the pauses in ChIs activity might powerfully enhance the salience of dopamine signaling (Threlfell et al., 2010) and transform the reward signal arising from dopaminergic neurons into a gating signal for LTD induction at MSNs (Wang et al., 2006). Further, the thalamic-induced burst-pause response of ChIs might provide a neural substrate for attentional shift and cessation of ongoing motor activity (Ding et al., 2010). Indeed, the patterned activity of ChIs has been suggested to differentially gate the cortical drive to striatopallidal and striatonigral MSNs. Upon thalamic stimulation, the initial burst response of ChIs triggers the transient suppression of cortical inputs to MSNs, through presynaptic muscarinic M2-class receptor activation, but also initiate a slower, muscarinic M1 receptor-dependent postsynaptic facilitation of striatopallidal MSNs. This facilitation extends during the pause response, when the cortical drive resumes, thus creating a late temporal window when the corticostriatal input can selectively drive activity in the striatopallidal network thought to control action suppression (Ding et al., 2010).
Muscarinic and nicotinic modulation of MSN activity
A very dense cholinergic innervation of the striatum arises from intrinsic ChIs. By tonically firing action potentials at about 5 Hz, these interneurons provide an ongoing ACh signal, that is rapidly terminated by acetylcholinesterase (AChE). ACh may act both at synaptic sites, predominantly onto distal dendrites and spine necks (Bolam et al., 1984; Phelps et al., 1985), and via volume transmission (Descarries et al., 1997; Koos and Tepper, 2002).
In the striatum, different subtypes of nACh receptors have been identified, containing a combination of the α4, α6, α7, and β2, β3 subunits (Wada et al., 1989; Seguela et al., 1993; for review, see Quik et al., 2007). In addition, both M1-like and M2-like muscarinic ACh (mACh) receptors, predominantly the M1 and M4 subtypes, are expressed at high density (Figure 2).
In MSNs, M1 receptor activation enhances NMDA-receptor-mediated currents, promoting cell depolarization and corticostriatal LTP (Calabresi et al., 2000), and increases the synchrony in the NMDA-induced network dynamics, via enhancement of persistent Na+ current (Carrillo-Reid et al., 2009). In addition, M1 receptors modulate HVA Ca2+ currents (Howe and Surmeier, 1995; Galarraga et al., 1999; Olson et al., 2005; Perez-Rosello et al., 2005; Perez-Burgos et al., 2008, 2010). Recently, M1 receptor activation has been suggested to have cell-specific effects on striatopallidal vs. striatonigral MSNs, due to specific characteristics of the downstream effectors (Chen et al., 2006; Shen et al., 2007; Day et al., 2008).
In addition to direct postsynaptic effects on MSNs, presynaptic ACh receptors regulate both glutamate and GABA release from striatal afferents. While mACh receptors inhibit neurotransmitter release, presynaptic nACh receptors exert the opposite effect (Calabresi et al., 1998a; Koos and Tepper, 2002; Zhou et al., 2002; Grilli et al., 2009; McClure-Begley et al., 2009).
The autonomous activity of ChIs ensures a sufficient level of endogenous ACh to tonically activate mACh and nACh receptors, thereby constantly influencing striatal activity. Through mACh receptor activation, ACh provides a presynaptic inhibitory tone on the excitatory glutamatergic drive onto MSNs (Pakhotin and Bracci, 2007). Indeed, a single spike in a ChI is able to induce a significant mACh receptor-mediated depression of glutamatergic synaptic currents in a MSN. However, a mechanism to limit this powerful inhibitory control of ChIs over the glutamatergic input to MSNs has been recently proposed to reside in the nicotinic excitation of striatal GABAergic interneurons (Sullivan et al., 2008).
Overall, nACh and mACh receptors would act to translate the pattern of the ongoing cholinergic activity into a strong influence over striatal output (Koos and Tepper, 2002): nACh receptor activation would rapidly affect the activity of MSNs, while the muscarinic impact might become more evident on a slower time scale, and in particular when additional extrasynaptic volume transmission extends the duration of the ACh signal, such as during periods of more intense cholinergic activity (Singer et al., 2002).
Striatal acetylcholine and synaptic plasticity
Enduring changes in synaptic efficacy at corticostriatal synapses are viewed as the cellular basis underlying motor learning and associative memory processes. HFS of corticostriatal afferents may induce either LTD or LTP at MSN synapses, depending on a variety of cell-specific mechanisms (Calabresi et al., 1992; Lovinger et al., 1993; Surmeier et al., 2009). Induction of LTD and LTP requires an intact nigrostriatal projection, and depends upon both dopamine and ionotropic glutamate receptor subtypes involved (Lovinger, 2010). Complex biochemical processes follow the activation of glutamatergic and dopaminergic receptors and their mutual interplay (Calabresi et al., 1994; Gerdeman et al., 2002).
M1 mACh receptors are abundantly expressed on dendrites and spines of MSNs (Figure 2), and are therefore likely to exert a relevant influence on synaptic plasticity (Hersch et al., 1994; Yan et al., 2001). In fact, activation of postsynaptic M1 muscarinic receptors increases MSN excitability, by reducing dendritic K+ currents (Galarraga et al., 1999; Shen et al., 2005). As a consequence, M1 receptor activation promotes MSN depolarization and plays a permissive role in corticostriatal LTP (Calabresi et al., 1999). Accordingly, the M1 receptor antagonist pirenzepine prevents LTP, whilst methoctramine, an M2-like receptor blocker, enhances the magnitude of this form of synaptic plasticity (Calabresi et al., 2000). In addition, M1 receptor activation reduces the opening of CaV1 channels, in response to depolarization, that is necessary for LTD induction (Calabresi et al., 1994; Choi and Lovinger, 1997; Kreitzer and Malenka, 2005). Indeed, LTD induction requires D2 receptor activation in order to pause ChI firing activity and reduce M1 receptor tone (Wang et al., 2006).
In summary, manipulation of ACh tone is expected to affect the direction of corticostriatal synaptic plasticity. In fact, loss of autoreceptor function in M2/M4 receptor knockout mice increases striatal ACh tone and impairs selectively LTD induction at MSN synapses. Accordingly, in these mice LTD can be restored by reducing ACh levels with hemicholinium-3, which depletes endogenous ACh (Bonsi et al., 2008).
Cholinergic Signaling in Disease States
Parkinson's disease
In the early 1960s anticholinergic drugs were introduced in the pharmacological treatment of PD, according to the evidence of an imbalance between dopaminergic and cholinergic transmission within the striatum (Barbeau, 1962; Duvoisin, 1967; Hornykiewicz and Kish, 1987).
Although the increased striatal ACh level has long been attributed to the removal of tonic inhibitory control by D2 receptors on ChIs (Maurice et al., 2004), recent experimental work has investigated in more detail ChI function in acute dopamine depletion models of PD (Fino et al., 2007; Salin et al., 2009). As expected, in dopamine-depleted animals ChIs displayed an increased excitability in vitro (Fino et al., 2007), and became highly synchronized in firing rhythmic bursts in vivo (Raz et al., 1996, 2001). This altered pattern of activity might result in periodic outbreaks of ACh release into the striatum which might not be readily hydrolyzed by AChE. Such alterations in ACh input are likely to underlie the loss of synaptic plasticity (Pisani et al., 2005) and to contribute to the pruning of spines (Shen et al., 2007) reported in MSNs from dopamine-depleted animals, contributing to imbalanced striatal outflow in the parkinsonian state.
Interestingly, recent experimental evidence revealed a novel mechanism by which mACh receptor signaling would disrupt striatal activity (Ding et al., 2006). “Regulators of G protein signaling” (RGS) proteins are GTPase accelerating proteins (GAPs), which terminate G protein coupling between receptors and effectors. Alterations in dopamine content have been shown to rapidly modify the expression of several RGS proteins. These authors report that dopamine depletion does not alter D2 dopamine receptor signaling in ChIs, but leads to a decreased mACh M4 receptor coupling to Ca2+ channels, thereby modifying ChIs excitability. Moreover, they show that this impaired coupling is caused by the selective upregulation of RGS4 expression (Ding et al., 2006).
A very recent paper by Ding et al. (2011) has suggested unexpected roles for ChIs also in the adverse motor effects, dyskinesias, induced by prolonged treatment of PD patients with the dopamine replacing agent 3,4-l-dihydroxphenylalanine methyl ester (l-DOPA). These authors have shown in PD rodent models that repeated l-DOPA exposure causes activation of extracellular signal-regulated kinase 1/2 (ERK) and, in turn, an increased basal firing rate and dopamine-dependent excitation in striatal ChIs. These specific responses of ChIs to chronic l-DOPA treatment correlated with the expression of dyskinesia. Accordingly, muscarinic receptor antagonism reduced l-DOPA-induced dyskinesia.
Dystonia
As in PD, anticholinergic drugs targeting mACh receptors are also effective in the treatment of another movement disorder, dystonia. DYT1 dystonia is a severe form of inherited dystonia, characterized by involuntary twisting movements and abnormal postures. Although the pathogenesis of this disabling disorder remains to be fully elucidated, an altered coupling of dopaminergic and cholinergic signaling has been recently demonstrated in the striatum of mice over-expressing the human protein torsinA with the mutation responsible for DYT1 dystonia (Pisani et al., 2006). In these mice, D2 receptor activation induces an excitatory, rather than inhibitory, effect in ChIs. This paradoxical effect was associated to an increase in the functional representation of CaV2 Ca2+ channels, that regulate Ca2+ entry and the physiological pacemaking activity of these interneurons, likely enhancing ACh release. Indeed, the activity of endogenous AChE was increased in the striatum of DYT1 mice, suggesting a compensatory mechanism to reduce an increased cholinergic tone. In accordance to the proposed role of ACh levels in determining the direction of corticostriatal synaptic plasticity (Bonsi et al., 2008), the elevation in cholinergic tone in DYT1 mice was correlated to the loss of LTD and synaptic depotentiation, and the enhancement of LTP (Martella et al., 2009). This notion was supported by the observation that these alterations were normalized by lowering ACh tone with hemicholinium-3, a depletor of endogenous ACh. Moreover, the clinical drug trihexyphenidyl as well as pirenzepine, both mACh M1 receptor antagonists, were effective in restoring normal synaptic plasticity. These observations might explain the efficacy of anticholinergic drugs in the treatment of dystonia.
Other movement disorders
Functional imaging and post-mortem studies have revealed a significant loss of striatal cholinergic markers in different basal ganglia disorders (Suzuki et al., 2002; Warren et al., 2005; Smith et al., 2006; Kataoka et al., 2010). Huntington's disease (HD) is an autosomal dominant neurodegenerative disease, caused by a mutation in the gene encoding Huntingtin, characterized by involuntary choreiform movements, behavioral and cognitive impairment. Though striatal ChIs have been reported to be spared during striatal degeneration in HD (Graveland et al., 1985), recent studies suggest that they might be functionally altered. Indeed, the levels of both the vesicular ACh transporter (VAChT) and choline acetyltransferase (ChAT) are markedly decreased in the striatum of HD transgenic mice, as well as in post-mortem striatal tissue from HD patients (Spokes, 1980; Suzuki et al., 2001a; Vetter et al., 2003; Smith et al., 2006). Moreover, in two experimental models of HD, 3-nitropropionic acid-treated rats and R6/2 transgenic mice, striatal ChIs did not express LTP (Picconi et al., 2006). Progressive supranuclear palsy (PSP) is a progressive neurodegenerative disease characterized by accumulation of tau protein and akinetic-rigid features, falls, supranuclear gaze palsy, and subcortical dementia. In this disorder, cholinergic dysfunction is indicated by reduced levels of ChAT and VAChT in post-mortem samples, as well as by loss of striatal ChIs (Suzuki et al., 2002; Warren et al., 2005).
Chronic clinical use of dopaminergic drugs is often associated with the development of different types of motor complications, such as dystonia, parkinsonism, hyperkinesia, and stereotyped behavior. These reactions to dopaminergic agents points to an imbalance between striatal ACh and dopamine levels. Accordingly, the treatment of choice for these complications is represented by cholinergic drugs (Sethi and Morgan, 2007; Cubo et al., 2008). In the case of motor stereotypy, as well as of Tourette's syndrome (TS), a childhood-onset neuropsychiatric disease characterized by motor and vocal tics (Graybiel and Canales, 2001) and by a reduced striatal volume (Peterson et al., 2003), a cholinesterase inhibitor has been reported to be effective in the clinical practice (Cubo et al., 2008). Notably, a recent stereological analysis of post-mortem brains showed a significant reduction in the number of ChIs in the sensorimotor regions of the striatum in TS patients (Kataoka et al., 2010). Accordingly, a key role of striatal ChIs has been demonstrated in the arrest of cocaine-induced motor stereotypy in a rat model, where pharmacological treatments restoring ACh release rapidly blocked movement dysfunction (Aliane et al., 2010). Interestingly, this study showed that, while the striatal dopamine/ACh balance is effective during the period of strong motor stereotypy (dopamine increases and ACh decreases), it becomes dissociated during the phase of motor recovery. Indeed, in this period dopamine level still remains high, while ACh returns to its basal level mirroring the decreasing intensity of stereotypy, suggesting an important role of cholinergic transmission in the arrest of motor stereotypy. In accordance with this hypothesis, pharmacological blockade of muscarinic receptors as well as lesion of ChIs significantly prolonged motor stereotypy.
Although simplistic, the striatal ACh/dopamine balance view finds support in clinical pharmacological evidence. To date, two major categories of drugs are successfully utilized in the management of most movement disorders: drugs interfering with either dopaminergic or cholinergic function, suggesting that the interplay between these transmitters is relevant to the maintenance of a correct motor control. In our view, a perspective based upon cholinergic dysfunction may prove useful to orientate clinical pharmacological reasoning.
Concluding Remarks
Clues from neurobiology, functional imaging studies and post-mortem data converge to suggest that both pathogenic features and clinical phenomenology of distinct movement disorders are closely related to dysfunction of striatal cholinergic signaling.
To date, therapeutic intervention to most of these disorders is unsatisfactory. In those conditions where an increased cholinergic activity is documented, targeting muscarinic receptors with more selective drugs is warranted. Indeed, both in PD and dystonia, enhancing M2/M4-mediated autoreceptor function, either by developing selective agonists, or by modulating CaV2 Ca2+ channels appears a promising strategy.
The development of new animal models, including transgenic mice, as well as muscarinic and nicotinic receptor knockout mice, is moving research to a level in which the physiology of the receptor subtypes could be addressed in vivo, offering new perspectives for their pharmacological manipulation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aliane V., Perez S., Bohren Y., Deniau J. M., Kemel M. L. (2010). Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain.[Epub ahead of print]. 10.1093/brain/awq285 [DOI] [PubMed] [Google Scholar]
- Aosaki T., Kimura M., Graybiel A. M. (1995). Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol. 73, 1234–1252 [DOI] [PubMed] [Google Scholar]
- Aosaki T., Kiuchi K., Kawaguchi Y. (1998). Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J. Neurosci. 18, 5180–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T., Miura M., Suzuki T., Nishimura K., Masuda M. (2010). Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr. Gerontol. Int. 10(Suppl. 1), S148–S157 [DOI] [PubMed] [Google Scholar]
- Aosaki T., Tsubokawa H., Ishida A., Watanabe K., Graybiel A. M., Kimura M. (1994). Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. 14, 3969–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P., Ravel S., Sardo P., Legallet E. (1998). Influence of predictive information on responses of tonically active neurons in the monkey striatum. J. Neurophysiol. 80, 3341–3344 [DOI] [PubMed] [Google Scholar]
- Apicella P., Scarnati E., Schultz W. (1991). Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp. Brain Res. 84, 672–675 [DOI] [PubMed] [Google Scholar]
- Barbeau A. (1962). The pathogenesis of Parkinson's disease: a new hypothesis. Can. Med. Assoc. J. 87, 802–807 [PMC free article] [PubMed] [Google Scholar]
- Bennett B. D., Callaway J. C., Wilson C. J. (2000). Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J. Neurosci. 20, 8493–8503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. D., Wilson C. J. (1998). Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J. Neurosci. 18, 8539–8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. D., Wilson C. J. (1999). Spontaneous activity of neostriatal cholinergic interneurons in vitro. J. Neurosci. 19, 5586–5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C., Mrzljak L., Smiley J. F., Pappy M., Levenson R., Goldman-Rakic P. S. (1995). Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. 15, 7821–7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam J. P., Hanley J. J., Booth P. A., Bevan M. D. (2000). Synaptic organisation of the basal ganglia. J. Anat. 196(Pt 4), 527–542 10.1046/j.1469-7580.2000.19640527.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam J. P., Ingham C. A., Izzo P. N., Levey A. I., Rye D. B., Smith A. D., Wainer B. H. (1986). Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res. 397, 279–289 10.1016/0006-8993(86)90629-3 [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Wainer B. H., Smith A. D. (1984). Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 12, 711–718 10.1016/0306-4522(84)90165-9 [DOI] [PubMed] [Google Scholar]
- Bonsi P., Cuomo D., Ding J., Sciamanna G., Ulrich S., Tscherter A., Bernardi G., Surmeier D. J., Pisani A. (2007). Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32, 1840–1854 10.1038/sj.npp.1301294 [DOI] [PubMed] [Google Scholar]
- Bonsi P., De Persis C., Calabresi P., Bernardi G., Pisani A. (2004). Coordinate high-frequency pattern of stimulation and calcium levels control the induction of LTP in striatal cholinergic interneurons. Learn. Mem. 11, 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P., Florio T., Capozzo A., Pisani A., Calabresi P., Siracusano A., Scarnati E. (2003). Behavioural learning-induced increase in spontaneous GABAA-dependent synaptic activity in rat striatal cholinergic interneurons. Eur. J. Neurosci. 17, 174–178 [DOI] [PubMed] [Google Scholar]
- Bonsi P., Martella G., Cuomo D., Platania P., Sciamanna G., Bernardi G., Wess J., Pisani A. (2008). Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J. Neurosci. 28, 6258–6263 10.1523/JNEUROSCI.1678-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Centonze D., Gubellini P., Bernardi G. (1999). Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology 38, 323–326 10.1016/S0028-3908(98)00199-3 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Centonze D., Gubellini P., Pisani A., Bernardi G. (1998a). Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur. J. Neurosci. 10, 3020–3023 10.1111/j.1460-9568.1998.00348.x [DOI] [PubMed] [Google Scholar]
- Calabresi P., Centonze D., Pisani A., Sancesario G., North R. A., Bernardi G. (1998b). Muscarinic IPSPs in rat striatal cholinergic interneurones. J. Physiol. 510(Pt 2), 421–427 10.1111/j.1469-7793.1998.421bk.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Centonze D., Gubellini P., Pisani A., Bernardi G. (2000). Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 23, 120–126 10.1016/S0166-2236(99)01501-5 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Maj R., Pisani A., Mercuri N. B., Bernardi G. (1992). Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 12, 4224–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Pisani A., Mercuri N. B., Bernardi G. (1994). Post-receptor mechanisms underlying striatal long-term depression. J. Neurosci. 14, 4871–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L., Tecuapetla F., Vautrelle N., Hernandez A., Vergara R., Galarraga E., Bargas J. (2009). Muscarinic enhancement of persistent sodium current synchronizes striatal medium spiny neurons. J. Neurophysiol. 102, 682–690 10.1152/jn.00134.2009 [DOI] [PubMed] [Google Scholar]
- Chang H. T., Kita H. (1992). Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 574, 307–311 10.1016/0006-8993(92)90830-3 [DOI] [PubMed] [Google Scholar]
- Chen Y., Yu F. H., Surmeier D. J., Scheuer T., Catterall W. A. (2006). Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron 49, 409–420 10.1016/j.neuron.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Choi S., Lovinger D. M. (1997). Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc. Natl. Acad. Sci. U.S.A. 94, 2665–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant C., Umbriaco D., Garcia S., Watkins K. C., Descarries L. (1996). Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 71, 937–947 10.1016/0306-4522(95)00507-2 [DOI] [PubMed] [Google Scholar]
- Cubo E., Fernandez Jaen A., Moreno C., Anaya B., Gonzalez M., Kompoliti K. (2008). Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: an 18-week, single-center, dose-escalating, prospective, open-label study. Clin. Ther. 30, 182–189 [DOI] [PubMed] [Google Scholar]
- Damsma G., Tham C. S., Robertson G. S., Fibiger H. C. (1990). Dopamine D1 receptor stimulation increases striatal acetylcholine release in the rat. Eur. J. Pharmacol. 186, 335–338 [DOI] [PubMed] [Google Scholar]
- Day M., Wokosin D., Plotkin J. L., Tian X., Surmeier D. J. (2008). Differential excitability and modulation of striatal medium spiny neuron dendrites. J. Neurosci. 28, 11603–11614 10.1523/JNEUROSCI.1840-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer P., Abercrombie E. D. (1996). Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J. Pharmacol. Exp. Ther. 277, 775–783 [PubMed] [Google Scholar]
- DeBoer P., Heeringa M. J., Abercrombie E. D. (1996). Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur. J. Pharmacol. 317, 257–262 [DOI] [PubMed] [Google Scholar]
- Deng P., Zhang Y., Xu Z. C. (2007). Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J. Neurosci. 27, 3148–3156 10.1523/JNEUROSCI.5535-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Gisiger V., Steriade M. (1997). Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 53, 603–625 [DOI] [PubMed] [Google Scholar]
- Dimova R., Vuillet J., Nieoullon A., Kerkerian-Le Goff L. (1993). Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience 53, 1059–1071 10.1016/0306-4522(93)90489-3 [DOI] [PubMed] [Google Scholar]
- Ding J., Guzman J. N., Tkatch T., Chen S., Goldberg J. A., Ebert P. J., Levitt P., Wilson C. J., Hamm H. E., Surmeier D. J. (2006). RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 9, 832–842 [DOI] [PubMed] [Google Scholar]
- Ding J. B., Guzman J. N., Peterson J. D., Goldberg J. A., Surmeier D. J. (2010). Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307 10.1016/j.neuron.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Won L., Britt J. P., Lim S. A., McGehee D. S., Kang U. J. (2011). Enhanced striatal cholinergic neuronal activity mediates l-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. U.S.A. 108, 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin R. C. (1967). Cholinergic-anticholinergic antagonism in parkinsonism. Arch. Neurol. 17, 124–136 [DOI] [PubMed] [Google Scholar]
- Fino E., Deniau J. M., Venance L. (2008). Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. J. Physiol. 586, 265–282 10.1113/jphysiol.2007.144501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E., Glowinski J., Venance L. (2007). Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci. Res. 58, 305–316 [DOI] [PubMed] [Google Scholar]
- Galarraga E., Hernandez-Lopez S., Reyes A., Miranda I., Bermudez-Rattoni F., Vilchis C., Bargas J. (1999). Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J. Neurosci. 19, 3629–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G. L., Ronesi J., Lovinger D. M. (2002). Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 5, 446–451 [DOI] [PubMed] [Google Scholar]
- Goldberg J. A., Teagarden M. A., Foehring R. C., Wilson C. J. (2009). Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J. Neurosci. 29, 8396–8407 10.1523/JNEUROSCI.5582-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. A., Wilson C. J. (2005). Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J. Neurosci. 25, 10230–10238 10.1523/JNEUROSCI.2734-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland G. A., Williams R. S., DiFiglia M. (1985). Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science 227, 770–773 10.1126/science.3155875 [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. (1990). Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 13, 244–254 10.1016/0166-2236(90)90104-I [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Aosaki T., Flaherty A. W., Kimura M. (1994). The basal ganglia and adaptive motor control. Science 265, 1826–1831 10.1126/science.8091209 [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Canales J. J. (2001). The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv. Neurol. 85, 123–131 [PubMed] [Google Scholar]
- Grilli M., Zappettini S., Raiteri L., Marchi M. (2009). Nicotinic and muscarinic cholinergic receptors coexist on GABAergic nerve endings in the mouse striatum and interact in modulating GABA release. Neuropharmacology 56, 610–614 10.1016/j.neuropharm.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Hersch S. M., Gutekunst C. A., Rees H. D., Heilman C. J., Levey A. I. (1994). Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 14, 3351–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O., Kish S. J. (1987). Biochemical pathophysiology of Parkinson's disease. Adv. Neurol. 45, 19–34 [PubMed] [Google Scholar]
- Howe A. R., Surmeier D. J. (1995). Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J. Neurosci. 15, 458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Sandoval O., Tecuapetla F., Unal B., Shah F., Koós T., Tepper J. M. (2010). Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J. Neurosci. 30, 6999–7016 10.1523/JNEUROSCI.5996-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A., Obinu M. C., Gessa G. L. (1993). Effects of cocaine and amphetamine on acetylcholine release in the hippocampus and caudate nucleus. Eur. J. Pharmacol. 238, 377–381 [DOI] [PubMed] [Google Scholar]
- Izzo P. N., Bolam J. P. (1988). Cholinergic synaptic input to different parts of spiny striatonigral neurons in the rat. J. Comp. Neurol. 269, 219–234 [DOI] [PubMed] [Google Scholar]
- Izzo P. N., Graybiel A. M., Bolam J. P. (1987). Characterization of substance P- and [Met]enkephalin-immunoreactive neurons in the caudate nucleus of cat and ferret by a single section Golgi procedure. Neuroscience 20, 577–587 10.1016/0306-4522(87)90111-4 [DOI] [PubMed] [Google Scholar]
- Jankovic J. (2006). Treatment of dystonia. Lancet Neurol. 5, 864–872 10.1016/S1474-4422(06)70574-9 [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Kalanithi P. S., Grantz H., Schwartz M. L., Saper C., Leckman J. F., Vaccarino F. M. (2010). Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 518, 277–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. (1993). Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 13, 4908–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Wilson C. J., Augood S. J., Emson P. C. (1995). Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 18, 527–535 10.1016/0166-2236(95)98374-8 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Wilson C. J., Emson P. C. (1989). Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol. 62, 1052–1068 [DOI] [PubMed] [Google Scholar]
- Koos T., Tepper J. M. (2002). Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci. 22, 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A. C., Malenka R. C. (2005). Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 25, 10537–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A. C., Malenka R. C. (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554 10.1016/j.neuron.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper S. R., Bolam J. P. (1992). Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51, 533–545 10.1016/0306-4522(92)90293-B [DOI] [PubMed] [Google Scholar]
- Lavoie B., Smith Y., Parent A. (1989). Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J. Comp. Neurol. 289, 36–52 [DOI] [PubMed] [Google Scholar]
- Lovinger D. M. (2010). Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58, 951–961 10.1016/j.neuropharm.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M., Tyler E. C., Merritt A. (1993). Short- and long-term synaptic depression in rat neostriatum. J. Neurophysiol. 70, 1937–1949 [DOI] [PubMed] [Google Scholar]
- Martella G., Platania P., Vita D., Sciamanna G., Cuomo D., Tassone A., Tscherter A., Kitada T., Bonsi P., Shen J., Pisani A. (2009). Enhanced sensitivity to group II mGlu receptor activation at corticostriatal synapses in mice lacking the familial parkinsonism-linked genes PINK1 or Parkin. Exp. Neurol. 215, 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone M. E., Armstrong D. M., Young S. J., Groves P. M. (1992). Ultrastructural examination of enkephalin and substance P input to cholinergic neurons within the rat neostriatum. Brain Res. 594, 253–262 10.1016/0006-8993(92)91132-X [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Minamimoto T., Graybiel A. M., Kimura M. (2001). Neurons in the thalamic CM–Pf complex supply striatal neurons with information about behaviorally significant sensory events. J. Neurophysiol. 85, 960–976 [DOI] [PubMed] [Google Scholar]
- Maurice N., Mercer J., Chan C. S., Hernandez-Lopez S., Held J., Tkatch T., Surmeier D. J. (2004). D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J. Neurosci. 24, 10289–10301 10.1523/JNEUROSCI.2155-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley T. D., King N. M., Collins A. C., Stitzel J. A., Wehner J. M., Butt C. M. (2009). Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by alpha4beta2 and alpha4alpha5beta2 nicotinic receptor subtypes. Mol. Pharmacol. 75, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. M., Mash D., Hersh L., Bothwell M., Geula C. (1992). Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J. Comp. Neurol. 323, 252–268 [DOI] [PubMed] [Google Scholar]
- Miura M., Saino-Saito S., Masuda M., Kobayashi K., Aosaki T. (2007). Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J. Neurosci. 27, 9721–9728 10.1523/JNEUROSCI.2993-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola S. M. (2007). The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl.) 191, 521–550 [DOI] [PubMed] [Google Scholar]
- Olson L., Seiger A., Fuxe K. (1972). Heterogeneity of striatal and limbic dopamine innervation: highly fluorescent islands in developing and adult rats. Brain Res. 44, 283–288 10.1016/0006-8993(72)90385-X [DOI] [PubMed] [Google Scholar]
- Olson P. A., Tkatch T., Hernandez-Lopez S., Ulrich S., Ilijic E., Mugnaini E., Zhang H., Bezprozvanny I., Surmeier D. J. (2005). G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci. 25, 1050–1062 10.1523/JNEUROSCI.3327-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald M. J., Oorschot D. E., Schulz J. M., Lipski J., Reynolds J. N. (2009). Ih current generates the afterhyperpolarisation following activation of subthreshold cortical synaptic inputs to striatal cholinergic interneurons. J. Physiol. 587, 5879–5897 10.1113/jphysiol.2009.177600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P., Bracci E. (2007). Cholinergic interneurons control the excitatory input to the striatum. J. Neurosci. 27, 391–400 10.1523/JNEUROSCI.3709-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos A., Probst A., Palacios J. M. (1985). Beta-adrenoceptor subtypes in the human brain: autoradiographic localization. Brain Res. 358, 324–328 10.1016/0006-8993(85)90977-1 [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A., Perez-Rosello T., Salgado H., Flores-Barrera E., Prieto G. A., Figueroa A., Galarraga E., Bargas J. (2008). Muscarinic M(1) modulation of N and L types of calcium channels is mediated by protein kinase C in neostriatal neurons. Neuroscience 155, 1079–1097 10.1016/j.neuroscience.2008.06.047 [DOI] [PubMed] [Google Scholar]
- Perez-Burgos A., Prieto G. A., Galarraga E., Bargas J. (2010). CaV2.1 channels are modulated by muscarinic M1 receptors through phosphoinositide hydrolysis in neostriatal neurons. Neuroscience 165, 293–299 10.1016/j.neuroscience.2009.10.056 [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T., Figueroa A., Salgado H., Vilchis C., Tecuapetla F., Guzman J. N., Galarraga E., Bargas J. (2005). Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J. Neurophysiol. 93, 2507–2519 10.1152/jn.00853.2004 [DOI] [PubMed] [Google Scholar]
- Peterson B. S., Thomas P., Kane M. J., Scahill L., Zhang H., Bronen R., King R. A., Leckman J. F., Staib L. (2003). Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch. Gen. Psychiatry 60, 415–424 [DOI] [PubMed] [Google Scholar]
- Phelps P. E., Houser C. R., Vaughn J. E. (1985). Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J. Comp. Neurol. 238, 286–307 [DOI] [PubMed] [Google Scholar]
- Picconi B., Passino E., Sgobio C., Bonsi P., Barone I., Ghiglieri V., Pisani A., Bernardi G., Ammassari-Teule M., Calabresi P. (2006). Plastic and behavioral abnormalities in experimental Huntington's disease: a crucial role for cholinergic interneurons. Neurobiol. Dis. 22, 143–152 [DOI] [PubMed] [Google Scholar]
- Pisani A., Bernardi G., Ding J., Surmeier D. J. (2007). Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 30, 545–553 10.1016/j.tins.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Pisani A., Bonsi P., Centonze D., Calabresi P., Bernardi G. (2000). Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J. Neurosci. 20, RC69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A., Bonsi P., Centonze D., Gubellini P., Bernardi G., Calabresi P. (2003a). Targeting striatal cholinergic interneurons in Parkinson's disease: focus on metabotropic glutamate receptors. Neuropharmacology 45, 45–56 10.1016/S0028-3908(03)00137-0 [DOI] [PubMed] [Google Scholar]
- Pisani A., Bonsi P., Centonze D., Martorana A., Fusco F., Sancesario G., De Persis C., Bernardi G., Calabresi P. (2003b). Activation of beta1-adrenoceptors excites striatal cholinergic interneurons through a cAMP-dependent, protein kinase-independent pathway. J. Neurosci. 23, 5272–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A., Centonze D., Bernardi G., Calabresi P. (2005). Striatal synaptic plasticity: implications for motor learning and Parkinson's disease. Mov. Disord. 20, 395–402 10.1002/mds.20394 [DOI] [PubMed] [Google Scholar]
- Pisani A., Martella G., Tscherter A., Bonsi P., Sharma N., Bernardi G., Standaert D. G. (2006). Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol. Dis. 24, 318–325 [DOI] [PubMed] [Google Scholar]
- Quik M., Bordia T., O'Leary K. (2007). Nicotinic receptors as CNS targets for Parkinson's disease. Biochem. Pharmacol. 74, 1224–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Feingold A., Zelanskaya V., Vaadia E., Bergman H. (1996). Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J. Neurophysiol. 76, 2083–2088 [DOI] [PubMed] [Google Scholar]
- Raz A., Frechter-Mazar V., Feingold A., Abeles M., Vaadia E., Bergman H. (2001). Activity of pallidal and striatal tonically active neurons is correlated in mptp-treated monkeys but not in normal monkeys. J. Neurosci. 21, RC128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. N., Hyland B. I., Wickens J. R. (2004). Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J. Neurosci. 24, 9870–9877 10.1523/JNEUROSCI.3225-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P., Lopez I. P., Kachidian P., Barroso-Chinea P., Rico A. J., Gomez-Bautista V., Coulon P., Kerkerian-Le Goff L., Lanciego J. L. (2009). Changes to interneuron-driven striatal microcircuits in a rat model of Parkinson's disease. Neurobiol. Dis. 34, 545–552 [DOI] [PubMed] [Google Scholar]
- Seguela P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 13, 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. D., Morgan J. C. (2007). “Drug-induced movement disorders,” in Parkinson's Disease and Movement Disorders, 5th Edn, eds Jankovic J., Tolosa E. (Philadelphia: Lippincott Williams and Wilkins; ), 394–408 [Google Scholar]
- Shen W., Hamilton S. E., Nathanson N. M., Surmeier D. J. (2005). Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J. Neurosci. 25, 7449–7458 10.1523/JNEUROSCI.1381-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Tian X., Day M., Ulrich S., Tkatch T., Nathanson N. M., Surmeier D. J. (2007). Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat. Neurosci. 10, 1458–1466 [DOI] [PubMed] [Google Scholar]
- Sidibe M., Smith Y. (1999). Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience 89, 1189–1208 10.1016/S0306-4522(98)00367-4 [DOI] [PubMed] [Google Scholar]
- Singer H. S., Szymanski S., Giuliano J., Yokoi F., Dogan A. S., Brasic J. R., Zhou Y., Grace A. A., Wong D. F. (2002). Elevated intrasynaptic dopamine release in Tourette's syndrome measured by PET. Am. J. Psychiatry 159, 1329–1336 [DOI] [PubMed] [Google Scholar]
- Smith A. D., Bolam J. P. (1990). The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 13, 259–265 10.1016/0166-2236(90)90106-K [DOI] [PubMed] [Google Scholar]
- Smith R., Chung H., Rundquist S., Maat-Schieman M. L., Colgan L., Englund E., Liu Y. J., Roos R. A., Faull R. L., Brundin P., Li J. Y. (2006). Cholinergic neuronal defect without cell loss in Huntington's disease. Hum. Mol. Genet. 15, 3119–3131 [DOI] [PubMed] [Google Scholar]
- Smith Y., Villalba R. (2008). Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov. Disord. 23(Suppl. 3), S534–S547 [DOI] [PubMed] [Google Scholar]
- Spokes E. G. (1980). Neurochemical alterations in Huntington's chorea: a study of post-mortem brain tissue. Brain 103, 179–210 10.1093/brain/103.1.179 [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Chen H., Morikawa H. (2008). Recurrent inhibitory network among striatal cholinergic interneurons. J. Neurosci. 28, 8682–8690 10.1523/JNEUROSCI.2411-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Plotkin J., Shen W. (2009). Dopamine and synaptic plasticity in dorsal striatal circuits controlling action selection. Curr. Opin. Neurobiol. 19, 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Desmond T. J., Albin R. L., Frey K. A. (2001a). Vesicular neurotransmitter transporters in Huntington's disease: initial observations and comparison with traditional synaptic markers. Synapse 41, 329–336 10.1002/syn.1089 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miura M., Nishimura K., Aosaki T. (2001b). Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J. Neurosci. 21, 6492–6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Desmond T. J., Albin R. L., Frey K. A. (2002). Cholinergic vesicular transporters in progressive supranuclear palsy. Neurology 58, 1013–1018 [DOI] [PubMed] [Google Scholar]
- Tepper J. M., Bolam J. P. (2004). Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 14, 685–692 [DOI] [PubMed] [Google Scholar]
- Tepper J. M., Tecuapetla F., Koós T., Ibáñez-Sandoval O. (2010). Heterogeneity and diversity of striatal GABAergic interneurons. Front. Neuroanat. 4:150. 10.3389/fnana.2010.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. M., Smith Y., Levey A. I., Hersch S. M. (2000). Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse 37, 252–261 [DOI] [PubMed] [Google Scholar]
- Threlfell S., Clements M. A., Khodai T., Pienaar I. S., Exley R., Wess J., Cragg S. J. (2010). Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J. Neurosci. 30, 3398–3408 10.1523/JNEUROSCI.5620-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vulpen E. H., van der Kooy D. (1998). Striatal cholinergic interneurons: birthdates predict compartmental localization. Brain Res. Dev. Brain Res. 109, 51–58 10.1016/S0165-3806(98)00012-1 [DOI] [PubMed] [Google Scholar]
- Vetter J. M., Jehle T., Heinemeyer J., Franz P., Behrens P. F., Jackisch R., Landwehrmeyer G. B., Feuerstein T. J. (2003). Mice transgenic for exon 1 of Huntington's disease: properties of cholinergic and dopaminergic pre-synaptic function in the striatum. J. Neurochem. 85, 1054–1063 10.1046/j.1471-4159.2003.01704.x [DOI] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. (1989). Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 284, 314–335 [DOI] [PubMed] [Google Scholar]
- Wang Z., Kai L., Day M., Ronesi J., Yin H. H., Ding J., Tkatch T., Lovinger D. M., Surmeier D. J. (2006). Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50, 443–452 10.1016/j.neuron.2006.04.010 [DOI] [PubMed] [Google Scholar]
- Warren N. M., Piggott M. A., Perry E. K., Burn D. J. (2005). Cholinergic systems in progressive supranuclear palsy. Brain 128, 239–249 10.1093/brain/awh391 [DOI] [PubMed] [Google Scholar]
- Wilson C. J. (2005). The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron 45, 575–585 10.1016/j.neuron.2004.12.053 [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Chang H. T., Kitai S. T. (1990). Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 10, 508–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. J., Goldberg J. A. (2006). Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J. Neurophysiol. 95, 196–204 [DOI] [PubMed] [Google Scholar]
- Yan Z., Flores-Hernandez J., Surmeier D. J. (2001). Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 103, 1017–1024 10.1016/S0306-4522(01)00039-2 [DOI] [PubMed] [Google Scholar]
- Yan Z., Surmeier D. J. (1996). Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 16, 2592–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Surmeier D. J. (1997). D5 dopamine receptors enhance Zn2+ -sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron 19, 1115–1126 10.1016/S0896-6273(00)80402-X [DOI] [PubMed] [Google Scholar]
- Yin H. H., Knowlton B. J. (2006). The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476 [DOI] [PubMed] [Google Scholar]
- Zhou F. M., Wilson C. J., Dani J. A. (2002). Cholinergic interneuron characteristics and nicotinic properties in the striatum. J. Neurobiol. 53, 590–605 10.1002/neu.10150 [DOI] [PubMed] [Google Scholar]