Abstract
Background
The rising life expectancy of cancer patients has led to a greater need for treatment of spinal metastases. Interdisciplinary collaboration is important so that each patient’s treatment can be properly tailored to the overall prognosis. The main factors to be considered are the histology of the primary tumor, potential spinal instability, and compression of neural structures.
Methods
We discuss the treatment options for spinal metastases on the basis of a selective literature review and our own extensive experience in an interdisciplinary tumor center.
Results
For spinal canal compression or impending spinal instability, the treatment of choice is decompression and stabilization, by either a dorsal approach (lumbar and thoracic spine) or a ventral approach (cervical spine). Radical ventral tumor resection is indicated only for solitary metastases in patients with a favorable long-range prognosis. If the tumor is radiosensitive, radiotherapy is given either as adjuvant treatment after surgery or as the primary treatment for multiple spinal metastases in the absence of an acute neurological deficit. Various fractionation schemes with different total radiation doses are used. Bisphosphonate treatment is an integral component of the overall treatment strategy.
Conclusion
The treatment of spinal metastases requires interdisciplinary collaboration and must be tailored to each patient’s overall prognosis.
In recent years, the odds of surviving most kinds of cancer have improved. Bone metastases often arise in patients whose quality of life is not otherwise markedly impaired by their neoplastic disease. The types of primary tumor that most frequently give rise to bone metastases are breast, prostate, and lung cancer, in that order (1). In 3% to 10% of all cases, the underlying primary tumor remains unknown (2– 4). Thus, the treatment of both symptomatic and asymptomatic spinal metastases is a matter of increasing clinical importance. Bone is the third most common site of metastases after the liver and the lungs, and about two-thirds of all bone metastases are located in the spine; accordingly, as many as 10% of all patients with malignant tumors suffer from spinal metastases at some point in the course of their disease (1). 10% to 20% of these patients have spinal cord compression due to a metastasis (e1).
The proper treatment of spinal metastases is a medical challenge requiring interdisciplinary collaboration. Treatment must be individually tailored for each patient in consideration of multiple factors including bony stability, the compression of neural structures, tumor radiosensitivity, pain, and, not least, the patient’s overall prognosis. There are various scoring systems for prognosis that are of only limited predictive value and cannot be used as anything more than a rough guide (5, 6). The prognosis with respect to survival essentially depends on the biology of the primary tumor: two-year survival rates for patients with spinal metastases range from 9% (lung cancer) to 44% (breast or prostate cancer) (4). In general, only 10% to 20% of patients with spinal metastases are still alive two years after these metastases are diagnosed. The physician must give due consideration to this fact when deciding upon the nature and invasiveness of any treatment that is to be provided.
Treatment strategy.
The treatment of spinal metastases requires an interdisciplinary treatment plan tailored to the needs of each patient.
In this article, we present the current therapeutic options for spinal metastases on the basis of a selective literature review as well as our own extensive experience in an interdisciplinary tumor center.
Learning objectives
Readers of this article should obtain
an overview of the various available options for the diagnostic evaluation of spinal metastases, and
a basic knowledge of current treatment strategies in the surgical oncology, radiotherapy, and chemotherapy of spinal metastases.
Diagnostic evaluation
The clinical manifestations of spinal metastases typically include any or all of the following:
local pain with or without radiation in a radicular or pseudoradicular pattern,
a neurological deficit,
spinal deformity,
a general decline of physical condition,
or no clinical manifestations at all (asymptomatic spinal metastases).
Local pain that first arises only at night and gradually increases in severity is often due to elevated intraosseous pressure caused by a metastasis. The size of the osteolytic change is correlated with pain intensity (7). If a growing metastasis destroys the involved bone and/or ligamentous soft tissues, the resulting secondary instability can cause pain that is precipitated by movement and mechanical stress.
Tumor-induced compression of a nerve root causes pain in a radicular distribution, while compression of the spinal cord causes long-tract deficits or conus medullaris syndrome and compression of the cauda equina causes cauda equina syndrome. The mass effect produced by a tumor comes from the vertebral body in about 90% of cases; thus, the corticospinal tracts are often the first long tracts of the spinal cord to be affected, as they are ventrally located within the cord. This explains why spastic paraparesis often arises before any sensory abnormalities are present. Bladder and/or bowel dysfunction resulting from compression of the conus medullaris, cauda equina, or both is often misinterpreted as a sequela of prostatic hypertrophy or weakness of the pelvic floor, particularly in elderly patients.
Diagnostic evaluation.
Only the combination of bone scanning, plain x-rays, and magnetic resonance imaging of the entire spine affords sufficient sensitivity and specificity.
When the clinical manifestations arouse suspicion of spinal metastases, the routine radiological investigations that should be performed include plain films, skeletal scintigraphy (bone scanning), and magnetic resonance imaging of the entire spine. Only the combination of all three techniques affords sufficient sensitivity and specificity. For example, in the case of prostate cancer, the sensitivity and specificity of bone scanning alone are 46% and 32%, respectively, while the corresponding figures for bone scanning combined with plain films are 63% and 64%, and for all three techniques combined 83% and 100% (8).
Osteolysis is visible in a plain x-ray film only when a tumor destroys 30% to 50% of a vertebral body. Magnetic resonance imaging with contrast medium is the best technique for distinguishing neoplastic from inflammatory and osteoporotic changes of the vertebrae. MRI of the entire spine should be performed (8), because spinal cord compression is present at more than one site in 1% of cases (9). Myelography is no longer routinely performed now that MRI is available. Nonetheless, myelography may provide valuable evidence of locally recurrent tumor if the MRI is rendered uninterpretable by artefacts such as those due to metallic implants.
Bone scanning and PET-CT can be used to detect further osseous metastases for tumor staging and to assess the metabolic activity of tumor tissue in further follow-up.
When the tumor is highly vascularized (e.g., hypernephroma metastases), preoperative angiography and embolization of the tumor vessels can be a useful aid to surgery.
Biopsy is indicated whenever the histological nature of the lesion and its degree of malignancy are uncertain. CT-guided needle biopsy frequently fails to yield enough representative tissue for diagnosis, particularly when only a small portion of the tumor mass is located outside of bone; thus, open biopsy is often a better option (10).
Treatment
Proper treatment planning must be based on an interdisciplinary evaluation of the patient’s overall disease situation, which is often a complex matter. Each of the following aspects must be considered individually:
Highly vascularized tumors.
When the tumor is highly vascularized (e.g., hypernephroma metastases), preoperative angiography and embolization of the tumor vessels can be a useful aid to surgery.
the clinical manifestations (pain, neurological deficit),
spinal stability,
the number of spinal metastases,
the degree of mobility that the patient desires or can reasonably expect to attain,
radio- and chemosensitivity of the tumor,
the patient’s prognosis for survival.
The literature contains a number of algorithms for treatment planning, yet there are no studies providing class I evidence to show that any particular procedure is the optimal one.
It is entirely clear, however, that clinical decisions should never be based on a single factor only, such as
the local extent of tumor,
the neurological findings,
the overall prognosis for survival,
the histology of the primary tumor,
or the extent of metastasis.
Tokuhashi proposed a scoring system based on individual scores in six different categories (Table) (5). He recommends radical tumor resection for a score of 9 or higher and palliative treatment for a score of 5 or lower. No recommendation is given for score of 6 to 8. Enkaoua studied the utility of the Tokuhashi score for assessing prognosis in a cohort of 71 patients (e2). He found a significant effect on mean survival time: patients scoring 7 or below lived an average of 5.3 months, while those scoring 8 or above lived an average of 23.6 months.
Table. The Tokuhashi Scoring System (5).
| Category | Options (%) | Points |
| General condition (Karnofsky index) | Poor (10–40) | 0 |
| Fair (50–70) | 1 | |
| Good (80–100) | 2 | |
| Number of extraspinal bony metastases | ≥ 3 | 0 |
| 1–2 | 1 | |
| 0 | 2 | |
| Number of spinal metastases | ≥ 3 | 0 |
| 2 | 1 | |
| 1 | 2 | |
| Organ metastases | Unresectable | 0 |
| Resectable | 1 | |
| None | 2 | |
| Primary tumor | Lung, stomach | 0 |
| Kidney, liver, uterus | 1 | |
| Thyroid, prostate, breast, rectum | 2 | |
| Spinal cord damage | Complete | 0 |
| Incomplete | 1 | |
| None | 2 | |
| Recommendation:≥ 9, radical tumor resection ≤ 5, palliative treatment | ||
Enkaoua further determined that mean survival time differed significantly depending on whether the metastasis was of an unknown primary tumor (2 months), thyroid carcinoma (33.1 months), or renal-cell carcinoma (8.6 months) (e2).
An updated version of the Tokuhashi scale lends greater weight in the assigning of points to the aggressiveness of the underlying neoplastic entity (1, e5).
In a study of 241 patients, Bauer and Wedin (11) developed the criteria described in the for Box estimating the patient’s life expectancy.
Box. Prognostication for patients with spinal metastases*1.
-
Criteria
No organ metastasis
No pathological fracture
Solitary skeletal metastasis
No lung cancer
The primary tumor is breast carcinoma, renal cell carcinoma, lymphoma, or myeloma
-
Prognosis
The one-year survival rate can be estimated from the number of the above criteria that are positive:
4–5 positive criteria → one-year survival 50%
2–3 positive criteria → one-year survival 25%
0–1 positive criteria → one-year survival 0%
*1 modified from (11)
Treatment with an orthosis
External stabilization with an orthosis is often performed in routine clinical practice in the hope of preventing pathological fractures or of avoiding the involvement of neural structures in case a fracture is already present.
The goal of treatment with orthoses is to put the spine in an extended position (reclination), redirecting forces dorsally in order to take mechanical stress off the weakened vertebral bodies. This works best at the thoracolumbar transition. External stabilization with an orthosis is biomechanically problematic at the craniocervical junction, at high thoracic levels, and below L3.
The goal of treatment with an orthosis.
The goal of treatment with orthoses is to put the spine in an extended position (reclination), redirecting forces dorsally in order to take mechanical stress off the weakened vertebral bodies.
The indication for an orthosis should be assessed critically and without excessive zeal for this form of treatment, as it may provide a questionable mechanical benefit at the expense of considerable discomfort for the patient.
Standard surgical technique.
Dorsal spinal decompression and stabilization is the standard surgical technique to treat symptomatic spinal cord metastases.
Surgery
The options for surgical treatment have improved markedly in recent years. The development of better implants and gentler anesthetic techniques has widened the spectrum of indications for surgery in patients suffering from lessened stability of the axial skeleton and/or clinically significant narrowing of the spinal canal. The anatomy of the spine (in contrast to the limbs) makes an oncologically radical tumor resection impossible in all but a small minority of cases. Thus, patients with a favorable overall prognosis should undergo postoperative radiotherapy to consolidate their treatment, even if a gross total resection has been achieved (e3). Preoperative radiotherapy, on the other hand, should be avoided because of the risk of impaired wound healing (e4).
Standard surgical technique for the thoracic and lumbar spine.
Dorsal spinal decompression and stabilization can be considered the standard surgical technique to treat metastatic disease of the thoracic and lumbar spine.
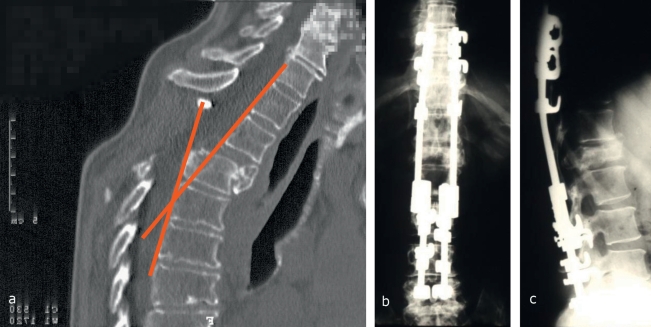
A variety of surgical methods are available to treat spinal metastases. Dorsal spinal decompression and stabilization can be considered the standard surgical technique to treat metastatic disease of the thoracic and lumbar spine (12). On the other hand, for cervical metastases, the leading method of treatment is clearly ventral decompression with corporectomy, vertebral body replacement, and ventral, stable-angle plate osteosynthesis. The main goals of the procedure are to reduce the volume of tumor and to resect the structures bordering the spinal canal dorsally (laminectomy and hemi-facetectomy) in order to prevent functional transection of the spinal cord (para- or tetraplegia). Its secondary goals are to stabilize the affected segment of the spine and to enable the patient to be mobilized without a corset. Discharge from the hospital should be possible in 10 to 14 days. Newly arisen (incomplete) para- or tetraplegia is an indication for emergency decompressive surgery. Impending or slowly progressive para- or tetraplegia, as well as segmental instability due to tumor infiltration into the dorsal edge of the vertebral body or into the pedicles, are likewise indications for emergency decompression and stabilization via a dorsal approach; the same is true when the spinal cord is compressed at more than one site. Decompression alone, without instrumentation, should be performed only in exceptional cases. The dorsal portion of the spinal column normally plays the role of a tension band that holds the spine upright; when it is not reconstructed, a markedly kyphotic postural abnormality of the affected motion segment(s) is the almost inevitable result (Figure 1).
Figure 1.
Spondylodesis with a hook-and-rod system
CT of the thoracic spine, revealing high-grade instability after laminectomy
Anteroposterior plain x-ray after repositioning with multisegmental dorsal spondylodesis employing a hook-and-rod system
The corresponding lateral plain x-ray
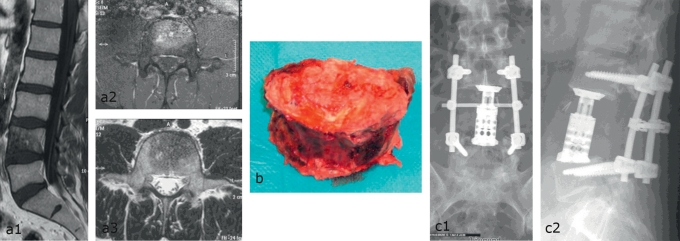
For patients with a solitary spinal metastasis who are in good general health and have a long life expectancy, the indicated procedure is ventral tumor resection (en bloc spondylectomy/total vertebrectomy) and primary stabilizing instrumentation (Figure 2). About 90% of metastatic tumor deposits are found in the ventral portion of the spine, and a ventral surgical approach therefore seems to be the most appropriate one. In some cases, neoadjuvant therapy may be needed beforehand to enable both the resection of the primary tumor and the proper treatment of the spinal metastasis. When the metastasis is derived from a primary tumor of a highly vascularized type, such as renal cell or thyroid carcinoma, preoperative embolization of the tumor vessels reduces blood loss, lowers surgical risk, and enables more precise dissection and more extensive resection of the tumor (e5). The anchoring of stabilizing metal implants to the vertebral bodies is more problematic from a ventral than from a dorsal approach, because the vertebral bodies consist mainly of spongiosa with relatively thin cortical bone, and because they are often osteoporotic as well. Improved spinal instrumentation is now available for the ventral approach to permit fixation for primary stabilization, so that patients can now be mobilized rapidly and without a corset. This is a major improvement in their quality of life. After (total or partial) vertebrectomy, the anterior column is not reconstructed with autologous bone, but rather with metal spacers, as the postoperative radiotherapy that will be needed to prevent tumor recurrence would also impair the fusion of any bony implant.
Figure 2.
Solitary intravertebral metastasis of breast carcinoma. Radical vertebrectomy and dorsoventral stabilization
Preoperative MR images showing exclusively intraosseous neoplasia: a1) sagittal plane, a2) and a3) axial planes
The resected vertebral body
Dorsal transpedicular stabilization and ventral vertebral body replacement: c1) anteroposterior and c2) lateral plain x-rays
Solitary spinal metastases.
For patients with a solitary spinal metastasis who are in good general health and have a long life expectancy, the indicated procedure is ventral tumor resection (en bloc spondylectomy/total vertebrectomy) and primary stabilizing instrumentation.
Vertebroplasty or kyphoplasty, i.e., the use of bone cement for the augmentation of vertebral bodies involved by metastatic disease, is a currently available minimally invasive technique for palliative treatment. As long as the tumor does not compress the adjacent neural structures, and painful destruction of the vertebral body is the main clinical concern, the placement of bone cement in the vertebral body can afford adequate segmental stabilization. The two techniques can also be combined with open decompression and dorsal stabilization when indicated. They have been shown to relieve pain and improve the quality of life, and they can be used as effective palliative treatment even for patients whose general condition is quite poor (13). On the other hand, their putative benefit with regard to spinal stability and neurological function, as well as their risks, have not yet been adequately documented by clinical studies.
Radiotherapy
The indications for percutaneous radiotherapy of spinal metastases are pain, the impending danger of fracture, and the impending danger of a neurological compression syndrome. Radiotherapy is also indicated postoperatively to prevent local recurrence. In conventional radiotherapy, the affected vertebral bodies are irradiated along with one or two vertebral bodies both cranial to the lesion and caudal to it. A modern linear accelerator apparatus is used. Radiotherapy of the cervical spine can be performed with lateral isocentric opposing fields in order to spare the larynx, trachea, and esophagus; the rest of the spine is usually irradiated with anterior-posterior-posterior-anterior (AP-PA) fields. Radiotherapy for spinal metastases has only rare and mild side effects, even when the individual doses are high (1, e6). In a patient collective with a median survival time of eight months (due to the totality of neoplastic disease, not just the spinal metastases), no late sequelae of radiotherapy are seen. Any surgically implanted stabilization material should be included in the target volume, because tumor cells may have been displaced intraoperatively together with this material during its implantation.
A substantial degree of pain relief is reported in 60% to 90% of cases, and total pain relief in 40% to 60% (14). This benefit with respect to pain takes effect 10 to 14 days after the beginning of radiotherapy in 70% of patients, and within three months in 90%.
Fractionation schemes.
A variety of fractionation schemes are used for the radiotherapy of spinal metastases, depending on the patient’s clinical manifestations and prognosis and on the goals of treatment.
The trabeculae that have been destroyed by the osteolytic process are replaced, at first, by connective tissue. Recalcification of osteolytic lesions can be observed two to three weeks after moderately dosed radiotherapy (20 to 30 Gy) and reaches its peak at two months (14). Occasionally, a drop of bone density by as much as 30% is seen immediately after radiotherapy and is then followed by a rapid rise of bone density. In contrast, healthy bone containing no tumor tissue does not manifest any change of mineral content after it has been irradiated (e6).
The reported rates of objective remission, defined as radiologically demonstrated recalcification one to six months after radiotherapy, are not fully comparable with one another because of methodological variations with respect to the precise measurements involved. They lie between 5% and 70%, with the highest remission rates reported for spinal metastases of cancer of the breast (62%), prostate gland (57%), lung (28%), and kidney (11%) (e7). Osteolytic lesions remineralize better in the truncal skeleton (about 60%) than in the limbs. The affected vertebral bodies may progressively lose height despite remineralization, because bone restitution takes place over a relatively long period of time. A bone scan can reveal evidence of objective remission (a decline of the pathologically high radionuclide uptake).
Effects of radiotherapy.
Radiotherapy is a highly effective standard technique for the treatment of spinal metastases because it helps relieve pain, prevent tumor recurrence, and promote recalcification of the affected vertebral bodies.
The currently available data on fractionated radiotherapy suggest that pain relief does not depend on the overall radiation dose, and that pain is relieved more rapidly if higher individual doses are administered. On the other hand, the antineoplastic effect and the secondary recalcification that follows it do, indeed, depend on the overall radiation dose. Individual doses should be no higher than 8 Gy, because higher doses can cause paraparesis. Fractionated radiotherapy is recommended if the goal of treatment is recalcification (which requires a high overall dose) in addition to pain relief. Multiple studies have documented the equivalent efficacy of various fractionation schemes with respect to the relief of pain (level I evidence) (e8– e10).
Rades et al. (15) performed a retrospective analysis of 1304 patients with spinal cord transection syndrome who underwent fractionated radiotherapy according to various different schemes (1 × 8 Gy, 5 × 4 Gy, 10 × 3 Gy, 15 × 2.5 Gy and 20 × 2 Gy), comparing their outcomes with respect to motor function, ability to walk, and in-field recurrences. There were no significant differences in outcome with respect to motor function or the regaining of the ability to walk. None of the fractionation schemes used were associated with any clinically significant acute or delayed radiotoxicity. The rate of in-field recurrences was found to be higher when the 1 × 8 Gy and 5 × 4 Gy schemes were used.
High-precision radiotherapeutic techniques.
If re-irradiation is needed when the patient has already undergone fractionated radiotherapy at a high overall dose, then the use of high-precision radiotherapeutic techniques should be considered in order to avoid late radiogenic injury.
If re-irradiation is needed when the patient has already undergone fractionated radiotherapy at a high overall dose, e.g., 10 × 3 Gy, then the use of high-precision radiotherapeutic techniques should be considered in order to protect the spinal cord optimally and avoid late radiogenic injury. Such techniques include, for example, extracranial stereotactic radiotherapy, radiosurgery (CyberKnife), dynamic-arc radiotherapy, and intensity-modulated radiotherapy (IMRT). In the clinical series of Ryu et al., 10 patients received conventional radiotherapy (10 × 2.5 Gy) as their initial treatment, followed by a radiosurgical (stereotactic) boost (e11). A substantial degree of pain relief was obtained by all 10 patients. Milker-Zabel et al. observed a clinically relevant response in 13 of 16 patients (81%) who had undergone re-irradiation with either IMRT or extracranial stereotactic radiotherapy (16). Gerszten et al. presented a retrospective series of 500 patients treated initially with CyberKnife radiosurgery (single-shot stereotactic irradiation at doses ranging from 12.5 to 25 Gy) (17). After a median follow-up interval of 21 months, 86% of patients still reported having much less pain than before their treatment.
Indications for the radiotherapy of bony metastases.
Radiotherapy is indicated as a treatment of bony metastases primarily for palliation in patients with a no more than moderately severe neurological deficit or multilocular involvement.
In summary, radiotherapy is indicated as a treatment of bony metastases primarily for palliation in patients with a no more than moderately severe neurological deficit or multilocular involvement. It can also be given postoperatively with curative intent after the incomplete resection of bony metastases. The response rates range from 60% to 90% and the indications are pain, chronically endangered stability, and the impending compression of neural tissue.
Pharmacotherapy
The level of activity of bone metastases is often correlated with the local or overall tumor mass, and, therefore, systemic treatment should always be considered in addition to local treatment. The basic principles of systemic treatment are as follows:
If the metastases are located exclusively in bone, then monotherapy with a well-tolerated drug is the treatment of choice.
For hormone-sensitive tumors, e.g., breast cancer, endocrine therapy should be considered.
When other organs aside from bone are involved as well, polychemotherapy is a reasonable option.
The goals of treatment.
The relief of pain, if present, is a major consideration in the treatment of spinal metastases.
The relief of pain, if present, is a major consideration in the treatment of spinal metastases. In general, pain due to bony metastases that are of the nociceptive type respond well to analgesic therapy according to the WHO algorithm and are particularly responsive to opiates (18). Spinal metastases, however, often also cause neuropathic pain with a radicular component (sensory deficit, burning pain, painful shock-like sensations). This type of pain requires the additional administration of anticonvulsants (e.g., gabapentin) and perhaps antidepressants as well (e.g., amitriptyline, doxepine) (1, e2, e3). In spinal cord compression syndromes, not only surgical intervention, but also the administration of glucocorticoids is of prime clinical importance. Steroids relieve the pain of spinal cord compression and reduce spinal cord edema through a mechanism involving prostaglandin inhibition. The initial dose must be high enough to be effective (e.g., 16 to 32 mg of dexamethasone per day); as a rule, the steroid dose can be gradually lowered starting four days after the initiation of treatment (19).
Bone-specific systemic treatment with bisphosphonates is a further important component of the treatment of bony metastases (20). Bisphosphonates inhibit bone resorption and thus exert a major beneficial effect in bone that is involved by tumor. They are effective in the treatment of bony metastases that are osteolytic, osteoplastic, or of a mixed nature. Studies currently in progress are addressing the question of their potential role as adjuvant therapy in an early stage of neoplastic disease before any bone metastases have been detected (21).
Bisphosphonates.
Bisphosphonates are an integral component of the current treatment of spinal metastases.
Many different bisphosphonates are available, and their antiresorptive activity varies. A distinction should be drawn between orally and parenterally administered bisphophonates. Even if only 5% of an orally administered bisphosphonate is actually absorbed, it can nonetheless bring about a good clinical result. Intravenously administered bisphosphonates have a more rapid onset of effect and are stored in bone for as long as 10 years.
The benefit of treating bony metastases with bisphosphonates is generally that they prevent skeletal complications. In women with metastatic breast cancer, oral zoledronate treatment lowers the frequency of bone-related events by about 40% and also brings about a significant degree of pain relief, a stabilization of physical performance ability, and an improved quality of life (20).
Although bisphosponates are generally well tolerated, a few possible side effects should be borne in mind. Orally administered bisphosphonates can cause gastrointestinal symptoms such as dyspepsia and eso-phagitis, as well as hypocalcemia (particularly in combination with aminoglycosides). Parenterally administered bisphosphonates, in particular, can impair renal function, especially when they are given as a rapid bolus in a small volume of fluid. An acute phase reaction can occur, as can osteonecrosis of the jaw, which occurs in as many of 1% of all patients. Jaw osteonecrosis is correlated with a number of other factors including mechanical injury through dental procedures, inflammatory states such as parodontosis, and nicotine and alcohol abuse.
Potential adverse effects of biphosphonates.
Oral adminstration: gastrointestinal symptoms such as dyspepsia and esophagitis
Parenteral administration: impaired renal function
Clinical trials have been carried out recently to evaluate the potential role of denosumab, a RANKL antibody, in the treatment of bone metastases, including spinal metastases, from various types of primary tumor. Denosumab interrupts the RANKL/RANK signaling pathway between osteoblasts and osteoclasts and thereby lessens the breakdown of bone. In a randomized phase III trial involving patients with breast cancer metastatic to bone, this antibody lowered the rate of fracture and osteolysis to a greater extent than bisphosphonates did (22). The frequency of these skeletal complications was significantly lower (hazard ratio [HR] 0.77; p = 0.001), and the time to the occurrence of the first event was also significantly longer (HR 0.82; p = 0.01). Similar findings (a lower overall frequency of bony complications and a prolonged time to the first event) were also made in patients with prostate carcinoma metastatic to bone, and with multiple myeloma (23). Denosumab was recently approved in the European Union for the treatment of loss of bone density secondary to antihormonal therapy (androgen deprivation) in men with prostate cancer who are at elevated risk of fractures. This was done mainly in consequence of the findings of a randomized double-blind phase III study that included 734 men who were receiving anti-androgenic therapy for prostate carcinoma. After two years of treatment with denosumab, their bone density increased by 5.6%, while that of patients receiving placebo instead of denosumab declined by 1.0% (1, e4). Moreover, only 1.5% of the patients in the denosumab group developed vertebral body fractures, compared to 3.9% in the placebo group (relative risk reduction, 62%). In the next few years, further study will be devoted to the long-term therapeutic effects and adverse effects of this interesting new class of agents for the treatment of bony metastases, including spinal metastases, from various types of primary tumor.
Figure 3.
Kyphoplasty of the L3 vertebral body after a pathological fracture due to metastatic breast cancer
Lateral plain film showing involvement of the L3 vertebral body by metastatic breast cancer with a pathological impression fracture of the superior endplate without involvement of the posterior edge of the vertebra
MRI of the same finding shown in a)
and d) Plain x-rays and e) CT showing the result after vertebral body augmentation with bone cement (kyphoplasty)
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 13/2011. The CME unit “Neonatal Screening for Metabolic Diseases and Endocrinopathies” (issue 1–2/2011) can be accesed until 21 February 2011. For issue 9/2011 we plan to offer the topic, “Central Venous Port Systems as an Integral Part of Chemotherapy”
Solutions to the CME questionnaire in issue 49/2010:
Trobisch P, Suess O, Schwab F: Idiopathic Scoliosis.
Answers: 1c, 2b+c, 3b, 4d, 5b, 6e, 7a, 8c, 9b, 10d
Answers b) and c) to question 2 are both correct.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the 2-year survival rate of patients with spinal metastases of lung cancer?
5%
9%
13%
17%
21%
Question 2
How extensively must a vertebral body be destroyed by a metastatic tumor for an abnormality to be visible in a plain x-ray?
5%
5–10%
20–30%
30–50%
> 70%
Question 3
Which of the following is an important consideration regarding the treatment of spinal metastases with orthoses?
External stabilization should be performed at the lumbosacral junction.
Orthoses should be used as often as possible, because the risk of fracture due to spinal metastases is high.
The spine should be put in an extended position (reclination) in order to redirect forces dorsally.
The indication for an orthosis is independent of the patient’s general state of health.
Treatment with an orthosis is indicated only when the spinal metastasis is a primary tumor.
Question 4
According to the Tokuhashi scoring system, under which of the following circumstances should spinal metastases be radically resected?
Incomplete spinal cord damage, primary tumor of the uterus, organ metastases unresectable, 2 spinal metastases, no extraspinal bony metastases, poor state of general health
No spinal cord damage, primary tumor of the kidney, resectable organ metastases present, 2 spinal metastases, 4 extraspinal bony metastases, poor state of general health
Complete spinal cord damage, primary tumor of the esophagus, no organ metastases, 3 spinal metastases, one extraspinal bony metastasis, fair state of general health
Incomplete spinal cord damage, primary tumor of the lung, resectable organ metastases present, 4 spinal metastases, more than 3 extraspinal bony metastases, good state of general health
No spinal cord damage, primary tumor is breast carcinoma, no organ metastases, one vertebral body metastasis, 2 extraspinal bony metastases, fair state of general health
Question 5
Which of the following procedures demonstrably relieves pain and improves quality of life in patients with certain kinds of spinal metastasis, and can also be used for palliation in patients whose general condition is poor?
Synovectomy
Kyphoplasty
Laminectomy
Osteomedullography
Corporectomy
Question 6
What is the maximum allowable single dose in the radiotherapy of spinal metastases?
4 Gy
5 Gy
6 Gy
7 Gy
8 Gy
Question 7
Which diagnosis is a criterion of exclusion for the administration of bisphosphonates?
Osteolytic bony metastases
Pathological osteoporotic fracture
Marked hypocalcemia
Osteoplastic spinal metastasis
Pathological vertebral body fracture due to metastasis
Question 8
What are typical complications of bisphosphonate therapy?
Pancreatitis, cholangiitis, hepatitis
Cystitis, prostatitis, nephrolithiasis
Arterial hypertension, CHD, hyperlipidemia
Dyspepsia, esophagitis, hypocalcemia, osteonecrosis of the jaw
Dysphagia, colitis, hyperkalemia
Question 9
What percentage of patients with spinal metastases are completely relieved of pain by radiotherapy?
10–20%
20–40%
40–60%
60–90%
90–100%
Question 10
Which of the following symptoms of spinal metastases in elderly men are often misinterpreted as being due to an enlarged prostate gland?
Bladder and bowel dysfunction
Reflux esophagitis
Biliary colic
Sciatica
Pelvic inflammation
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin: A prospective study of a diagnostic strategy. J Bone Joint Surg [Am] 1993;75:1276–1281. doi: 10.2106/00004623-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Rades D, Fehlauer F, Veninga T, et al. Functional outcome and survival after radiotherapy of metastatic spinal cord compression in patients with cancer of unknown primary. Int J Radiat Oncol Biol Phys. 2007 Feb 1;67(2):532–537. doi: 10.1016/j.ijrobp.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 4.Ulmar B, Huch K, Kocak T, Catalkaya S, Naumann U, Gerstner S, Reichel H. The prognostic influence of primary tumour and region of the affected spinal segment in 217 surgical patients with spinal metastases of different entities. Z Orthop Ihre Grenzgeb. 2007 Jan–Feb;145(1):31–38. doi: 10.1055/s-2007-960506. [DOI] [PubMed] [Google Scholar]
- 5.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15(11):1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Tomita K, Kawahara N, Kobayashi T, et al. Surgical stategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy DR, Mantyh PW. Skeletal Complications of Malignancy Bone Cancer Pain. Clin Orthop Rel Res. 2002;415:279–288. [Google Scholar]
- 8.Lecouvet FE, Geukens D, Stainier A, et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007 Aug 1;25(22):3281–3187. doi: 10.1200/JCO.2006.09.2940. [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Lichter AS. A caution about the use of MRI to diagnose spinal cord compression. N Engl J Med. 1990;322(8):556–557. doi: 10.1056/NEJM199002223220817. [DOI] [PubMed] [Google Scholar]
- 10.Datir A, Pechon P, Saifuddin A. Imaging-guided percutaneous biopsy of pathologic fractures: a retrospective analysis of 129 cases. AJR Am J Roentgenol. 2009;193(2):504–508. doi: 10.2214/AJR.08.1823. [DOI] [PubMed] [Google Scholar]
- 11.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66(2):143–146. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 12.Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58(4):245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 13.Lemke DM, Hacein-Bey L. Metastatic compression fractures—vertebroplasty for pain control. J Neurosci Nurs. 2003;35(1):50–55. [PubMed] [Google Scholar]
- 14.Adamietz IA, Feyer P. Bamberg M, Molls M, Sack H, editors. Radioonkologie Klinik. Palliative Radiotherapie. Zuckschwerdt W, Verlag GmbH. 2009:1065–1106. [Google Scholar]
- 15.Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366–3375. doi: 10.1200/JCO.2005.04.754. [DOI] [PubMed] [Google Scholar]
- 16.Milker-Zabel S, Zabel A, Thilmann C, et al. Clinical results of re-treatment of vertebral bone metastases by stereotactic conformal radiotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55(1):162–167. doi: 10.1016/s0360-3016(02)03864-6. [DOI] [PubMed] [Google Scholar]
- 17.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 18.WHO (1996) World Health Organisation. Geneva: Cancer pain relief, with a guide to opiod availability. [Google Scholar]
- 19.Klaschik E. Husebo S, Klaschik E, editors. Schmerztherapie und Symptomkontrolle in der Palliativmedizin. Palliativmedizin, Grundlagen und Praxis. Springer Verlag. (4th edition) 2006:203–307. [Google Scholar]
- 20.Schmid P, Possinger K. Supportive Therapie von Knochenmetastasen. Bremen, London, Boston: Uni-Med Verlag; 2005. Pharmakologie der Bisphosphonate; pp. 78–88. [Google Scholar]
- 21.Neville-Webbe HL, Coleman RE. Bisphosphonates and RANK ligand inhibitors for the treatment and prevention of metastatic bone disease. Eur J Cancer. 2010;46:1211–1222. doi: 10.1016/j.ejca.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Stopeck A, de Boer R, Fujiwara Y, et al. Cancer Res 2009; 69. (Suppl. 24) abstract 22. [Google Scholar]
- 23.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- e1.Siegal T, Siegal T. Current considerations in the managment of neoplastic spinal cord compression. Spine. 1989;14:223–228. doi: 10.1097/00007632-198902000-00015. [DOI] [PubMed] [Google Scholar]
- e2.Enkaoua EA, Doursounian L, Chatellier G, Mabesoone F, Aimard T, Saillant G. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine. 1997;22(19):2293–2298. doi: 10.1097/00007632-199710010-00020. [DOI] [PubMed] [Google Scholar]
- e3.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005 Aug 20-26;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- e4.Ghogawala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001;26(7):818–824. doi: 10.1097/00007632-200104010-00025. [DOI] [PubMed] [Google Scholar]
- e5.Hess T, Kramann B, Schmidt E, Rupp S. Use of preoperative vascular embolisation in spinal metastasis resection. Arch Orthop Trauma Surg. 1997;116(5):279–282. doi: 10.1007/BF00390053. [DOI] [PubMed] [Google Scholar]
- e6.Koswig S, Budach V. Remineralization and pain relief in bone metastases after different radiotherapy fractions (10 times 3 Gy vs 1 time 8 Gy). A prospective study. Strahlenther Onkol. 1999;175:500–508. doi: 10.1007/s000660050061. [DOI] [PubMed] [Google Scholar]
- e7.Rieden K, Adolph J, Lellig U, Winkel K. The radiotherapeutic effect on bone metastases in relation to the frequency of metastases, sites of metastases and histology of the primary tumor. Strahlenther Onkol. 1989;165(5):380–385. [PubMed] [Google Scholar]
- e8.Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55(3):594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- e9.Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy — a systematic review of randomised trials. Clin Oncol. 2003;15(6):345–352. doi: 10.1016/s0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- e10.Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- e11.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- e12.Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic syndromes. Drugs. 2000;60:1029–1052. doi: 10.2165/00003495-200060050-00005. [DOI] [PubMed] [Google Scholar]
- e13.Sindrup SH, Jensen TS. Antidepressants in the treatment of neuropathic pain: Neuropathic pain: pathophysiology and treatment. Progr Pain Res Manage. In: Hansson PT, Fields RG, Hill RG, Marchetti P, editors. Vol. 21. Seattle: IASP Press; 2001. pp. 169–183. [Google Scholar]
- e14.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Tokuhashi Y, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- e16.Adamietz IA, Feyer P. Palliative Radiotherapie: Radioonkologie - Klinik W. In: Bamberg M, Molls M, Sack H, editors. Zuckschwerdt Verlag. 2nd edition. München Wien New York; 2009. pp. 1065–1106. [Google Scholar]