Abstract
Corticosterone (CORT) release from the adrenal glands in response to acutely stressful stimuli is well-characterized, however several non-experimental, environmental stressors can also engender a CORT response. The aim of this study was to investigate an acute activation of the HPA axis in pair-housed animals in response to separation. We observed a rapid significant increase in CORT in the animal remaining in the home cage following cage mate removal, that was not caused by cage opening and transient removal of cage mate. In addition, we examined this response in both control, non-stressed animals and in animals subjected to chronic variable stress (CVS) and found that although basal levels of CORT differed between control and CVS animals, there was no significant difference in the acute CORT levels between the control and CVS animals after separation, indicating that this environmental event is perceived as acutely stressful in both conditions. Furthermore, we examined the time course of CORT activation and found that CORT levels rapidly rise within minutes of separation peaking at 15 minutes and returning to baseline by 90 minutes. The results of this study demonstrate that separation can induce an acute stress response in the remaining cage mate measured by increased CORT and should be considered in molecular, behavioral, and electrophysiological studies.
Keywords: Cohort Removal, Corticosterone, Stress, Pair-housed
1. Introduction
Alterations to the social environment of the laboratory rat can have a significant influence on the welfare of the individual cage-mates. Previous research has shown that non-invasive laboratory procedures such as cage cleaning, or handling and transport, which briefly disrupts the social environment of the cage, results in neurochemical, hormonal, and behavioral alterations typically seen in response to an acute stress exposure [1, 11, 12, 14]. Activation of the hypothalamic-pituitary-adrenal (HPA) axis, a key element of the acute stress response, involves the release of corticosterone (CORT) from the adrenal cortex that circulates throughout the body exerting action both peripherally and centrally [reviewed in 18]. Time course studies reveal that acute exposure to stressful stimuli results in an increase in CORT within minutes of the stressor exposure both peripherally [3, 28] as well as in brain structures like the hippocampus and prefrontal cortex [10, 12].
In the brain, the effects of CORT are mediated by two types of intracellular receptors: the mineralcorticoid receptor (MR) and the glucocorticoid receptor (GR) [18]. Activation of these intracellular receptors by CORT causes nuclear translocation of the receptor and the initiation of genomic events that lead to long-lasting neurophysiological changes in these areas [2]. In addition, emerging evidence suggests that GRs are capable of exerting rapid neuronal effects within minutes of exposure to CORT, suggesting a non-genomic mechanism via the activation of novel membrane-bound GRs in the hippocampus [19, 26, 31] and the hypothalamus [13]. Therefore, environmental disturbances that result in a rapid increase in CORT may mediate significant changes within the brain structures that cannot be directly attributed to the experimental manipulation.
One such environmental change is the sequential removal of rodents from a group-housed cage. Several studies have shown that cohort removal generates an acute physiological reaction known as stress-induced hypothermic response [6, 21]. In addition, behavioral changes have been observed in response to cohort removal including increases in anxiety-like behavior and increases in agonistic behavior [8]. Furthermore, neuropeptides associated with activation of the limbic system demonstrate an increase in response to separation including prolactin [29], somatostatin [7], and most importantly two hormonal markers of HPA activation, adrenal corticotrophin hormone (ACTH) and CORT [8, 30].
Increased CORT levels have been reported in response to long-term (< 24 hours) pair separation in several species that form monogamous male-female pair bonds, including Siberian dwarf hamsters [9] and zebra finches [25]. In addition, a rapid increase in HPA axis activation was observed upon separation of same-sex social partners in cattle [5], marmosets [23] as well domestic chickens [15]. Together, these studies suggest that pair separation is stressful and the stressful response is evolutionarily conserved across species that exhibit social behavior.
A critical component of research involving stress and the effects of CORT is the comparison of results found under application of the stressor (high CORT) to control, stress-free (low CORT) conditions. The goal of this study was to examine if there is a disparity in HPA activation between control animals and animals subjected to chronic variable stress (CVS), a rodent model of chronic stress, and to determine the time course of activation of the HPA axis in pair-housed animals sequentially removed from their cage. In addition, although it has been established that group-housed cohort removal results in an increase in CORT levels in non-stressed control animals, to our knowledge, the immediate CORT response and time course following separation of pair-housed rats has not been reported. Therefore, we aimed to assess the time course of CORT activity in the periphery in response to separation by increasing the interval between cage mate removal and the measurement of plasma CORT.
2. Methods
2.1. Animals
Male Wistar rats (42 days of age upon arrival) were purchased from Harlan Inc. (Indianapolis, IN). On arrival, animals were housed in pairs on a 12:12 light/dark cycle (lights on at 0700 hours) and received food and water ad libitum. All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tulane University Institutional Animal Care and Use Committee.
2.2. Experiment 1 Cage Mate Removal
For experiments involving chronic variable stress (CVS), animals were allowed 14 days to habituate in the vivarium. At 56 days of age, animals were randomly assigned to CVS and Control (non-stressed) conditions. CVS was conducted using a modified method previously reported [17]. Briefly, the CVS paradigm consisted of twice-daily exposure to randomly assigned stressors applied over 14 consecutive days. Morning stressors were administered between 0830h and 1130h, and afternoon stressors were administered between 1330h and 1630h. Overnight stressors (social isolation or social crowding) began immediately after cessation of the afternoon stressor and concluded with the start of the next day’s morning stressor. CVS stressors consisted of warm swim (20 min at 31-33°C), cold swim (10 min at 16-18°C), cold room (1 hr at 4°C, two rats per cage without bedding), rotation (1 hr at 100 rpm), social isolation (overnight, one rat per cage), and social crowding (overnight, 6 rats per cage). With the exception of the overnight stressors, the daily stressors were applied in a semi-randomized manner with each stressor being assigned equally over the 14 days. Control animals were handled and weighed daily. For sacrifice, animals were transported from the vivarium to the lab and allowed two hours to settle prior to experimental manipulation. The first animal in each cage was removed and rapidly decapitated and trunk blood collected 10 minutes prior to the sacrifice of the second animal. Animals were killed between 0900h and 1000h, when CORT levels are lowest.
2.3. Experiment 2 Corticosterone Time Course
For experiments investigating the time course of CORT activation during sacrifice, animals were pair-housed for 14 days on arrival and handled daily until sacrifice. At 56 days of age, animals were killed as described above by rapid decapitation and trunk blood was collected for CORT determination. The second animal in each cage was sacrificed either 0, 5, 15, 30, or 90 minutes after the removal and sacrifice of the first animal in each cage. To control for the effect of cage opening on the CORT response, an additional control group was added in which the cage was opened, one animal was picked up, and then immediately returned to the cage. Both animals in this group were then killed 15 mins later. Animals were sacrificed between 0900h and 1000h, when CORT levels are lowest.
2.4. Corticosterone Radioimmunoassay
For CORT measurements, trunk blood was allowed to coagulate at room temperature for 90 minutes. Samples were centrifuged at 2000 × g for 15 min, serum collected and samples stored at −20°C. Samples were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for corticosterone determination by 125I Corticosterone radioimmunoassay. The average limit of detection was 10.5 ng/ml. To provide the most conservative estimate for statistical comparisons, samples in which hormone levels were below the detectable limit (n=3 for corticosterone) were set to the individual detection limits of each assay. Sample values were calculated from known standards run within each assay, with an average intra-assay variation (%CV) of 3.8%. Inter-assay variation was 7.6%.
2.5. Statistical Analysis
Statistics were performed using Graph Pad Prism 5 one-way analysis of variance (ANOVA) with subsequent Tukey post-hoc test to determine significance. Unpaired t-tests were performed where appropriate. A p value of less than 0.05 was considered to indicate a significant difference.
3. Results
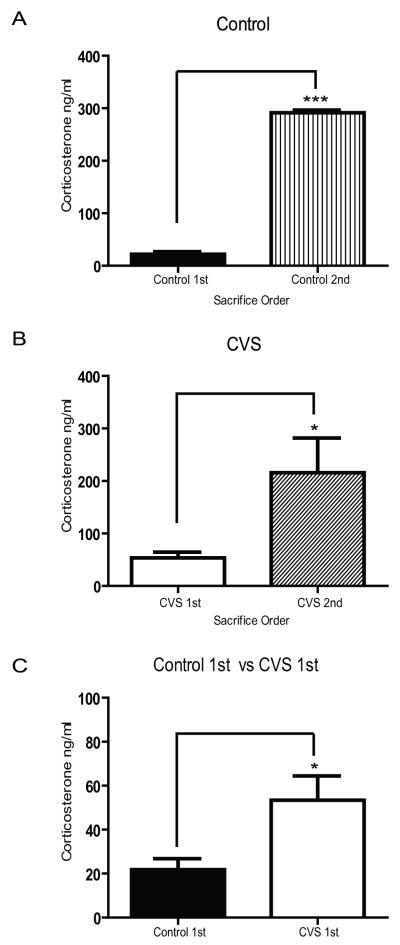
In order to determine the activation profile of the HPA axis through the measurement of circulating CORT in pair-housed rats, we removed trunk blood and measured CORT under basal conditions in a subset of the separated animals 10 minutes before the CORT measurement in the remaining cage mate. As shown in Figure 1A, a significant increase in plasma CORT levels was observed in the second animal remaining in each cage under control conditions (n=4, 291.7 ± 4.8 ng/nl) compared to the first animal (n=8, 21.8 ± 5.0 ng/nl), (p<0.0001). A similar pattern was observed in CVS animals (Fig. 1B) with the remaining cage mate demonstrating significantly higher levels of CORT (n=5, 215.7 ± 66.2 ng/nl) compared to the first animal (n=8, 53.4 ± 11.1 ng/nl), (p<0.05). As was previously shown [17, 24], chronically stressed animals exhibited significantly higher basal circulating levels of CORT over control animals (Fig. 1C, p<0.05). However, there was no significant difference in the high CORT levels between control and CVS animals from the remaining cage mate at the 10 minute time point (p=0.36). This suggests that despite the discrimination in basal CORT tone observed between control and CVS animals, the remaining cage mate demonstrated acutely elevated levels of circulating CORT regardless of the experimental condition. Furthermore, these results show that separation in pair-housed animals represents an acutely stressful situation.
Figure 1.
Effects of sequential removal of animals on plasma corticosterone in control (A) or CVS (B) pair-housed animals. Basal corticosterone levels (C) were significantly different between control and CVS exposed animals. *p<0.05, ***p<0.001.
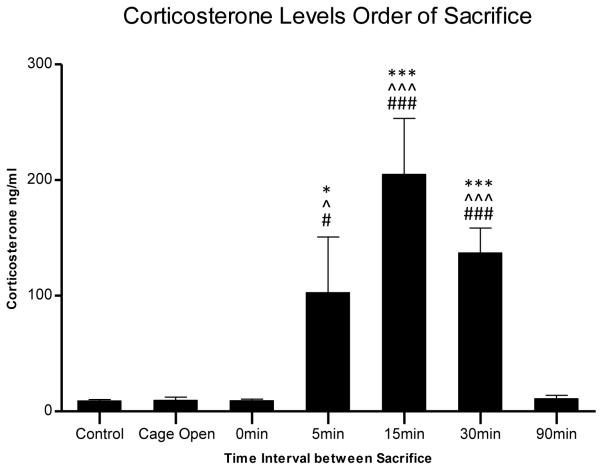
To determine the time course of CORT activation in pair-housed animals, control non-stressed animals were housed in pairs for 14 days. The animals were separated and CORT was measured at 0 min, 5 min, 15 min, 30 min or 90 min intervals following the separation of control animals. Figure 2 shows that CORT levels remained at basal levels for both control animals (n=4, 9.21 ± 1.7 ng/nl) and their cage mates that were simultaneously removed (time point 0; n=4, 10.29 ± 1.1 ng/nl). As expected, following separation a significant increase in CORT was observed in the remaining animals at the 5 min interval (time point 5; n=3, 102.7 ± 48.1 ng/nl), with a peak at the 15 min interval (time point 15; n=4, 205.0 ± 48.3 ng/nl) and remaining significantly elevated through the 30 min interval [(time point 30; n=4, 136.9 ± 21.6 ng/nl), p<0.001, F(6,30)=17.07, ANOVA). Animals removed at the 90 min time interval demonstrated CORT levels similar to both controls and the 0′ time point (time point 90; n=4, 10.9 ± 3.0 ng/nl), suggesting that CORT levels return to baseline by this time point. The lack of difference in CORT levels between control animals and time point 0 min cage mates suggests that concurrent removal and sacrifice of cage mates prevents HPA activation observed at the 5 min, 15 min, and 30 min time point delays. An additional group was added to control for the effect of cage opening and transient removal. For this group, the cage was opened and one animal was picked up and immediately returned to the cage. Both animals were killed 15 min later, the time point in which we observed the peak in CORT levels with cage mate separation. However, as Figure 2 shows these animals demonstrated CORT levels (n=4, 9.6 ± 2.7 ng/nl) similar to both control and time point 0 min animals following cage opening suggesting that the observed increases in CORT levels seen at the 5 min, 15 min and 30 min time point intervals are a result of cage mate separation and not due to the environmental disturbance to the cage.
Figure 2.
Time course of plasma corticosterone activation in pair-housed animals between the removal of the first animal (control) to the second animal removed at variable time point intervals (0 min, 5 min, 15 min, 30 min, and 90 min). *p<0.05, ***p<0.001 compared to the first animal removed in each pair (control); ^p<0.05, ^^^p<0.001 compared to cage opened control animals; #p<0.05, ###p<0.001 compared to 0 min time interval animals.
4. Discussion
The present study aimed to evaluate the effects of separation of pair-housed rats on the plasma CORT response. Our study revealed that the removal of a cage mate in pair-housed male Wistar rats resulted in a rapid elevation of CORT in the remaining animal. It is well established that chronic stress results in an alteration of HPA axis feedback and subsequently high circulating plasma levels of CORT [17, 24]. We observed the effect of separation on CORT in both CVS and non-stressed control animals suggesting that this effect is independent of HPA dysregulation normally seen in chronically stressed animals. In addition, we also report here the time course of CORT activation following separation. We observed that CORT levels in the animal remaining in the cage significantly rise to acute stress levels following cage mate removal peaking at 15 minutes and returning to baseline by 90 minutes. This effect is mediated by separation and not simply cage opening and transient removal as the CORT levels in these animals were at non-stressed baseline CORT levels similar to control animals. The duration of the specific stress response caused by cage mate removal is transient, as CORT levels return to baseline within 90 minutes after cage mate removal. This result contrasts with a prolonged CORT response during 90 minutes of restraint stress that return to baseline within 90 minutes after cessation of the restraint [3, 16, 28]. The return to baseline is most likely mediated by the feedback inhibition of the HPA axis after stressor removal.
Activation of the HPA axis and the ultimate release of CORT from the adrenal glands demonstrate a broad feedback system throughout the body acting on receptors both peripherally and centrally [18]. The binding of CORT to its receptors triggers a cascade of events with a multitude of neurobiological and behavioral changes in response to acute stress levels of CORT such as those observed in this study [4, 20, 22]. Recent studies have demonstrated that in addition to the rise in plasma CORT, several limbic structures such as the hippocampus and hypothalamus can exhibit a rapid rise in CORT within minutes of the onset of an acute stressor [10, 12]. These rapid rises in CORT can activate non-genomic effects mediated through the membrane GR. Although few studies have investigated the specific actions of this non-classical GR, evidence suggests that CORT is capable of engendering functional and behavioral effects through the activation of this receptor. In slices of the paraventricular nucleus of the hypothalamus, activation of a putative membrane GR exerts rapid inhibitory effects including the suppression of presynaptic glutamate release and the facilitation of GABA inputs [13]. Additionally, application of CORT to hippocampus slices causes an increase in glutamate synaptic inputs to CA1 pyramidal cells [19]. Long-term potentiation (LTP), the cellular correlate of learning and memory, also demonstrates rapid enhancement in response to application of CORT, an effect that was not blocked with the use of antagonists to intracellular glucocorticoid receptors [32]. Taken together with the results of this study demonstrating an acute rise in CORT in response to cohort removal, it is possible that molecular and electrophysiological differences, particularly those directly related to GR activation, could be inadvertently confounded by cohort removal during sacrifice. Furthermore, to avoid a possible confound of cohort removal during experimental procedures, the results of this study suggest that pair-housed rats should be concomitantly sampled to avoid HPA activation and subsequent increase in circulating CORT levels.
In addition to the neurophysiological effects of acute alterations in CORT, behavioral effects including increased locomotor activity in a novel environment [27] and reversed serial memory retrieval pattern on a hippocampus-dependent memory task [10] have been demonstrated in rodents with rapid increases in CORT. Furthermore, recent evidence from Roozendaal suggests that the effects of stress on memory enhancement are mediated by the rapid actions of the membrane GR [26]. The results of this experiment strongly suggest that the stress of cohort removal and the potential behavioral effects that result should be considered when interpreting the results of behavioral experiments. Furthermore, an important component of experiments examining the behavioral effects of chronic stress application is that they operate under the assumption of basal, non-stress levels of CORT in control animals. The rise in CORT in both control and chronically stressed animals following cohort removal suggests that the experimenter could observe acute behavioral effects directly related to the elevated CORT from cage-mate removal and not due to the effects of experimental manipulation, leading to difficulty in result interpretation. We acknowledge that the experimental design we employed involved the transport of all the animals to another room 2 hours prior to experimental manipulation, a process which in itself could be considered an acute stressor. However this scenario is unavoidable for many laboratories particularly given the size and space requirements for many behavioral paradigms. Therefore understanding the HPA reactivity and response time course to these conditions is important to appropriate experimental design.
Conclusions
The results of this study suggest that the effects of a minor stressor, such as the removal of a cage mate, can have profound effects on the activation of the HPA axis in both chronically stressed animals and control animals, including a rapid rise in plasma CORT levels. Taken together with what is known about the rapid effects of CORT, the central effect of this CORT response could lead to unexpected changes within the brain and have direct implications for electrophysiological, behavioral, and molecular studies. These results and the increase in CORT caused by cage mate removal should be considered in both behavioral as well as molecular studies.
Acknowledgements
The authors would like to thanks Dan Liu and Emily Evans for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- [2].Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- [3].Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- [4].Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- [5].Boissy A, Le Neindre P. Behavioral, cardiac and cortisol responses to brief peer separation and reunion in cattle. Physiol Behav. 1997;61:693–699. doi: 10.1016/s0031-9384(96)00521-5. [DOI] [PubMed] [Google Scholar]
- [6].Borsini F, Lecci A, Volterra G, Meli A. A model to measure anticipatory anxiety in mice? Psychopharmacology (Berl) 1989;98:207–211. doi: 10.1007/BF00444693. [DOI] [PubMed] [Google Scholar]
- [7].Brodin E, Rosen A, Schott E, Brodin K. Effects of sequential removal of rats from a group cage, and of individual housing of rats, on substance P, cholecystokinin and somatostatin levels in the periaqueductal grey and limbic regions. Neuropeptides. 1994;26:253–260. doi: 10.1016/0143-4179(94)90079-5. [DOI] [PubMed] [Google Scholar]
- [8].Burman O, Owen D, Abouismail U, Mendl M. Removing individual rats affects indicators of welfare in the remaining group members. Physiol Behav. 2008;93:89–96. doi: 10.1016/j.physbeh.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [9].Castro WL, Matt KS. Neuroendocrine correlates of separation stress in the Siberian dwarf hamster (Phodopus sungorus) Physiol Behav. 1997;61:477–484. doi: 10.1016/s0031-9384(96)00456-8. [DOI] [PubMed] [Google Scholar]
- [10].Chauveau F, Tronche C, Pierard C, Liscia P, Drouet I, Coutan M, Beracochea D. Rapid stress-induced corticosterone rise in the hippocampus reverses serial memory retrieval pattern. Hippocampus. 2009 doi: 10.1002/hipo.20605. [DOI] [PubMed] [Google Scholar]
- [11].Crockett CM, Bowers CL, Shimoji M, Leu M, Bowden DM, Sackett GP. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. J Comp Psychol. 1995;109:368–383. doi: 10.1037/0735-7036.109.4.368. [DOI] [PubMed] [Google Scholar]
- [12].Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [13].Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- [14].Dobrakovova M, Jurcovicova J. Corticosterone and prolactin responses to repeated handling and transfer of male rats. Exp Clin Endocrinol. 1984;83:21–27. doi: 10.1055/s-0029-1210308. [DOI] [PubMed] [Google Scholar]
- [15].Feltenstein MW, Lambdin LC, Webb HE, Warnick JE, Khan SI, Khan IA, Acevedo EO, Sufka KJ. Corticosterone response in the chick separation-stress paradigm. Physiol Behav. 2003;78:489–493. doi: 10.1016/s0031-9384(03)00030-1. [DOI] [PubMed] [Google Scholar]
- [16].Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72:114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- [17].Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- [18].Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [19].Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- [21].Lecci A, Borsini F, Volterra G, Meli A. Pharmacological validation of a novel animal model of anticipatory anxiety in mice. Psychopharmacology (Berl) 1990;101:255–261. doi: 10.1007/BF02244136. [DOI] [PubMed] [Google Scholar]
- [22].Meller E, Shen C, Nikolao TA, Jensen C, Tsimberg Y, Chen J, Gruen RJ. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64. doi: 10.1016/s0006-8993(03)02866-x. [DOI] [PubMed] [Google Scholar]
- [23].Norcross JL, Newman JD. Effects of separation and novelty on distress vocalizations and cortisol in the common marmoset (Callithrix jacchus) Am J Primatol. 1999;47:209–222. doi: 10.1002/(SICI)1098-2345(1999)47:3<209::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [24].Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007;146:1734–1742. doi: 10.1016/j.neuroscience.2007.03.027. [DOI] [PubMed] [Google Scholar]
- [25].Remage-Healey L, Adkins-Regan E, Romero LM. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm Behav. 2003;43:108–114. doi: 10.1016/s0018-506x(02)00012-0. [DOI] [PubMed] [Google Scholar]
- [26].Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sandi C, Venero C, Guaza C. Novelty-related rapid locomotor effects of corticosterone in rats. Eur J Neurosci. 1996;8:794–800. doi: 10.1111/j.1460-9568.1996.tb01264.x. [DOI] [PubMed] [Google Scholar]
- [28].Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seggie J, Brown GM. Twenty-four-hour resting prolactin levels in male rats: the effect of septal lesions and order of sacrifice. Endocrinology. 1976;98:1516–1522. doi: 10.1210/endo-98-6-1516. [DOI] [PubMed] [Google Scholar]
- [30].Trullas R, Havoundjian H, Zamir N, Paul S, Skolnick P. Environmentally-induced modification of the benzodiazepine/GABA receptor coupled chloride ionophore. Psychopharmacology (Berl) 1987;91:384–390. doi: 10.1007/BF00518197. [DOI] [PubMed] [Google Scholar]
- [31].Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- [32].Wiegert O, Joels M, Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn Mem. 2006;13:110–113. doi: 10.1101/lm.87706. [DOI] [PubMed] [Google Scholar]