INTRODUCTION
The intestinal microbiota consisting of the complex and dynamic communities of microbes is a component of the human ecosystem, which profoundly influences health through effects on nutrition, immunity, intestinal epithelial homeostasis and intestinal development. However, the composition of the ‘normal healthy intestinal microbiota’ is not currently clearly defined. Likewise, the characteristics of the host–microbial interaction that contribute to beneficial effects on human health and disruption of this interaction that leads to various disease states are poorly understood. Recent studies using metagenomic analysis of gut microbiome have identified unique bacterial genome sequences that functionally associate with host health.1, 2 Furthermore, analysis of the known bacterial genomic sequences, such as Lactobacillus strains, predicts secreted and cell surface proteins with potential regulatory effects on intestinal cells.3, 4 Therefore, functional studies of microbe-derived proteins are needed to determine whether the nature of the microbe-derived molecules is as important as the specific microbial composition of the gut. For example, two novel Lactobacillus rhamnosus GG (LGG)-derived proteins have been cloned and shown to prevent cytokine-induced intestinal injury and apoptosis.5
Regulation of signalling pathways to induce diverse cellular responses serves as one of the mechanisms by which microbes and microbial products exert their action on the host. Intestinal microbial regulation of nuclear factor (NF)-κB signalling is one of the best-studied pathways determining intestinal homeostasis and diseases. NF-κB is a key transcriptional factor controlling the expression of genes mediating inflammatory and anti-apoptotic responses (reviewed by Hayden et al6). Growing evidence suggests that ‘optimal’ NF-κB activity plays a significant role in maintaining normal intestinal homeostasis. However, hyper-activation of NF-κB results in chronic intestinal inflammatory disorders (reviewed by Spehlmann and Eckmann7).
It is well known that toll-like receptors (TLRs), a class of membrane receptors that sense extracellular microbes through recognition of microbial products, trigger anti-pathogen signalling cascades in intestinal epithelial cells and mucosal immune cells. In the best understood pathway, activation of one of the TLR adaptor proteins, MyD88, leads to NF-κB activation, which defends against most pathogens to maintain intestinal homeostasis and prevent injury.8, 9 One integrating target of TLRs is the activation of NF-κB. Since studies regarding TLRs have recently been extensively reviewed,10, 11 this article will not address microbial product activation of NF-κB through these pathways.
This review focuses on new insights into intestinal microbe-derived molecules that inhibit NF-κB activation and its transcriptional activity for disease prevention and treatment. The biological consequences of NF-κB signalling regulated by these molecules on innate and adaptive immune responses in intestinal epithelial cells and immune cells involved in intestinal protection will be discussed (figure 1). The majority of studies referenced in this review are from publications since 2007.
Figure 1.
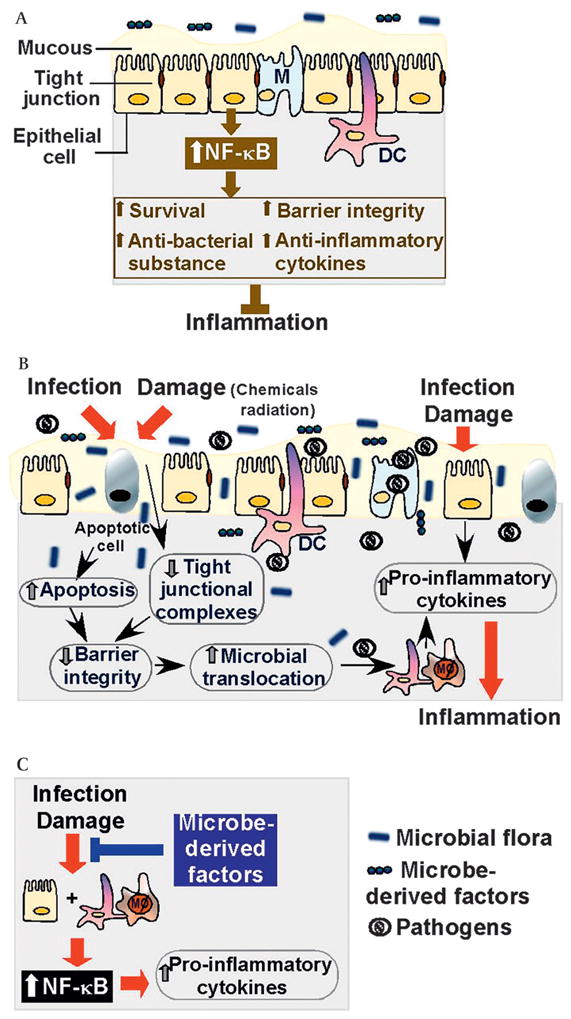
Impact of NF-κB regulatory microbial products in the intestine. Intestinal epithelial cells and their physical and biochemical adaptations, including tight junctions and the mucus layer, form an integral barrier separating host tissues from the external environment. (A) Protective roles of optimal NF-κB activation in intestinal epithelial cells. (B) Infection and damage to the intestinal epithelium disrupt the integrity leading to microbial translocation, which stimulates pro-inflammatory cytokine production by lamina propria mononuclear cells, such as dendritric cells and macrophages. In addition, infection and damage induce intestinal epithelial cells to produce inflammatory cytokine. (C) NF-κB activation is one of the mechanisms of increased pro-inflammatory cytokine production by lamina propria mononuclear cells and intestinal epithelial cells. Microbe-derived factors suppress pro-inflammatory cytokine production by inhibiting NF-κB activation to attenuate intestinal inflammation. DC, dendritic cell; Mϕ, macrophage; NF-κB, nuclear factor kappa B.
BASIC MECHANISMS OF REGULATING NF-κB activation and its transcriptional activity
The NF-κB family of transcriptional factors consists of five members, p50, p52, p65 (RelA), c-Rel and RelB, which share an N-terminal Rel homology domain responsible for homo- and heterodimerisation and DNA binding. The positive regulation of gene expression requires the transcription activation domain present only in p65,c-Rel and RelB. However, p50 and p52, which lack this domain, may repress transcription unless they associate with p65, c-Rel or RelB. Complex elements are involved in regulating NF-κB activation and its transcriptional activity towards targeted gene expression.
The signalling pathways involved in regulating NF-κB activation have been extensively reviewed.12, 13 Under normal conditions, NF-κB dimers are held in an inactive state in a cytoplasmic complex with inhibitor of κB (IκB) family members, including IκBα, IκBβ, IκBε, IκBζ, IκBNS and Bcl3. NF-κB activation is induced when IκB undergoes phosphorylation, targeting for ubiquitination and proteosome-mediated degradation. Thus, NF-κB dimers are released for nuclear translocation and transcriptional activation.
IκB is directly phosphorylated by the multimeric protein kinase complex, IκB kinase (IKK). the IKK complex consists of two catalytic subunits, IKKα and IKKβ, and a regulatory protein, IKKγ/NEMO. Upon stimulation by various stimuli, such as ligation of cytokine receptors and TLRs by proinflammatory cytokines and microbial components, respectively, adaptor proteins, including TRAF and RIP, are recruited to the IKK complex for activation of IKK through NF-κB-inducing kinase (NIK) and mitogen-activated protein kinase/ERK kinase kinase-1 (MEKK1). Recently, additional novel mechanisms of activating NF-κB by IκB-independent pathways have been described. Both IKKα and IKKβ have been implicated in the direct phosphorylation of NF-κB proteins, especially for p65 subunit.14
Once in the nucleus, NF-κB binds to κB-cis-acting elements present on a subset of genes, and together with other DNA binding proteins to trigger transcriptional activity. Specificity in transcriptional responses is controlled by the combination of NF-κB hetero- or homo-dimers, which are regulated by IκB proteins. Recent studies have identified additional factors to determine NF-κB-regulated genes for a given stimulus, which include ankirin15 and p38 mitogen-activated protein kinase.16 The known genes regulated by NF-κB encode a variety of proteins, including proinflammatory cytokines, survival and proliferative proteins, adhesion molecules, and growth factors.
NF-κB as a regulator of diverse intestinal responses
NF-κB is a key regulator of diverse intestinal responses. Inflammation is a fundamental physiological process that protects the host from pathological microbial infection. NF-κB modulates expression of many genes with important functions in Inflammation through regulating innate and adaptive immune responses. In addition, the anti-apoptotic products of NF-κB protect tissue from damage, which may indirectly exert an anti-inflammatory action. However, inappropriately or excessive immune responses result in persistent tissue damage, leading to chronic Inflammation. Thus, deficiency in or hyper-activation of NF-κB results in chronic intestinal inflammatory disorders.
Protective roles of NF-κB
The intestinal epithelium forms a barrier against pathological microorganisms and toxic substances and actively participates in epithelial–immune system cross-talk to maintain host health. Studies using mice with deletion of IKKγ/NEMO in intestinal epithelial cells show that deficiency of NF-κB activation causes spontaneous intestinal Inflammation. In addition, these mice have increased intestinal epithelial apoptosis, disruption of intestinal epithelial integrity, and impaired expression of antimicrobial peptides, which weaken epithelial defence leading to increased bacterial translocation into mucosa to induce persistent Inflammation.17 In another study, mice with a deletion of IKKβ in intestinal epithelial cells developed severe intestinal Inflammation with increased production of several dendritic cell-derived pro-inflammatory cytokines. Furthermore, these mice showed a reduced expression of thymic stromal lymphopoietin in intestinal epithelial cells and failed to develop T helper type 2 (TH2) response and eradication of pathogens after Trichuris infection.18 Similarly, specific loss of the NF-κB p65 subunit in the intestinal epithelium increased the susceptibility to DSS-induced colitis.19 Furthermore, Raf, a regulator of a number of cellular responses to promote a healthy colon epithelium has been shown to protect against DSS-induced colitis by promoting colon epithelial cell survival through activating NF-κB.20 These results indicate that NF-κB signalling in the intestinal epithelium is crucial in maintaining intestinal homeostasis and preventing acute Inflammation in the intestinal tract.
NF-κB has also been shown to protect against epithelial cell apoptosis during radiation-induced intestinal injury. Flagellin, a polypeptide derived from Salmonella and which interacts with TLR5 and activates NF-κB, protects mice from this acute intestinal radiation-induced damage. These protective effects are abolished by flagellin derivatives that fail to stimulate NF-κB activation.21 The protective role of NF-κB in thermal skin injury-induced intestinal tissue damage has been indicated since loss of intestinal epithelial IKKβ exacerbates the mucosal damage with increased epithelial apoptosis.22 These studies emphasise the cell survival role of NF-κB in intestinal epithelial cells to protect the intestinal tract from acute inflammatory and injurious challenges.
Inflammatory roles of NF-κB
It is important to note that excessive activation of NF-κB leads to proinflammatory cytokine and chemokine ‘over-production’ and chronic inflammation. It appears that protective or destructive outcomes of NF-κB activation in intestinal Inflammation depend upon the underlying pathophysiological mechanisms and the primary cell types involved. One group reported that acute Inflammation is aggravated by deletion of IKKβ in intestinal epithelial cells and by an IKKβ inhibitor. In a mouse model of chronic colitis, ablation of IKKβ in the intestinal epithelium has no impact. However, IKKβ deficiency in macrophages and neutrophils attenuates inflammation.23 Another finding showed that Enterococcus faecalis- and Escherichia coli-induced experimental colitis in interleukin (IL)10−/− mice is attenuated by blocking TLR-induced NF-κB in mucosal immune cells, including T cells, dendritic cells, neutrophils and macrophages.24 In fact, clinical studies show that NF-κB protein expression level of NF-κB and activation state of NF-κB are markedly increased in patients with chronic inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease. In addition, NF-κB activation strongly affects the course of mucosal Inflammation through its ability to promote the expression of several proinflammatory cytokines (reviewed by Atreya et al25).
On the basis of findings regarding inflammatory roles of NF-κB in chronic Inflammation, inhibition of NF-κB has been proposed as a therapeutic target. However, considering the opposing functions of NF-κB during acute and chronic Inflammation, inhibition of excess, but not basal NF-κB activation, or tissue specific inhibition of NF-κB, such as in intestinal immune cells, but not in epithelial cells, may constitute a better approach for manipulating NF-κB as a therapeutic target.
COMMENSAL MICROBES REGULATING HOST HEALTH
The human gastrointestinal tract houses complex populations of commensal bacteria, fungi and protozoa in continuous contact with intestinal epithelial cells. In humans, colonisation of the intestinal microbiota occurs immediately after birth and is modified during the first one or two years of life. The intestinal microbiota is relatively stable over a person’s lifetime. The intestine provides a suitable environment with stable temperature and rich nutrients for microbes to thrive. Microbe-derived signals are essential for normal intestinal functions, including regulation of intestinal development, nutrition, epithelial homeostasis and immune responses (reviewed by Vanderpool et al26).
Intestinal development
Much of the knowledge regarding the active cross-talk between the host and microbial flora has been derived from gnotobiotic studies. In the absence of microbes there are profound deficiencies in intestinal epithelial and mucosal immunological development and functions, including the inability to generate proper immune responses to protect against infection and Inflammation (reviewed by Wagner27). Recent findings show that impaired development and maturation of isolated lymphoid follicles in germ-free mice can be re-induced following the introduction of gut bacteria.28 Furthermore, a surface carbohydrate molecule of Bacteroides fragilis, polysaccharide, has been found to play a role in modulating the intestinal immune system maturation.29 It has been reported that monocolonisation of B fragilis is sufficient to correct several defects found in germ-free animals, including systemic T cell deficiencies and H1/TH2 imbalances. However, a polysaccharide mutant of B fragilis does not restore these immunological functions.29
Nutrition
It has been well known that the intestinal microbiota promotes host nutritional status, including promotion of polysaccharide digestion and uptake of nutrients by intestinal cells (reviewed by Yan and Polk30). Studies of lean and obese mice suggest that the gut microbiota affects energy balance by influencing the efficiency of calorie harvest from the diet, and the usage and storage of this harvested energy.31, 32 More recent studies indicate that there may be a core gut microbiome, which exists at the level of shared genes, including an important component involved in various metabolic functions. Deviations from this core are associated with aberrant physiological states, such as obesity.33
Intestinal epithelial homeostasis
Intestinal epithelial cells, along with their physical and biochemical adaptations, including tight junctions and the mucus layer, form an integral barrier to separate host tissues from the external environment. Intestinal microbes facilitate the protective roles of intestinal epithelial cells through promoting proliferation, migration, survival, barrier integrity, antimicrobial substance secretion, and competition for pathogen interaction with epithelial cells (reviewed by Vanderpool et al26 and Yan and Polk30). Findings regarding intestinal microbes regulating signalling in intestinal epithelial cells in vivo provide a mechanism through which microbes are recognised by epithelial cells without inducing inflammatory responses. For example, although pathogens interact with TLR to activate NF-κB leading to Inflammation, commensal bacteria, such as L casei, suppress invasive Shigella flexneri-induced transcription of inflammatory cytokines, chemokines and adhesion molecules in intestinal epithelial cells through inhibiting NF-κB activation.34
Immune responses
Commensal microbes play a key role in defining and maintaining the delicate balance between necessary and excessive defence mechanisms including innate and adaptive immune responses. Both in vitro and in vivo studies have shown effects of intestinal commensal microbes on host immune functions: upregulation of immune function may improve the ability to fight infections; downregulation may prevent the onset of intestinal inflammation (reviewed by Vanderpool et al26). Recent studies into the mechanisms of intestinal immune modulation by the microbiota reveal that ATP generated by intestinal bacteria specifically increases the production of IL17, but not interferon (IFN)γ, in the colon.35 Furthermore, a single molecule, polysaccharide A (PSA) made by Bacteriodes fragilis, has been found to protect against experimental colitis through inducing anti-inflammatory cytokine, IL10, production.36
NF-κB is a target for the effects of microbes on immune function. For example, L reuteri, LGG, B infantis and L salivarius suppress tumour necrosis factor (TNF)- or S typhimurium-induced IL8 gene expression and secretion by intestinal epithelial cells in a NF-κB-dependent manner.37–39 Further studies reveal that L casei suppresses invasive S flexneri-induced transcription of inflammatory cytokines, chemokines and adhesion molecules in intestinal epithelial cells through modulation of the ubiquitin/proteasome pathway to stabilise IκB and thus inhibit NF-κB nuclear translocation.34 Another mechanism of probiotic regulation of NF-κB transcriptional activity in the nucleus is through activation of peroxisome proliferators activated receptor (PPAR)γ.40 B thetaiotaomicron induces nuclear export of the RelA subunit of NF-κB associated with PPARγ.
Recent in vivo studies reveal that Bifidobacterium infantis drives the generation and function of Treg to suppress LPS-induced NF-κB activation and S typhimurium infection.41
REGULATION OF NF-κB signalling by intestinal microbe-derived molecules
A significant finding from recent studies is the recognition that not only intact live microbes, but also factors derived from these microbes regulate host health with implications for disease prevention and treatment. For example, two novel soluble proteins produced by LGG exert inhibitory effects on TNF-induced apoptosis in intestinal epithelial cells through activation of anti-apoptotic Akt activation.5 Although these two proteins have not been found to regulate NF-κB signalling, these findings suggest that functional characterisation of microbial molecules is important for understanding the mechanisms of microbial action.
Microbe-derived soluble factors in the culture supernatant
One mechanism of microbial beneficial effects is through the suppression of NF-κB signalling to limit excessive inflammation; therefore, investigators are starting to define the contribution of microbe-derived soluble factors in this response. Soluble factors released by B breve C50 in the culture supernatant reduce TNF-induced cytokine production through inhibition of NF-κB and AP-1-dependent transcription in intestinal epithelial cells. These soluble factors and dendritic cells conditioned by these soluble factors prevent trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice. However, these effects were not found in B breve ATCC 15698, L rhamnosus ATCC 10863 and Eubacterium rectale L15.42 Factors present in conditioned media of L plantarum, but not L acidophilus, L paracasei, B fragilis, B breve, E coli F18 or EPEC inhibit TNF-induced NF-κB binding capacity, IκB degradation, and proteasome activity in intestinal epithelial cells, macrophages and dendritic cells.43 Another interesting study shows an inverse relationship between the number of Facecalibacterium prausnitzii and the risk of postoperative recurrence of ileal Crohn’s disease. Importantly, culture supernatant, not the bacterium, inhibits IL1β-induced NF-κB activation and IL8 production in intestinal epithelial cells. Both bacterium and supernatant exert anti-inflammatory effects in TNBS-induced colitis in mice.44 Furthermore, L reuteri-secreted factors promote TNF-induced apoptosis and anti-apoptotic protein production (Bcl-2 and Bcl-xL) in human myeloid cells by inhibiting NF-κB activation and enhancing MAPK signalling. These secreted proteins inhibit TNF-induced NF-κB activation through inhibition of IκB ubiquitination.45
These studies indicate the strain specific secretion of factors to regulate NF-κB activation.42, 43 Microbe-derived soluble factors also function in multiple cell types to regulate NF-κB activity, including intestinal epithelial cells and mucosal immune cells.42–45 Further characterisation of these various bioactive factors will enhance our understanding of the cross-talk between intestinal microbiota and the gut. In this case, multiple factors derived from microbes are presumed to function on NF-κB signalling.
Reactive oxygen species
Hydrogen peroxide derived from commensal microbes, such as several Lactobacillus strains, has long been considered as a non-specific antimicrobial defence mechanism to control the microbial ecosystem associated with mucosa in the intestine and the female vagina. Recent studies, however, demonstrate the role of reactive oxygen species (ROS) in intracellular signalling mechanisms to specifically regulate host inflammatory responses. L crispatus M247-derived hydrogen peroxide acts as a signal-transducing molecule to activate PPARλ leading to suppression of NF-κB activity in colon epithelial cells. However, other Lactobacillus strains produce little hydrogen peroxide and fail to activate PPARλ.46 Interestingly, another group has reported that viable commensal bacteria, such as L rhamnosus, E coli and B thetaiotaomicron, induce rapid and reversible ROS generation from intestinal epithelial cells that block cullin-1 neddylation, a critical step in the ubiquitination system, leading to suppression of NF-κB activation.47 Furthermore, bacterium-fermented products, short-chain fatty acids, such as butyrate, exert an effect on the induction of ROS from intestinal epithelial cells to downregulate NF-κB activation through the same mechanism.48 Thus, ROS generated by microbes and intestinal epithelial cells upon interaction with microbes may also serve as a regulatory second messenger molecule determining NFκB-mediated inflammatory responses in the intestine.
Pathogen type III secretion system-delivered effector proteins
However, it should be noted that several intestinal disorders induced by infections are associated with inhibition of NF-κB to disrupt host defence mechanisms by pathogen-derived factors. Therefore, NF-κB stands at the point to control inflammation and the consequence of NF-κB activation depends on the stimulators to initiate this process at certain conditions.
Several Gram-negative bacterial pathogens use a type 3 secretion system, which resembles a needle-like molecular structure to connect pathogens with host cells and deliver effector proteins into host cells. These effector proteins disrupt signalling pathways essential for host cells to elicit an immune response by inhibiting the signalling transduction pathways, including NF-κB. Ubiquitination of phosphorylated IκB is required for NF-κB nuclear translocation and activation. Several pathogens have been shown to produce effectors to disrupt IκB degradation at different stages of ubiquitination pathways. For example, YopJ, a Yersinia species effector protein, acts as a de-ubiquitinating enzyme for ubiquitinated IκB to prevent its degradation in macrophages.49 Furthermore, it has been shown that YopJ also functions as an acetyl-transferase to binding and acetylates critical residues in the activation loop of mitogen-activated protein kinase kinase (MAPKK)6 and IKKβ, respectively, thereby preventing these residues from being phosphorylated, which leads to blockade of MAPKK and NF-κB activation.50 Therefore, NF-κB serves as a key signalling pathway for pathogens to escape host defence mechanisms.
FUTURE STUDIES
Recent advances in understanding the action of some microbe-derived factors in the supernatants of bacterial culture and bacterial conditioned media on NF-κB signalling provide insight into developing novel treatment options in the future. However, information regarding identification and characterisation of these factors is lacking. In addition, studies focused on determining the mode(s) of action of microbes and microbe-derived molecules in the inhibition of NF-κB in intestinal cells and model systems of gastrointestinal diseases are essential for developing hypothesis-driven clinical trials to study clinical efficacy. Since NF-κB exerts opposing functions on intestinal epithelial cells and mucosal immune cells during acute and chronic Inflammation, the development of approaches for specific delivery of these factors to target cells represents an important advance needed to move from the promise of these initial investigations to clinical application of novel therapies.
Footnotes
Competing interests None.
Provenance and peer review Commissioned; externally peer reviewed.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, et al. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarova K, Slesarev A, Wolf Y, et al. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A. 2006;103:15611–16. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaillou S, Champomier-Verges MC, Cornet M, et al. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol. 2005;23:1527–33. doi: 10.1038/nbt1160. [DOI] [PubMed] [Google Scholar]
- 5.Yan F, Cao H, Cover TL, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 7.Spehlmann ME, Eckmann L. Nuclear factor-kappaB in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25:92–9. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- 8.Brown SL, Riehl TE, Walker MR, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 13.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 15.Goto A, Matsushita K, Gesellchen V, et al. Akirins are highly conserved nuclear proteins required for NF-kappaB-dependent gene expression in drosophila and mice. Nat Immunol. 2008;9:97–104. doi: 10.1038/ni1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappaB recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 17.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 18.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrecher KA, Harmel-Laws E, Sitcheran R, et al. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–99. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 20.Edelblum KL, Washington MK, Koyama T, et al. Raf protects against colitis by promoting mouse colon epithelial cell survival through NF-kappaB. Gastroenterology. 2008;135:539–51. doi: 10.1053/j.gastro.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LW, Chen PH, Chang WJ, et al. IKappaB-kinase/nuclear factor-kappaB signaling prevents thermal injury-induced gut damage by inhibiting c-Jun NH2-terminal kinase activation. Crit Care Med. 2007;35:1332–40. doi: 10.1097/01.CCM.0000261891.30360.F0. [DOI] [PubMed] [Google Scholar]
- 23.Eckmann L, Nebelsiek T, Fingerle AA, et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:15058–63. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karrasch T, Kim JS, Muhlbauer M, et al. Gnotobiotic IL-10−/−NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–32. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 25.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1585–96. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- 27.Wagner RD. Effects of microbiota on GI health: gnotobiotic research. Adv Exp Med Biol. 2008;635:41–56. doi: 10.1007/978-0-387-09550-9_4. [DOI] [PubMed] [Google Scholar]
- 28.Bouskra D, Brezillon C, Berard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 29.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Yan F, Polk DB. Commensal bacteria in the gut: learning who our friends are. Curr Oppin Gastroenterol. 2004;20:565–71. doi: 10.1097/00001574-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tien MT, Girardin SE, Regnault B, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–37. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 35.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 36.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 37.Ma D, Forsythe P, Bienenstock J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun. 2004;72:5308–14. doi: 10.1128/IAI.72.9.5308-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hara AM, O’Regan P, Fanning A, et al. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology. 2006;118:202–15. doi: 10.1111/j.1365-2567.2006.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Li N, Caicedo R, et al. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752–6. doi: 10.1093/jn/135.7.1752. [DOI] [PubMed] [Google Scholar]
- 40.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear–cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol. 2004;5:104–12. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 41.O’Mahony C, Scully P, O’Mahony D, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heuvelin E, Lebreton C, Grangette C, et al. Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS One. 2009;4:e5184. doi: 10.1371/journal.pone.0005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrof EO, Claud EC, Sun J, et al. Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm Bowel Dis. 2009;15:1537–47. doi: 10.1002/ibd.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer C, Kosters A, Sethi G, et al. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10:1442–52. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 46.Voltan S, Martines D, Elli M, et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology. 2008;135:1216–27. doi: 10.1053/j.gastro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Wu H, Collier-Hyams LS, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–66. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Wu H, Collier-Hyams LS, et al. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–46. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou H, Monack DM, Kayagaki N, et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappaB activation. J Exp Med. 2005;202:1327–32. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee S, Keitany G, Li Y, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–14. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]


