Abstract
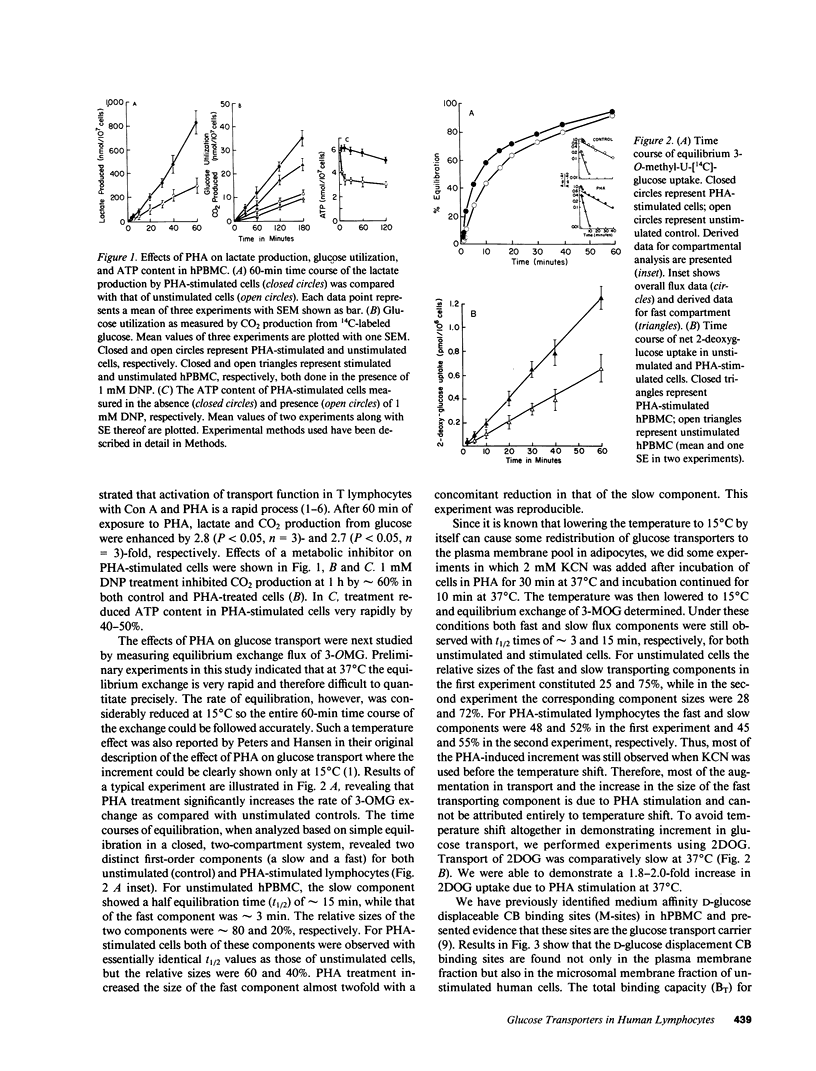
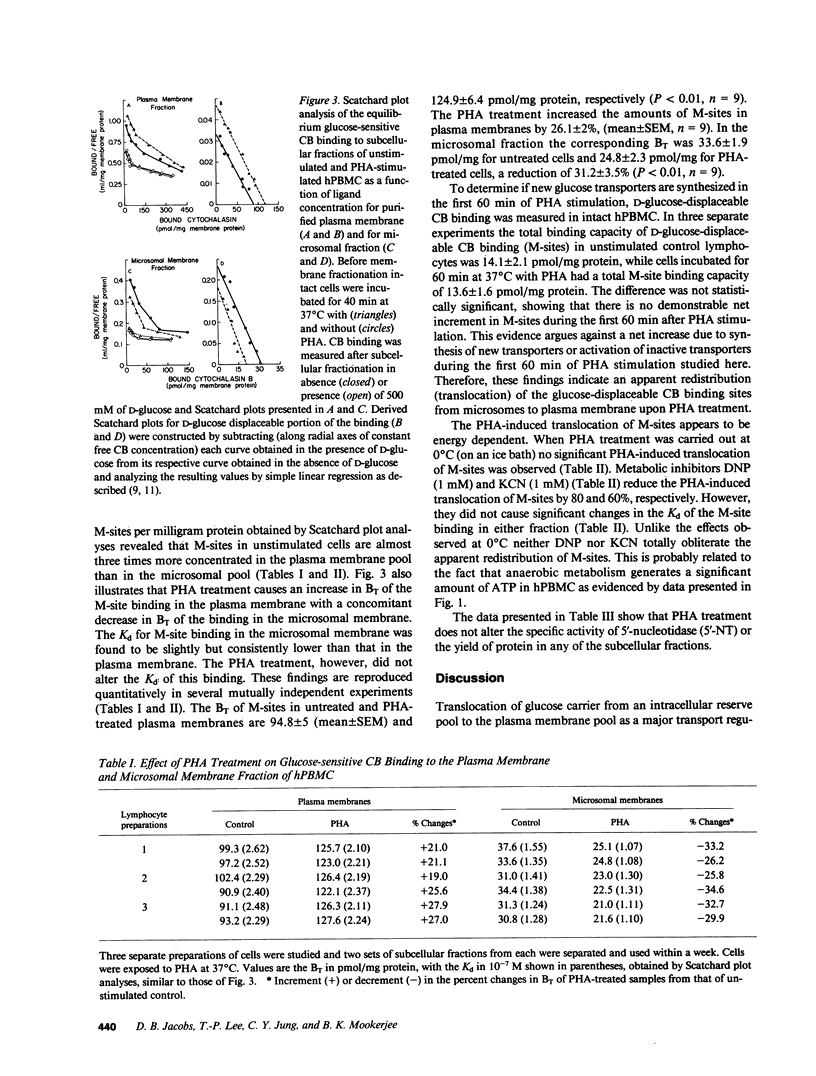
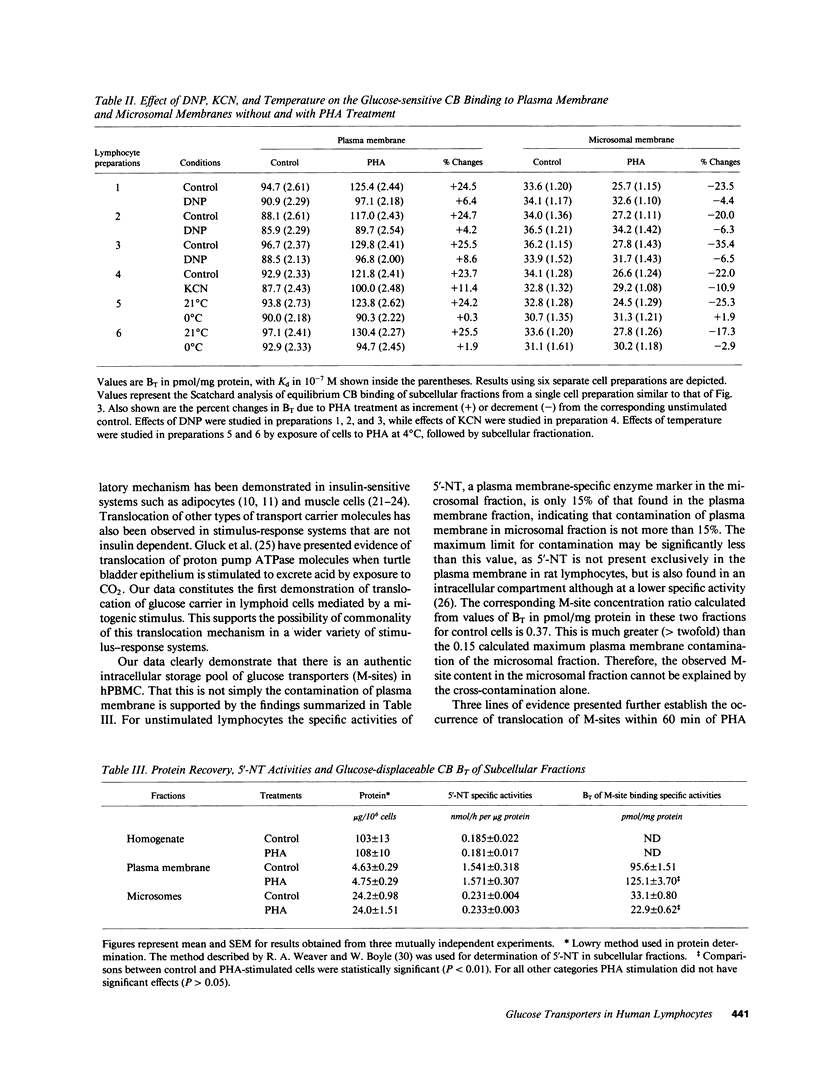
The present study examines the effects of phytohemagglutinin stimulation of a population of human (h) PBMC enriched in lymphocytes (hPBMC) on D-glucose displaceable cytochalasin B binding sites or medium-affinity sites (M-sites) in relation to glucose transport. Previously we have shown that M-sites are glucose transporters in hPBMC (Mookerjee, B.K., et al. 1981. J. Biol. Chem. 256:1290-1300). Equilibrium exchange of 3-O-methyl D-glucose in unstimulated cells revealed two populations with fast and slow flux rates. Phytohemagglutinin stimulates flux rates by converting part of the slow flux population to the fast flux population. M-sites occur in two distinct pools, one in plasma membrane and the other in microsomal fraction. Phytohemagglutinin treatment increases the plasma membrane pool size of M-sites with a concomitant reduction in the microsomal pool size without affecting the binding affinities or the total number of M-sites/cell. Data presented in this paper demonstrate that there are two pools of glucose transporters in these cells and phytohemagglutinin stimulation induces an energy-dependent net translocation of glucose transporters from an intracellular reserve pool to the plasma membrane, which accounts for greater than 60% of the increment in glucose transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman J. H. Role of insulin in the intermediary metabolism of the activated thymic-derived lymphocyte. J Clin Invest. 1981 Jun;67(6):1636–1642. doi: 10.1172/JCI110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. M., Goldenberg H., Frantz C., Morré D. J., Keenan T. W., Crane F. L. Comparison of NADH-linked cytochrome C reductases of endoplasmic reticulum, golgi apparatus and plasma membrane. Int J Biochem. 1979;10(8):723–731. doi: 10.1016/0020-711x(79)90218-0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Weidemann M. On the stimulation of rat thymocyte 3-O-methyl-glucose transport by mitogenic stimuli. J Cell Physiol. 1978 Sep;96(3):303–308. doi: 10.1002/jcp.1040960305. [DOI] [PubMed] [Google Scholar]
- Jacobs D. B., Mookerjee B. K., Jung C. Y. Furosemide inhibits glucose transport in isolated rat adipocytes via direct inactivation of carrier proteins. J Clin Invest. 1984 Nov;74(5):1679–1685. doi: 10.1172/JCI111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Kay J. E. The importance of changes in membrane transport in lymphocyte activation by phytohaemagglutinin. Biochem Soc Trans. 1976;4(6):1120–1122. doi: 10.1042/bst0041120. [DOI] [PubMed] [Google Scholar]
- Kono T., Suzuki K., Dansey L. E., Robinson F. W., Blevins T. L. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981 Jun 25;256(12):6400–6407. [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. Calcium transport and calcium-ATPase activity in human lymphocyte plasma membrane vesicles. J Biol Chem. 1981 Jun 25;256(12):6148–6154. [PubMed] [Google Scholar]
- Martz A., Mookerjee B. K., Jung C. Y. Insulin and phorbol esters affect the maximum velocity rather than the half-saturation constant of 3-O-methylglucose transport in rat adipocytes. J Biol Chem. 1986 Oct 15;261(29):13606–13609. [PubMed] [Google Scholar]
- Mookerjee B. K., Cuppoletti J., Rampal A. L., Jung C. Y. The effects of cytochalasins on lymphocytes. Identification of distinct cytochalasin-binding sites in relation to mitogenic response and hexose transport. J Biol Chem. 1981 Feb 10;256(3):1290–1300. [PubMed] [Google Scholar]
- Mookerjee B. K., Jung C. Y. Effects of cytochalasins on lymphocytes: mechanism of inhibition of rosette formation. J Immunol. 1983 Sep;131(3):1126–1130. [PubMed] [Google Scholar]
- Mookerjee B. K., Jung C. Y. The effects of cytochalasins on lymphocytes: mechanism of action of Cytochalasin A on responses to phytomitogens. J Immunol. 1982 May;128(5):2153–2159. [PubMed] [Google Scholar]
- Oka Y., Czech M. P. Photoaffinity labeling of insulin-sensitive hexose transporters in intact rat adipocytes. Direct evidence that latent transporters become exposed to the extracellular space in response to insulin. J Biol Chem. 1984 Jul 10;259(13):8125–8133. [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. 2. Stimulation of "facilitated diffusion" of 3-O-methyl-glucose. Eur J Biochem. 1971 Apr 30;19(4):509–513. doi: 10.1111/j.1432-1033.1971.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim Biophys Acta. 1983 Dec 19;763(4):393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- Slaunwhite W. R., 3rd, Goldman J. K., Bernardis L. L. Sequential changes in glucose metabolism by adipose tissue and liver of rats after destruction of the ventromedial hypothalamic nuclei: effect of three dietary regimens. Metabolism. 1972 Jul;21(7):619–631. doi: 10.1016/0026-0495(72)90086-8. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Identification of the D-glucose-inhibitable cytochalasin B binding site as the glucose transporter in rat diaphragm plasma and microsomal membranes. Biochim Biophys Acta. 1983 Apr 21;730(1):49–56. doi: 10.1016/0005-2736(83)90315-2. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Watanabe T., Smith M. M., Robinson F. W., Kono T. Insulin action on glucose transport in cardiac muscle. J Biol Chem. 1984 Nov 10;259(21):13117–13122. [PubMed] [Google Scholar]
- Weaver R. A., Boyle W. Purification of plasma membranes of rat liver. Application of zonal centrifugation to isolation of cell membranes. Biochim Biophys Acta. 1969 Apr;173(3):377–388. doi: 10.1016/0005-2736(69)90003-0. [DOI] [PubMed] [Google Scholar]
- Whitesell R. R., Johnson R. A., Tarpley H. L., Regen D. M. Mitogen-stimulated glucose transport in thymocytes. Possible role of Ca++ and antagonism by adenosine 3':5'-monophosphate. J Cell Biol. 1977 Feb;72(2):456–469. doi: 10.1083/jcb.72.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell R. R., Regen D. M. Glucose transport characteristics of quiescent thymocytes. J Biol Chem. 1978 Oct 25;253(20):7289–7294. [PubMed] [Google Scholar]
- Yasmeen D., Laird A. J., Hume D. A., Weidemann M. J. Activation of 3-O-methyl-glucose transport in rat thymus lymphocytes by concanavalin A. Temperature and calcium ion dependence and sensitivity to puromycin but to cycloheximide. Biochim Biophys Acta. 1977 Nov 7;500(1):89–102. doi: 10.1016/0304-4165(77)90049-6. [DOI] [PubMed] [Google Scholar]



