Abstract
Background & Aims
Increased inflammatory cytokine levels and intestinal epithelial cell apoptosis leading to disruption of epithelial integrity are major pathologic factors in inflammatory bowel diseases. The probiotic bacterium Lactobacillus rhamnosus GG (LGG) and factors recovered from LGG broth culture supernatant (LGG-s) prevent cytokine-induced apoptosis in human and mouse intestinal epithelial cells by regulating signaling pathways. Here, we purify and characterize 2 secreted LGG proteins that regulate intestinal epithelial cell antiapoptotic and proliferation responses.
Methods
LGG proteins were purified from LGG-s, analyzed, and used to generate polyclonal antibodies for immunodepletion of respective proteins from LGG-conditioned cell culture media (CM). Mouse colon epithelial cells and cultured colon explants were treated with purified proteins in the absence or presence of tumor necrosis factor (TNF). Akt activation, proliferation, tissue injury, apoptosis, and caspase-3 activation were determined.
Results
We purified 2 novel proteins, p75 (75 kilodaltons) and p40 (40 kilodaltons), from LGG-s. Each of these purified protein preparations activated Akt, inhibited cytokine-induced epithelial cell apoptosis, and promoted cell growth in human and mouse colon epithelial cells and cultured mouse colon explants. TNF-induced colon epithelial damage was significantly reduced by p75 and p40. Immunodepletion of p75 and p40 from LGG-CM reversed LGG-CM activation of Akt and its inhibitory effects on cytokine-induced apoptosis and loss of intestinal epithelial cells.
Conclusions
p75 and p40 are the first probiotic bacterial proteins demonstrated to promote intestinal epithelial homeostasis through specific signaling pathways. These findings suggest that probiotic bacterial components may be useful for preventing cytokine-mediated gastrointestinal diseases.
Inflammatory bowel diseases (IBD) are characterized by increased production of inflammatory cytokines, epithelial cell apoptosis, and immune cell infiltration, leading to disruption of the intestinal epithelial integrity.1 Therefore, remission of these disorders requires both decreased apoptosis and restitution of the damaged epithelium. Recent studies reveal several potential therapeutic approaches to induce restitution of the damaged epithelium. Growth factors2-5 and cytokines6,7 have been reported to modulate these processes by regulating proliferation,3 migration,2 and apoptosis.6,7
Increasing evidence suggests that some commensal bacteria enhance intestinal epithelial homeostasis and barrier integrity. Indeed, commensal bacteria regulate a number of host processes, including nutrition, development, and immune responses, that are relevant for both health and disease.8 Therefore, manipulation of intestinal bacterial flora has been used as an alternative health approach for disease prevention and treatment.9 Living microorganisms in the intestinal tract that benefit the host are termed probiotics.10 Recent studies indicate that some Lactobacillus species function as probiotics and induce sustained remission in ulcerative colitis and pouchitis.11-14 Lactobacillus rhamnosus GG (LGG), a bacterium used in the production of yogurt, is one of the best-studied Lactobacillus strains in clinical trials for IBD.
The presumed first target of probiotic actions is the intestinal epithelial cell. Probiotic bacteria stimulate several intestinal epithelial cell protective responses, including enhancement of epithelial barrier function,15,16 mucin synthesis and secretion,17,18 inhibition of enteropathogenic Echerichia coli binding,17 and cell survival.19 However, the mechanisms regulating epithelial responses to probiotics are complex and mostly unknown. We have used LGG to investigate molecular mechanisms by which probiotics regulate intestinal epithelial cells. We previously reported that LGG prevents cytokine-induced apoptosis in both human and mouse intestinal epithelial cells through activating Akt and inhibiting p38 mitogen-activated protein kinase (MAPK) activation.19 Akt plays a central role in promoting cell survival by inactivation of several proapoptotic pathways, including Bad, caspase 9, and caspase 3, and stimulating cell proliferation by activation of cell cycle regulators, such as cyclin/cyclin-dependent kinase (CDK).20,21 We further found that soluble factors recovered from LGG broth culture supernatant (LGG-s) activate Akt in a phosphatidylinositol-3′-kinase (PI3K)-dependent manner and prevent cytokine-mediated apoptosis.19 One recent report has shown that soluble factors present in LGG-s induce cytoprotective heat shock protein synthesis in intestinal epithelial cells.22 However, to our knowledge, the specific components of LGG-s that promote intestinal epithelial health have not been identified. Therefore, the purpose of this study was to purify and characterize LGG-derived soluble proteins that regulate intestinal epithelial cell proliferation and survival.
Here, we report that 2 proteins (p75 and p40) purified from LGG-s stimulate Akt activation, promote cell growth, and inhibit tumor necrosis factor (TNF)-induced epithelial cell apoptosis in cultured cells and ex vivo colon organ culture models. Furthermore, although a number of Lactobacilli display antiapoptotic activity when in contact with epithelial cells, only those strains producing soluble p75 and p40 conferred this response in the absence of cell contact. The present studies provide novel insight into the molecular mechanisms of probiotic regulation of intestinal epithelial cells. These findings suggest that it may be possible to use probiotic bacterial products for gastrointestinal disease prevention and treatment.
Materials and Methods
Bacterial Culture, LGG-s, and LGG Conditioned Cell Culture Media Preparation
Lactobacillus rhamnosus GG (ATCC 53103), Lactobacillus casei 334, (ATCC 334), Lactobacillus casei 393 (ATCC 393), and Lactobacillus acidophilus (ATCC 4356) were cultured in Lactobacillus MRS broth at 37°C19,23 according to ATCC guidelines. Bacteria were harvested from MRS broth by centrifugation and washed twice with phosphate-buffered saline (PBS). Following centrifugation, LGG-s was passed through a 0.2-μm filter. LGG-conditioned cell culture media (LGG-CM) were generated by incubating LGG (107colony-forming units (CFU)/mL) in RPMI or Dulbecco’s modified Eagle medium (DMEM) cell culture medium at 37°C for 2 hours; the media were then centrifuged twice, and the supernatant was filtered (0.2 μm).
Purification of Proteins From LGG-s
To purify proteins under native conditions from LGG-s, LGG-s was loaded onto UNOsphere S ion exchange media (Bio-Rad Laboratories, Hercules, CA), which was prepared according to the manufacturer’s instructions. Bound proteins were eluted using 30 mmol/L Tris, pH 7.3, containing sequential concentrations of NaCl (100 to 800 mmol/L). Eluted fractions were collected and subjected to SDS-PAGE and stained with Colloidal Blue Stain kit (Invitrogen Corporation, Carlsbad, CA). Eluted fractions containing proteins were then concentrated using Amicon Ultra-4 centrifugal filter devices (Millipore, Bedford, MA), with a 5-kilodalton molecular weight limit cut-off, and the > 5-kilodalton molecular weight fraction was retained for study. Protein concentrations were determined by using a DC protein assay (Bio-Rad Laboratories). From 200 mL of LGG broth culture (107 CFU/mL), approximately 1 mg of p40 and 0.75 mg of p75 were purified. The concentration of purified proteins was adjusted to 0.1 mg/mL using PBS.
Antibody Generation and Immunodepletion of p75 and p40 From LGG-CM
To prepare p75 and p40 as antigens for generating antibodies, chromatographically purified p75 and p40 were separated on an SDS-PAGE gel, and p75 and p40 bands were excised and electroeluted from the gels using a Model 422 Electro-Eluter (Bio-Rad Laboratories) according to the manufacturer’s instructions. SDS in the eluted fractions was removed by Bio-Beads SM-2 adsorbents (Bio-Rad Laboratories). Polyclonal antibodies against p75 and p40 were generated by injecting rabbits with purified p75 or p40 proteins, respectively (Strategic Biosolutions, Newark, DE). p75 and p40 Antibodies were conjugated to Protein A/G beads (Santa Cruz Biotechnology, Santa Cruz, CA) by incubating antibodies with beads in PBS for 2 hours at 4°C. To sequentially immunodeplete p75 and p40 from LGG-CM, LGG-CM was incubated with anti-p75 antibody-conjugated beads for 4 hours at 4°C. After removing anti-p75 antibody-conjugated beads, CM was incubated with anti-p40 antibody-conjugated beads for another 4 hours. Preimmune serum was used as the negative control. The amounts of p75 and p40 present in LGG-CM or immunodepleted LGG-CM were detected by Western blot analysis with anti-p75 and anti-p40 antibodies.
N-Terminal and Internal Peptide Sequence Analysis of p75 and p40
p75 and p40 Proteins were purified as described previously, separated by SDS-PAGE, and transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc). N-terminal peptide sequence analysis of p75 and p40 by the Edman degradation methodology was performed at the Iowa State University Protein Facility (Ames, IA). Internal peptide sequences of p75 and p40 were determined by performing in-gel digestion of the proteins with trypsin, followed by analysis of the resulting peptides by matrixassisted laser desorption/ionization (MALDI), time-of-flight (TOF) tandem mass spectrometry (MALDI-TOF/MS/MS) and liquid chromatography-tandem mass spectrometry (LC/MS/MS) at the Vanderbilt University Proteomics Core of the Digestive Disease Research Center, Nashville, TN.
Analysis of the LGG Genes Encoding p75 and p40
N-terminal and internal peptide sequences of p75 and p40 were compared with protein sequences in the NCBI microbial genome database using BLAST analysis. The most closely related proteins were encoded by 2 genes in the genome of Lactobacillus casei 334 (Genbank accession numbers COG0791 and COG3883). Multiple primer pairs were designed based on the sequences of these L casei genes and flanking DNA sequences in the L casei genome. LGG genomic DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI), and this DNA was used as a template for polymerase chain reaction (PCR). PCR was carried out for 30 cycles, each including 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C. PCR products were excised from agarose gels and purified using a QIAquick Gel Extraction Kit (QIAGEN Company, Valencia, CA). PCR products were cloned into pGEM-T Easy vector (Promega Corporation), plasmids were transformed into E coli DH5α, and nucleotide sequences were then determined.
Cell Culture and Cell Treatment
Young adult mouse colon (YAMC) epithelial cells or kinase suppressor of Ras−1 knockout (KSRI−/−) mouse colon epithelial (MCE) cells were maintained in RPMI 1640 media with 5% fetal bovine serum (FBS) and 5 U/mL of murine interferon (IFN)-γ on collagen-coated plates and grown under permissive conditions at 33°C with 5% CO2.24 Before all experiments, cells were transferred to 37°C (nonpermissive) conditions with 0.5% FBS, IFN-γ-free media for 16 hours. The human colonic epithelial carcinoma cell line, HT 29 cells, was grown in DMEM media supplemented with 10% FBS at 37°C. Cells were serum starved (0.5%) at 37°C for approximately 16 hours before experiments. Cells were treated with purified p75, p40, LGG-CM, or 107 CFU/mL of bacteria (20:1 ratio of bacteria to cells) for 2 hours or 1 hour before TNF (100 ng/mL) treatment. To compare the effects of soluble factors, cells were cultured in transwells with bacteria placed in the upper chamber, separated by a permeable filter (0.2 μm pore size).
Preparation of Cellular Lysates
After treatment, cell monolayers were washed twice with ice-cold PBS and then scraped into cell lysis buffer (20 mmol/L HEPES [pH 7.5], 1 mmol/L orthovandate, 50 mmol/L β-glycerolphosphate, 10 mmol/L sodium pyrophosphate, with leupeptin [10 μg/mL], aprotinin [10 μg/mL], PMSF [18 μg/mL], and 1% Triton-X-100). The scraped suspensions were centrifuged (14,000g, 10 minutes) at 4°C, and protein content was determined using DC protein assay (Bio-Rad Laboratories). Cellular lysates were mixed with Laemmli sample buffer, and proteins were separated by SDS-PAGE for Western blot analysis with anti-p75, anti-p40, anti-phospho-Ser 473 (P)-Akt, anti-Akt, anti-phospho-Tyr180/182 (P)-p38 MAPK, antiinhibitor of nuclear factor κB (IκB) α (Cell Signaling Technology, Beverly, MA), and anti-phospho-Thr183/Tyr185 (P)-extracellular signal-regulated kinase (ERK)1/2 MAPK (Promega, Madison, MI).
Colon Organ Culture
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Vanderbilt University. Six- to 8-week-old C57BL/6 mice were killed; the colon was opened and washed with sterile PBS and DMEM media, and then cut into 4 × 4 mm pieces. The colon explants were laid on Netwell inserts (membrane mesh size of 500 μmol/L; Corning Incorporated Life Sciences, Acton, MA) with the serosal layer facing the insert. DMEM containing 0.5% FBS was filled to a point just over the epithelium and incubated at 37°C with 5% CO2 for 2 hours before treatment. At the end of the experiment, colon tissue was fixed in 4% paraformaldehyde at 4°C overnight before sectioning. Paraffin-embedded tissue sections were stained with H&E for light microscopic assessment of epithelial injury or for apoptosis assays.
Apoptosis Assays
Apoptosis was detected in colon tissue slides by ApopTag In Situ Oligo Ligation (ISOL) Kit (Intergen Company, Purchase, NY) using T4 DNA ligase according to the manufacturer’s guidelines or by using antiactive caspase-3 antibody (BD Biosciences, Palo Alto, CA) staining and reagents provided in the Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA). Differential interference contrast (DIC) microscopy was used as we have previously reported.24 Apoptotic cells were determined by counting the absolute number of positive stained cells in at least 300 colonic crypts.
Apoptosis in cell lines was detected by 2 methods. ApopTag In Situ Apoptosis Detection Kits (TUNEL; Intergen Company) and DAPI staining were described previously.24,25 The slides were observed by fluorescence microscopy, and the number of positive stained cells within a population of at least 500 cells was counted to determine the proportion of apoptotic cells. For Annexin V-FITC staining, attached cells were dissociated using Accutase (Innovative Cell Technologies, Inc., San Diego, CA) and double stained with Annexin V-FITC and propidium iodide (Calbiochem/EMD Biosciences, Darmstadt, Germany) according to the respective manufacturer’s instructions. The percentage of cells positive for Annexin V and propidium iodide was determined by flow cytometry.
Cell Proliferation Assays
After YAMC cells cultured in 96-well dishes were treated with LGG, purified p75, or purified p40 for 24 hours, cells were incubated with CellTiter 96 AQueous One Solution Reagent (Promega Corporation) for 1 hour to label viable cells. The absorbance at 590 mm was detected using a 96-well plate reader. Cell numbers were determined by comparing the absorbance of samples to the standard cell-absorbance curve generated for each experiment. The change in the number of control cells from the start to the end of an experiment was standardized as 100%. The changes in the treated cells were reported as a percentage relative to the untreated control.
Cell proliferation was also determined by immunostaining of cells on chamber slides with antiproliferative cell nuclear antigen (PCNA) antibody (Santa Cruz Biotechnology) using reagents provided in the Vectastain ABC kit (Vector Laboratories, Inc.) and visualization of cells by DIC microscopy. At least 500 cells were counted to determine the percentage of cells with positive PCNA nuclei.
Statistical Analysis
The statistical significance of the difference between mean values was determined using paired Student t test analysis. The level of statistical significance was set at P < .05. Data were analyzed as the mean ± SD.
Results
Purification of 2 proteins, p75 and p40, From LGG-s
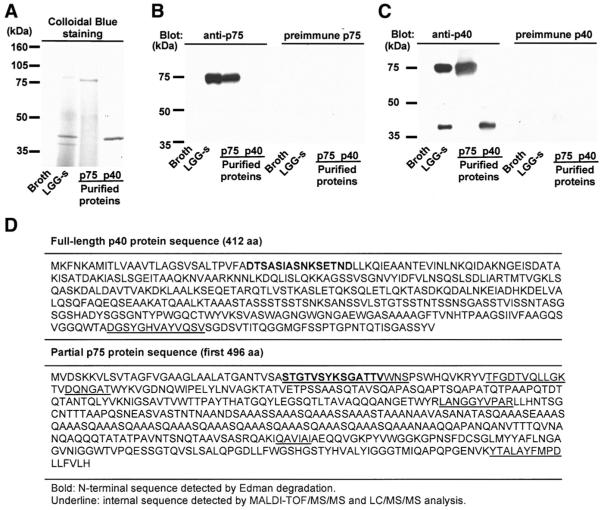
Soluble factors recovered from LGG-s and LGG-CM have been shown to regulate intestinal epithelial signaling pathways and biologic functions,19,22 but the identities of these factors are unknown. Therefore, we pursued characterization of these soluble factors to provide insight into the molecular mechanisms of probiotic bacterial actions on host cells. As a first step, proteins in the culture supernatant of LGG broth cultures were analyzed by SDS-PAGE and Coomassie blue staining. Two of the most abundant proteins had molecular masses of approximately 75 kilodaltons and 40 kilodaltons. To purify these proteins from LGG-s, filtered LGG-s was loaded onto UNOsphere S ion exchange media with negatively charged functional groups, and bound proteins were eluted using 30 mmol/L Tris (pH 7.3) containing progressively increasing concentrations of NaCl from 0.1 mol/L to 0.8 mol/L. Eluted proteins were analyzed by SDS-PAGE to identify proteins contained in each fraction (data not shown). The 75-kilodalton protein (p75) and 40-kilodalton protein (p40) eluted from the cation exchange resin at NaCl concentrations of 0.25 mol/L and 0.5 mol/L, respectively (Figure 1A). SDS-PAGE analysis indicated that the chromatography procedure successfully separated p75 and p40.
Figure 1.
Purification and sequencing of p75 and p40 purified from LGG-s. Filtered LGG culture supernatant was loaded onto a cation exchange column. Proteins bound to exchange media were eluted using Tris buffer containing sequential concentrations of NaCl (100–800 mmol/L). Eluted proteins were separated by SDS-PAGE and stained with Colloidal Blue Staining Kit (A, lanes 3 and 4). Proteins present in concentrated fractions of broth and LGG-s using a 5-kilodalton cut-off filter are shown (A, lanes 1 and 2, respectively). Polyclonal antibodies against p75 or p40 were generated as detailed in the Materials and Methods section and used in Western blot analysis (B and C). N-terminal sequences (bold) and internal peptide sequences (underline) of p75 and p40 were detected by Edman degradation or MALDI-TOF/MS/MS and LC/MS/MS analysis, respectively (D). LGG genetic sequences encoding p75 and p40 were determined as described in the Materials and Methods section, and predicted amino acid sequences were deduced from the nucleotide sequences (D).
Anti-p75 and anti-p40 polyclonal antibodies were generated using chromatographically purified p75 or p40, respectively. Western blot analysis indicated that the anti-p75 antiserum recognized p75 only (Figure 1B), and the anti-p40 antiserum reacted with both p40 and p75 (Figure 1C). Preimmune sera did not react with either p75 or p40 (Figures 1B and 1C). Immunoblotting experiments indicated that the purified p75 preparation was not contaminated with p40, and the purified p40 preparation was not contaminated with p75 (Figures 1B and 1C).
Characterization of p75 and p40 Proteins
As a first step in characterizing p75 and p40, N-terminal sequences and internal peptide sequences of these proteins were determined, as described in the Materials and Methods section. When compared with sequences in the NCBI microbial genome database, the p75 and p40 peptide sequences were most closely related to 2 proteins predicted to be encoded by the genome of L casei 334 (NCBI GeneBank accession numbers COG0791 and COG3883, respectively). Multiple oligonucleotide primers were designed based on the sequences of the corresponding L casei genes and flanking DNA sequences in the L casei genome, and these primers were used to PCR-amplify related sequences from LGG genomic DNA.
Sequence analysis of 1 set of cloned PCR products revealed the presence of a 1236-base pair (bp) open reading frame (ORF), predicted to encode a 412 amino acid residue protein with a calculated molecular mass of 42 kilodaltons (Figure 1D). The experimentally determined N-terminal amino acid sequence of LGG p40, as well as an internal peptide sequence of p40, was identified within the deduced amino acid sequence encoded by this ORF (Figure 1D). The deduced full-length amino acid sequence of p40 was 79% identical to the sequence of a 396 amino acid protein of unknown function in L casei 334 (NCBI GeneBank COG3883).
Sequence analysis of another set of cloned PCR products revealed the presence of a partial ORF that was > 1488 bp in length; the full-length ORF was not successfully amplified. The experimentally determined N-terminal amino acid sequence of p75 and internal peptide sequences of p75 were identified within the deduced amino acid sequence encoded by this partial ORF (Figure 1D). The deduced amino acid sequence of p75 was most closely related to a 493 amino acid cell wall-associated hydrolase of L casei 334 (NCBI GeneBank COG0791) and exhibited 70% and 93% identity to 2 different regions of this L casei 334 protein, respectively. The predicted molecular mass of the full-length cell wall-associated hydrolase of L casei 334 (49 kilodaltons) differs substantially from the molecular mass of the LGG p75 protein.
An analysis of the LGG p40 and p75 gene sequences and the experimentally determined N-terminal amino acid sequences of the encoded proteins indicates that both genes encode proteins with N-terminal signal sequences. The presence of signal sequences is consistent with the hypothesis that p40 and p75 are actively secreted into the culture supernatant by LGG. The p40 gene sequence and the partial p75 gene sequence do not show significant relatedness, and the experimentally determined N-terminal amino acid sequences of these 2 proteins are not related. Thus, based on the available sequence data, there is no evidence to suggest that p40 is a degradation product of p75. However, it is possible that there could be sequence similarity between p40 and the uncharacterized C-terminal portion of p75.
p75 and p40 Stimulate Akt Activation in Intestinal Epithelial Cells
Because we have reported that factors recovered from LGG-s stimulate Akt activation,19 we first determined the effect of p75 and p40 on this signal transduction pathway. Cells were treated with various concentrations of p75 and p40, and activation of Akt was determined by Western blot analysis using an antibody against phosphorylated Akt. We found that p75 at 10 –1000 ng/mL and p40 at 1–100 ng/mL stimulated Akt activation in a concentration-dependent manner in either mouse or human colon epithelial cells (Figure 2A). p75 At 100 ng/mL and p40 at 10 ng/mL were chosen for subsequent experiments in this paper because these are the minimal concentrations for inducing detectable Akt activation. As controls, we tested the flow-through fraction from the ion exchange column and the fraction eluted from the column with 0.1 mol/L NaCl: neither of these preparations contained p75 or p40, and neither of these preparations activated Akt (data not shown). As with LGG-s, Akt activation by p75 or p40 was PI3K-dependent because PI3K inhibitors LY294002 and Wortmannin (data not shown) blocked p75 and p40 activation of Akt (Figure 2B). Because we have previously reported that LGG, but not LGG-CM, inhibits TNF-stimulated activation of p38,19 we tested whether the purified LGG-s proteins modulate this signaling pathway. Similar to LGG-CM, p75 and p40 showed no effect on TNF activation of p38 (Figure 2D). Furthermore, p75 and p40 were also not able to induce ERK1/2 or p38 activation or IκBα degradation (15-minute to 4-hour treatments, 2-hour treatment data are shown in Figure 2D) or inhibit TNF effects on these pathways (Figure 2D). Therefore, purified p75 and p40 are selective in their activity for regulating intestinal epithelial cell signal transduction.
Figure 2.
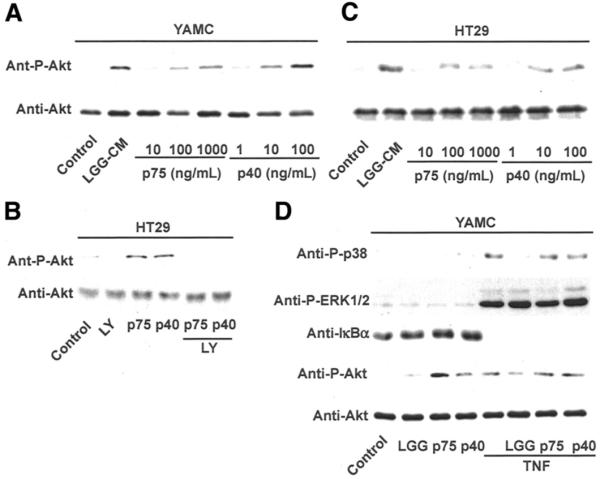
p75 and p40 Stimulate Akt activation in mouse and human colon epithelial cells. YAMC (A and D) and HT29 (B and C) cells were treated with purified p75 or p40 at the indicated concentrations for 2 hours in the presence or absence of 30-minute pretreatment of PI3K inhibitor LY294002 (10 μmol/L; C). Akt, p38, ERK1/2 MAPK activation, and IκBα degradation were detected by Western blot analysis of cellular lysates with indicated antibodies. Data are representative of 5 separate experiments.
p75 and p40 Inhibit TNF-Induced Intestinal Epithelial Cell Apoptosis and Organ Culture Lesions
To determine the biologic roles of purified proteins from LGG-s, we next evaluated the effects of p75 and p40 on cytokine-induced apoptosis in intestinal epithelial cells. We detected intestinal epithelial cell apoptosis and distinguished it from necrosis by using 3 different methods. TUNEL and ISOL assays measure apoptosis by specifically labeling fragmented genomic DNA with terminal deoxynucleatidyl transferase or T4 DNA ligase, respectively. Annexin V staining detects apoptosis based on FITC-conjugated Annexin V specifically binding to phosphatidylserine once it is exposed to the outer layer of the plasma membrane during the apoptotic process.26
TNF-induced apoptosis detected by TUNEL assay in KSRI−/− MCE cells, a mouse colon cell line null for KSRI expression that undergoes apoptosis following TNF treatment,24 was inhibited by either p75 or p40 (Figure 3A and B). As expected, TNF-induced apoptosis was also inhibited by LGG coculture.19 These results were confirmed in HT29 cells, a human intestinal cell line, by Annexin V-FITC staining. The “cytokine cocktail” combination of TNF, interleukin (IL)-1α, and IFN-γ induced apoptosis in HT29 cells, which was reversed by either p75 or p40 treatment (Figures 3C and D).
Figure 3.
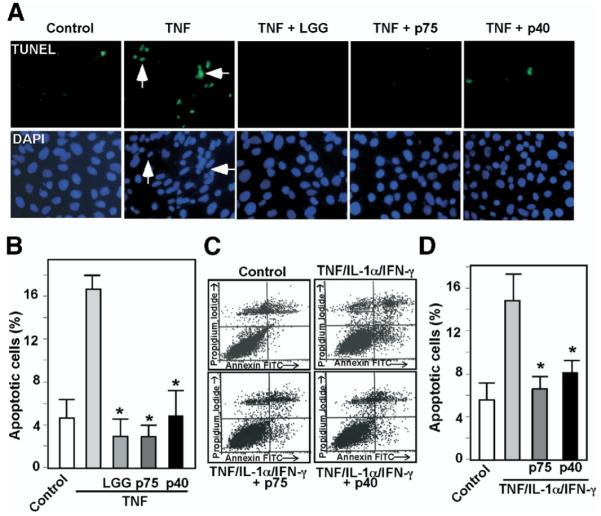
p75 and p40 Inhibit cytokine-induced apoptosis in intestinal epithelial cells. KSRI−/− MCE cells (A and B) or HT29 cells (C and D) were treated with TNF (100 ng/mL) for 6 hours or the “cytokine cocktail” combination of TNF (100 ng/mL), IL-1-α (10 ng/mL), and IFN-γ (100 ng/mL) for 16 hours, respectively, in the presence or absence of 1-hour pretreatment with viable LGG, p75 (100 ng/mL), or p40 (10 ng/mL). LGG, p75, and p40 were maintained during the entire course of cytokine treatment in all experiments shown in this paper. KSR−/− MCE cells were fixed for TUNEL with apoptotic nuclei labeled with FITC and DAPI staining (A). FITC and DAPI labeled images were taken from the same field. Arrows indicate representative apoptotic nuclei. The percentage of cells undergoing apoptosis is shown (B). HT29 cells were dissociated and stained with Annxin V-FITC and propidium iodide and analyzed by flow cytometry (C). Results are shown as density plots with Annxin V-FITC vs propidium iodide (D). Viable cells have low Annexin V-FITC and low propidium iodide staining (lower left quadrant); early apoptotic cells have high Annexin V-FITC and low propidium iodide staining (lower right quadrant); late apoptotic cells have high Annexin V-FITC and high propidium iodide staining (upper right quadrant); and necrotic cells have low Annexin V-FITC and high propidium iodide staining (upper left quadrant). The early apoptotic cell populations in the lower right quadrant are shown in D. *P < .01 compared with TNF (B) or the “cytokine cocktail” (D), respectively. Experiments were performed on at least 3 separate occasions.
To determine the potential of p75 and p40 to regulate colon epithelial homeostasis, we performed colon organ culture and observed the direct effects of p75 and p40 on colon epithelium ex vivo as detailed in the Materials and Methods section. Histologic sections prepared for injury assessment showed that TNF induced massive mucosal necrosis and disruption of epithelial integrity in colon explants. Coculture with p75, p40, or LGG with TNF restored colonic epithelial integrity and the crypt structure of the colon crypts (Figure 4).
Figure 4.
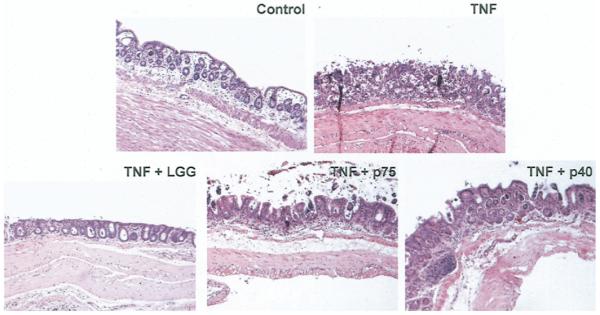
p75 and p40 Rescue TNF-induced epithelial damage in cultured mouse colon explants. Colon explants derived from 6- to 8-week old C57BL/6 mice were cultured in DMEM containing 0.5% FBS and treated with TNF (100 ng/mL) for 24 hours in the presence or absence of LGG, p75 (100 ng/ mL), or p40 (10 ng/mL). Paraffin-embedded tissue sections were stained with H&E for light microscopic assessment of epithelial damage (original magnification, ×10). Images shown are representative of 7 mice in each group.
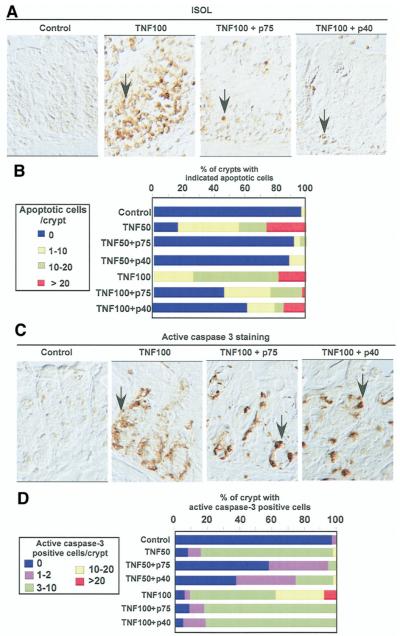
Coculture of colon tissue explants with TNF induced colon epithelial apoptosis with 75% of crypts containing more than 10 apoptotic cells. This effect was decreased 3-fold by p75 or p40 cotreatment (P < .005, Figures 5A and B). Because caspase-3 is a major regulator of the apoptotic program and PI3K-dependent Akt activation has been shown to prevent TNF-induced caspase-3 activation,27 we determined the effect of p75 and p40 on caspase-3 activity using immunostaining with an antiactive caspase-3 antibody. TNF stimulated caspase-3 activation in intestinal epithelial cells, which was significantly inhibited by p75 or p40 coculture (Figures 5C and D).
Figure 5.
p75 and p40 Inhibit TNF-induced apoptosis in cultured mouse colon explants. Mouse colon explants prepared as in Figure 4 were treated with TNF (50 ng/mL, TNF50 or 100 ng/mL, TNF100) for 24 hours in the presence or absence of LGG, p75 (100 ng/mL), or p40 (10 ng/mL). Paraffin-embedded tissue sections were studied for apoptosis using ISOL staining. Apoptotic nuclei labeled with peroxidase were visualized using DIC microcopy (A). Caspase-3 activity was determined by immunohistochemistry using antiactive caspase-3 antibody (C). The percentage of crypts with indicated apoptotic nuclei (B) or positive active caspase-3 (D) cells is shown. Arrows indicate examples of ISOL or caspase-3-positive cells. The percentage shown here is the average representing 5 independent experiments. All images were taken with ×40 magnification.
Thus, in mouse and human intestinal epithelial cell lines and cultured mouse colon explants using 3 different apoptotic assays, both p75 and p40 exert significant inhibitory effects on TNF-induced apoptosis. These effects may be involved in protective roles on TNF-induced intestinal epithelial lesions.
p75 and p40 Stimulate Intestinal Epithelial Cell Growth
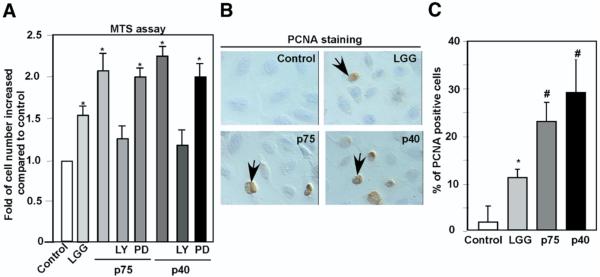
The homeostatic balance between proliferation and apoptosis regulates normal gastrointestinal epithelial morphology and function. Probiotic bacteria have been shown to enhance intestinal epithelial cell proliferation.28 Furthermore, PI3K and Akt have been reported to play a critical role in regulating this proliferative response in intestinal epithelial cells.29 Therefore, we evaluated the effects of p75 and p40 on cellular proliferation using a 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)– based proliferation assay. Both p75 and p40 promoted YAMC cell growth, which was inhibited by the PI3K inhibitor LY294002 but not the MEK inhibitor PD98059 (Figure 6A). To further characterize p75 and p40 effects on intestinal cellular proliferation, PCNA staining was used to detect proliferative nuclei. p75 and p40 Enhanced the number of PCNA-positive nuclei (Figure 6B and C). These results suggest that p75 and p40 help to restore intestinal epithelial integrity after TNF-induced injury through not only preventing apoptosis but also enhancing proliferation.
Figure 6.
p75 and p40 Promote intestinal epithelial cell proliferation. YAMC cells plated in 96-well dish were treated with LGG, p74 (100 ng/mL), or p40 (10 ng/mL) for 24 hours. At the end of treatment, viable cells were counted using MTS-based assays. The change in the number of control cells from the start to the end of an experiment was standardized as 100%. Changes in the treated cells were reported as a percentage relative to the untreated control (A). Cells cultured on chamber slides treated as indicated were immunostained with anti-PCNA antibody. Peroxidase-labeled positive cells, indicated by arrows, were observed by DIC microscopy (B). At least 500 cells were counted to determine the percentage of PCNA-positive cells (C). *P < .01, #P < .001 compared with control. Data represent at least 3 separate experiments.
Immunodepletion of p75 and p40 From LGG-CM Blocks LGG-CM Biologic Effects on Intestinal Epithelial Cells
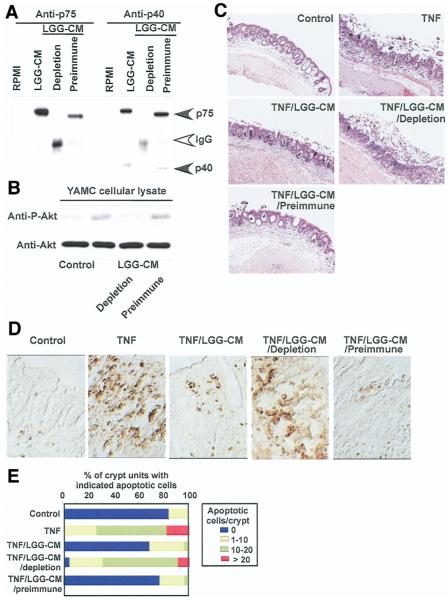
To test by another approach whether p75 and p40 are required for the regulatory effects of LGG-CM on intestinal epithelial cells, antibodies against p75 and p40 were used for sequential immunoprecipitation to immunodeplete both p75 and p40 from LGG-CM. Immunoprecipitation with these 2 antibodies, but not preimmune sera, efficiently removed p75 and p40 from LGG-CM (Figure 7A). These immunodepleted LGG-CM fractions were used to treat cells or colon culture explants. Immunodepletion of p75 and p40 from LGG-CM attenuated Akt activation (Figure 7B). Furthermore, LGG-CM, but not immunodepleted LGG-CM, prevented TNF-induced ulcerative lesions, including mucosal necrosis and disruption of epithelial integrity (Figure 7C) and epithelial apoptosis in colon organ cultures (Figure 7D and E). Individual immunodepletion of either p75 or p40 partially attenuated Akt activation and the antiapoptotic response (data not shown). These results indicate that p75 and p40 are required for LGG-CM regulation of intestinal epithelial cells and suggest that they may have redundant functions.
Figure 7.
Immunodepletion of p75 and p40 blocks LGG-CM’s antiapoptotic effects on colon epithelial cells. Immunodepletion of p75 and p40 was performed by sequential immunoprecipitation of LGG-CM with anti-p75 and p40 antibodies, characterized in Figure 1, to remove both p75 and p40 from LGG-CM. Preimmune sera were used as a control. Proteins present in LGG-CM, LGG-CM immunodepleted with antibodies (LGG-CM depletion), or preimmune sera (LGG-CM preimmune) were separated by SDS-PAGE for Western blot analysis with anti-p75 and p40 antibodies (A). LGG-CM, LGG-CM depletion, and LGG-CM preimmune were used to treat YAMC cells to detect Akt activation as shown in Figure 2 (B) or C57BL/6 mouse colon explants described as in Figure 5 in the presence or absence of TNF (100 ng/mL) for 24 hours. Paraffin-embedded tissue sections were stained with H&E for light microscopic assessment of epithelial damage (C; original magnification, ×10) and ISOL staining to detect epithelial cell apoptosis using DIC microscopy (D; original magnification, ×40). The percentage of crypts with indicated apoptotic cells is shown (E). Data represent mean scores from at least 3 experiments.
Strain-Specific Expression of p75 and p40 by Lactobacilli
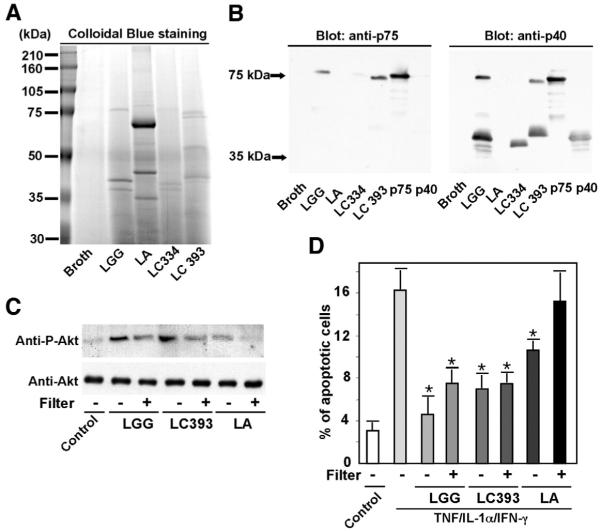
Because we have reported that probiotic Lactobacilli regulation of cell survival is strain specific,19 we next investigated whether expression of soluble p75 and p40 proteins was also strain specific. To address this question, we studied 3 other strains of Lactobacillus—Lactobacillus casei 334, Lactobacillus casei 393, and Lactobacillus acidophilus—which are known to regulate intestinal function16,30 or lymphocyte proliferation and immunity.31 Conditioned-media derived proteins from 105 of these respective Lactobacillus strains were separated by SDS-PAGE (Figure 8A). p75 and p40 Antisera recognized proteins of similar size in culture supernatant from L casei 393-CM, but no immunoreactive proteins were detected in culture supernatant from L acidophilus-CM (Figure 8B). The p40 antiserum recognized a 38-kilodalton protein produced by L casei 334-CM (Figure 8B), which is consistent with relatedness between LGG p40 and a protein of unknown function encoded by L casei (NCBI GeneBank COG3883). Weak reactivity of the p75 antiserum with a 75-kilodalton L casei protein was detectable only if films were overexposed (data not shown).
Figure 8.
Production of p75 and p40 by Lactobacilli is strain specific. Concentrated proteins recovered from indicated bacterial-conditioned cell culture media using a 5-kilodalton cut-off filter were separated by SDS-PAGE and stained with Colloidal Blue Staining Kit (A), and Western blot analysis of these proteins with anti-p75 and anti-p40 antibodies was performed (B). To test the effects of bacteria-derived soluble factors on colon cells, the indicated bacteria were separated from YAMC cells by 0.2-μmol/L filters during coculture experiments for 2 hours. Cellular lysates were collected and Akt activation determined as in Figure 2 (C). To test these soluble factors’ effects on preventing apoptosis, bacteria were separated in transwell cocultures as in C, with HT29 cells for 1 hour followed by 16-hour cotreatment with “cytokine cocktail” containing TNF (100 ng/mL), IL-1α (10 ng/mL), and IFN-γ (100 ng/mL). Cells were then prepared for apoptosis assays using Annexin V-FITC staining as in Figure 3. The percentage of the early apoptotic cell populations is shown (D). LC334, Lactobacillus casei 334; LC393, Lactobacillus casei 393; LA, Lactobacillus acidophilus. *P < .01, compared with TNF/IL-1α/IFN-γ. Data represent 3 separate experiments.
To investigate the effects of soluble factors produced by these Lactobacillus species on epithelial cells, bacteria were cocultured with the epithelial cells but were physically separated by a filter (0.2 μmol/L) so that any observed effects would be mediated by soluble factors. Although all of the strains stimulated Akt activation and inhibited cytokine-induced apoptosis when in direct contact with epithelial cells, LGG, L casei 334, and L casei 339 but not L acidophilus supernatants promoted Akt activation (Figure 8C) and prevented TNF-induced apoptosis (Figure 8D). Strain-specific production of soluble p75 and p40 proteins and the correlation between expression of these proteins and observable phenotypes provides further evidence that these bacterial components can promote intestinal epithelial cell survival and inhibit cytokine-induced apoptosis.
Discussion
Probiotics have recently received clinical attention for their potential to prevent and/or treat IBD.9 We previously showed that LGG-derived soluble factors regulate cell survival signaling and inhibit cytokine-induced apoptosis in intestinal epithelial cells.19 Here, we report purification of 2 LGG-derived soluble proteins (p75 and p40) and demonstrate that both of these proteins suppress cytokine-induced colon epithelial apoptosis and injury. These findings provide a molecular basis for therapeutic application of probiotic bacterial products for inflammation-mediated intestinal disorders. Future in vivo studies applying p75 and p40 to regulate intestinal inflammatory responses in animal models will help to determine the feasibility of their use as novel treatments for IBD.
Although LGG has been shown to induce remission and prevent recurrence of IBD in patients14 and in animal models of colitis,12 a clinical trial designed to test the efficacy of LGG as an adjunct to standard therapy in children with Crohn’s disease showed no beneficial effect of LGG in maintaining remission.32 These results emphasize a current problem regarding the use of probiotic therapy, namely the difficulty determining the bioavailability of bacteria in the gastrointestinal tract. In addition, use of live probiotic bacteria raises concerns because of several cases of bacteremia associated with probiotic therapy in very young33 and immunocompromised patients.34 Therefore, one approach to address these questions may be to use probiotic bacterial-derived proteins as novel therapeutic agents for treatment of IBD and other inflammation-related disorders.
Commensal bacteria engage in active cross talk with the intestinal epithelium to promote epithelial development,35 facilitate nutrient digestion and uptake by epithelial cells,36 and exert protective roles on intestinal inflammation.37 The known mechanisms for these functions include enhancing production of anti-inflammatory cytokines, blocking production of proinflammatory cytokines, antagonism to pathogenic bacteria,18 increasing secretory-IgA production,38 and maintaining barrier function.15,16 However, the specific bacterial factors that attenuate epithelial inflammatory responses remain unclear. We provide 2 lines of evidence to support the importance of p75 and p40 in regulating intestinal homeostasis. First, we show that the Lactobacillus strain Lactobacillus acidophilus, whose culture supernatants do not contain p75 and p40, requires bacterial-cell contact for both Akt activation and suppression of cytokine-induced apoptosis (Figure 8). Consistent with these findings, Resta-Lenert and Barrett reported that Lactobacillus acidophilus regulation of epithelial barrier function depends on bacterial-cell interaction.15 Second, immunodepletion experiments show that loss of p75 and p40 from LGG-CM eliminates LGG-CM antiapoptotic effects on colon epithelial cells (Figure 7).
It should be noted that, when we analyzed proteins present in LGG-s, we focused on p75 and p40 because they are the 2 major bands seen on SDS-PAGE analysis of concentrated LGG-s (Figures 1A and 8A). LGG-s also contained a 17-kilodalton protein, which was recognized by anti-p40 antibody in Western blot analysis (data not shown). However, this 17-kilodalton protein did not bind to the ion exchange medium and therefore was not detected in chromatographically purified p75 or p40 fractions (data not shown). We speculate that this 17-kilodalton protein may be a degradation product of p40 but does not contain the functional domain of p40 required for Akt activation.
Only a partial ORF encoding LGG p75 was characterized in this study, and, therefore, it is difficult to compare fully the similarity between p75 and p40. However, 2 observations suggest that p75 and p40 may share some identical peptide sequences: (1) analysis of trypsin-digested peptides from p75 and p40 samples by MALDI-TOF/MS revealed several identical peaks in both preparations (data not shown), and (2) the p40 antibody recognized p75 in Western blot analysis (Figures 1B and 1C, Figure 8B). Therefore, further studies of the C-terminal portion of p75 will be required to determine whether there is any sequence similarity between these 2 proteins.
Bacterial regulation of host responses through the production of biologically active products has been described in several other bacteria. Staphylococcal aureus produces lipoteichoic acid to prevent delayed-type hypersensitivity reactions through activation of the platelet-activating factor receptor.39 In addition, Salmonella protects epithelial cells from apoptosis by sustained activation of Akt through the effector protein SopB.40 Interestingly, low-molecular-weight factors secreted by LGG (<10 kilodaltons) have been reported to stimulate Hsp25 and Hsp72 production by intestinal epithelial cells.22 However, we found that LGG-s containing factors > 5 kilodaltons, but not LGG-s filtrate with factors <5 kilodaltons, stimulates Akt activation.19 It is possible that multiple factors in LGG-s may regulate different epithelial cellular responses.
One long-term goal of these studies is to understand the molecular basis of p75 and p40 regulation of signaling pathways and cellular responses leading to inhibition of intestinal inflammation. Commensal bacteria inhibit inflammatory responses in part through nuclear factor (NF) κB,41,42 but we have not detected any effects of LGG, p75, or p40 on nuclear factor-κB activation in intestinal epithelial cells (Figure 2D).19 Because p75 and p40 activate Akt in a PI3K-dependent manner (Figure 2B), it is important to elucidate mechanisms of signaling pathways and downstream targets determining cellular survival in intestinal epithelial cells.
In summary, this report shows that purified p75 and p40 from LGG-s protect intestinal epithelial cells from apoptosis, promote proliferation, and activate Akt in a PI3K-dependent manner in both cell and organ culture models. These soluble protein effects are further confirmed by the fact that only Lactobacillus strains that produce p75 and p40 in their supernatant show independence of direct bacterial-cell interaction for these regulatory effects. To date, p75 and p40 are the first identified probiotic bacterial soluble proteins regulating intestinal epithelial homeostasis through specific cellular signal transduction pathways. These findings support a potential application of native bacterial components to prevent cytokine-mediated gastrointestinal injury and disease.
Acknowledgments
Supported by NIH grants DK 065744 (to F.Y.), DK56008 (to D.B.P.), and DK58404 (Vanderbilt University Digestive Disease Research Center); Atticus Trust; Vanderbilt University Medical Center Imaging Core Research Laboratory grant (CA-68485); and the Department of Veterans Affairs.
Abbreviations used in this paper
- DIC
differential interference contrast
- ERK
extracellular signal-regulated kinase
- IκB
inhibitor of nuclear factor κB
- ISOL
in situ oligo ligation
- KSR1
kinase suppressor of Ras-1
- LGG
Lactobacillus rhamnosus GG
- LGG-CM
LGG-conditioned cell culture media
- LGG-s
LGG broth culture supernatant
- MAPK
mitogen-activated protein kinase
- MCE
mouse colon epithelial
- PCNA
proliferative cell nuclear antigen
- PI3K
phosphatidylinositol-3′-kinase
- TNF
tumor necrosis factor
- YAMC
young adult mouse colon
References
- 1.Sartor RB. Mucosal immunology and mechanisms of gastrointestinal inflammation. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 7th ed. I. Saunders; Philadelphia: 2002. pp. 21–51. [Google Scholar]
- 2.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura M, Okazaki K, Nishio A, Nakase H, Tamaki H, Uchida K, Nishi T, Asada M, Kawasaki K, Fukui T, Yoshizawa H, Ohashi S, Inoue S, Kawanami C, Hiai H, Tabata Y, Chiba T. Therapeutic effects of rectal administration of basic fibroblast growth factor on experimental murine colitis. Gastroenterology. 2005;128:975–986. doi: 10.1053/j.gastro.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.McCole DF, Rogler G, Varki N, Barrett KE. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 6.Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNF-α neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Down-regulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumor necrosis factor α antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan F, Polk DB. Commensal bacteria in the gut: learning who our friends are. Curr Opin Gastroenterol. 2004;20:565–571. doi: 10.1097/00001574-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Lilly DM, Stillwell RH. Probiotics: Growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 11.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Dieleman LA, Goerres MS, Arends A, Sprengers D, Torrice C, Hoentjen F, Grenther WB, Sartor RB. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52:370–376. doi: 10.1136/gut.52.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol. 2004;4:5. doi: 10.1186/1471-230X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 19.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor α, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272–1280. doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan F, John SK, Polk DB. Kinase suppressor of Ras determines survival of intestinal cells exposed to tumor necrosis factor. Cancer Res. 2001;61:8668–8675. [PubMed] [Google Scholar]
- 26.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Trencia A, Perfetti A, Cassese A, Vigliotta G, Miele C, Oriente F, Santopietro S, Giacco F, Condorelli G, Formisano P, Beguinot F. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol Cell Biol. 2003;23:4511–4521. doi: 10.1128/MCB.23.13.4511-4521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, Sakata T. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig Dis Sci. 1999;44:2119–2123. doi: 10.1023/a:1026647024077. [DOI] [PubMed] [Google Scholar]
- 29.Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, Bourdet-Sicard R, Sansonetti PJ, Pedron T. Antiinflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 31.Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017), and Bifidobacterium lactis (HN019) Br J Nutr. 2000;83:167–176. doi: 10.1017/s0007114500000210. [DOI] [PubMed] [Google Scholar]
- 32.Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, Murray KF, Oliva-Hemker M, Rosh JR, Tolia V, Zholudev A, Vanderhoof JA, Hibberd PL. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–839. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 33.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–181. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 34.Apostolou E, Kirjavainen PV, Saxelin M, Rautelin H, Valtonen V, Salminen SJ, Ouwehand AC. Good adhesion properties of probiotics: a potential risk for bacteremia? FEMS Immunol Med Microbiol. 2001;31:35–39. doi: 10.1111/j.1574-695X.2001.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 35.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 37.Dotan I, Rachmilewitz D. Probiotics in inflammatory bowel disease: possible mechanisms of action. Curr Opin Gastroenterol. 2005;21:426–430. [PubMed] [Google Scholar]
- 38.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings SD, Perkins SM, Howard KM, Ishii S, Shimizu T, Travers JB. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J Clin Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 41.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-g and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. Epub December 21, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Neish AS. Molecular aspects of intestinal epithelial cell-bacterial interactions that determine the development of intestinal inflammation. Inflamm Bowel Dis. 2004;10:159–168. doi: 10.1097/00054725-200403000-00015. [DOI] [PubMed] [Google Scholar]