Abstract
Robotic surgery is a cutting edge and minimally invasive procedure, which has generated a great deal of excitement in the urologic community. While there has been much advancement in this emerging technology, it is safe to say that robotic urologic surgery holds tremendous potential for progress in the near future. Hence, it is paramount that urologists stay up-to-date regarding new developments in the realm of robotics with respect to novel applications, limitations and opportunities for incorporation into their practice. Robot-assisted surgery provides an enhanced 3D view, increased magnification of the surgical field, better manual dexterity, relatively bloodless field, elimination of surgeon′s tremor, reduction in a surgeon′s fatigue and mitigation of scattered light. All these factors translate into greater precision of surgical dissection, which is imperative in providing better intraoperative and postoperative outcomes. Pioneering work assessing the feasibility of robotic surgery in urology began in the early 2000's with robot-assisted radical prostatectomy and has since expanded to procedures such as robot-assisted radical cystectomy, robot-assisted partial nephrectomy, robot-assisted nephroureterectomy and robot-assisted pyeloplasty. A MEDLINE search was used to identify recent articles (within the last two years) and publications of specific importance, which highlighted the recent developments and future direction of robotics. This review will use the aforementioned urologic surgeries as vehicles to evaluate the current status and future role of robotics in the advancement of the field of urology.
Keywords: Bladder, cystectomy, laparoscopy, minimally invasive surgery, nephroureterectomy, nephrectomy, prostate, prostatectomy, pyeloplasty kidney, robotics
INTRODUCTION
Urology is a dynamic surgical discipline, which has undergone many developments and refinements over the past few decades. The advent of laparoscopic surgery was a major breakthrough in the urologic landscape and provided a minimally invasive alternative to conventional open procedures.[1] The decreased intraoperative estimated blood loss (EBL), shorter hospital stay and quicker return to function makes laparoscopic urologic surgery extremely appealing to physicians and patients alike.[2] The bottleneck with respect to laparoscopic urologic surgery seems to be the relatively long learning curve that is required for a surgeon to achieve proficiency. [2]
Robotic surgery using the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA) seems to remedy this extended learning curve [3] while offering the same, if not better, advantages as laparoscopic surgery.[2] The wristed instruments allow for improved dexterity in the confines of the pelvis and the robot is able to correct any tremor the surgeon may have.[2] In addition, optimal port placement translates into non-collision of the robotic arms and can be instrumental in performing a more precise surgery.[4] This, in conjunction with a 3D camera that can confer 15-fold magnification of the surgical field, allows for better preservation of critical anatomical structures, which translates into enhanced intraoperative and postoperative outcomes.[5] Numerous studies show that robotic surgery offers less intraoperative EBL, shorter hospital stays, less postoperative pain, decreased medical complications and quicker return to function than its open counterpart.[2,5,6] The frequently cited drawbacks of robotic surgery in the status quo relate to issues of cost, lack of tactile feedback and over-reliance on the patient side surgeon.[7] Recent advances such as the Fourth arm are beginning to provide more freedom to the robotic console surgeon,[8] while widespread adoption by more high-volume centres across the globe will help mitigate costs. The need for tactile feedback is somewhat remedied by the enhanced visual field and dexterity of robotic instrumentation, and it is important to note that the patient side surgeon has tactile feedback, which can provide insight into how to assist the console operator. New developments such as single-port and natural-orifice approaches are expanding the realm of possibility for this cutting-edge modality and, in our opinion, underscore the immense interest regarding the use of robotics in urology.[9]
This article will provide an overview of recent developments in various robotic procedures and then explore the future direction of robotic surgery in the development of the field of urology.
METHODS
A MEDLINE search was performed for keywords such as “robotics”, “robot-assisted surgery”, “laparoscopy”, “robot-assisted radical prostatectomy”, “robot-assisted radical cystectomy”, “robot-assisted pyeloplasty”, “robot-assisted partial nephrectomy”, “robot-assisted pyelolithotomy”, to name a few. Articles were selected that fit the scope of the topic with special consideration given to articles published in 2009-2010, and also landmark papers in the field and publications describing the future outlook of robot-assisted urologic surgery.
PROSTATE
Robot-assisted radical prostatectomy
Robot-assisted radical prostatectomy (RARP) is rapidly gaining acceptance in the urologic community as a safe and efficacious treatment option for localised prostatic adenocarcinoma with comparable oncological outcomes as open and laparoscopic counterparts. [10] RARP seems to have overtaken retropubic radical prostatectomy (RRP) as the treatment of choice due to patient preference for minimally invasive surgical options.[5] Patient intrigue coupled with aggressive marketing of robotic surgery means that the majority of prostatectomies performed in the status quo utilises the da Vinci platform.[5] RARP has come a long way since the first large series appeared in the literature,[11] and a recent analysis by Menon et al. found that RARP provided acceptable rates of biochemical recurrence at 5 years for clinically localised prostate cancer.[12] This study was especially promising as it included a large cohort of patients for analysis and demonstrated that when an experienced and well-trained surgeon performs RARP, adequate long-term oncologic efficacy is obtained. [12] Newly emerging evidence reinforces this point, with RARP having lower rates of positive surgical margins than RRP and laparoscopic radical prostatectomy (LRP).[10]
In one of the largest studies to date, Menon et al. found that their Vattikuti Institute prostatectomy (VIP) technique achieved comparable oncological outcomes to conventional nerve-sparing modalities but offered 84% of patients total urinary control at a mean 12-month follow-up, with a further 8% using liners for reassurance purposes. [13] Furthermore, the VIP technique utilised the increased dexterity of the robot′s wristed instruments and high-magnification 3D view to ensure preservation of the lateral prostatic fascia, which conferred better erectile function postoperatively as compared to conventional open surgery. [11,13] The aforementioned results were corroborated in a meta-analysis from various high-volume centres, which revealed that RARP had better return of urinary continence and improved sexual function postoperatively than after open and laparoscopic modalities.[10] Patel et al. recently reported that the age of the patient had a significant effect on potency after RARP, with younger men having quicker return to sexual function at 6 weeks, 3, 6 and 12 months postoperatively.[14] Complication rates after RARP in a recent study of 2,500 patients were found to be 5.08%, with the vast majority of complications being either Clavien grade I or II. [15] RARP also seemed to have decreased intraoperative EBL, risk of intraoperative transfusion and anastomotic strictures[5] in comparison with RRP. [10] Coupled with the fact that RARP seems to have a shorter learning curve than LP,[16] it appears that the use of robotic surgery in the realm of localised prostatic adenocarcinoma will reach ever greater heights.
BLADDER
Robot-assisted radical cystectomy
In 2010, there are estimated to be over 70,000 new cases of cancer of urinary bladder in the US, which account for over 14,000 deaths.[17] The probability of developing carcinoma of the bladder increases as an individual gets older; moreover, in patients over the age of 80, bladder cancer becomes the fourth highest killer.[17] Therefore, in older patients who are acceptable surgical candidates, it is imperative that we utilise surgical techniques that will minimise stress inflicted to the body and allow for a smoother return to function. Robot-assisted radical cystectomy (RARC) offers an attractive minimally invasive alternative to the current gold standard of open radical cystectomy (ORC) for muscle-invasive bladder cancer and high-risk non-muscle-invasive disease.[18] The interest generated since the initial description of RARC[19–21] has been immense and larger case series are now appearing in the literature. Pruthi et al. reported their initial experience with 100 patients who underwent RARC and found that there were no positive surgical margins and that the mean hospital stay was 4.9 days, with mean bowel movement being at 2.8 days.[22] The complication rate appeared to be 36%, with 8% of these being Clavien grade III or higher.[22] In a mean follow-up of 21 months, the authors reported that 15 patients manifest recurrent malignancy with 6 individuals succumbing to their disease.[22] The same group reported the first prospective randomised trial of ORC versus RARC in 41 patients and found that there was no significant difference in postoperative complication rate (33% RARC vs. 50% ORC; P = 0.279) and mean hospital stay (5.1 days RARC vs. 6.0 days ORC; P = 0.239).[23] The investigators reported that RARC had a longer operative time than ORC (4.2 vs. 3.5 hr; P < 0.001 ), but that there was less intraoperative EBL associated with RARC (258 vs. 575 ml; P < 0.001).[23] RARC also appeared to confer quicker time-to-bowel movement and time to flatus with less use of narcotic analgesics for pain relief.[23] This landmark study used a prospective randomised clinical trial to demonstrate that RARC was not inferior in comparison to ORC and matched up favourably with respect to various intraoperative and postoperative outcomes.[23] While there is still much work that needs to be done to assess long-term oncological outcomes, RARC is an evolving technique that affords patients and physicians alike an efficacious minimally invasive treatment option in the treatment of bladder cancer.
Robot-assisted partial cystectomy
Robot-assisted partial cystectomy (RAPC) for the treatment of malignant bladder lesions was recently performed in three patients and found to be technically feasible with acceptable intraoperative and postoperative outcomes.[24] We have had similar experience at our institution with RAPC in three patients and found that mean operative time, mean EBL and average hospital stay were all within satisfactory limits. [25] The procedure also seems to confer satisfactory short-term oncological outcomes and provides a bladder sparing surgical option in select patients.
KIDNEY
Robot-assisted partial nephrectomy
Robot-assisted partial nephrectomy (RAPN) was first described in 2004 by Gettman et al.[26] It has since enjoyed widespread adoption at many high-volume centres. Recent evidence suggests that RAPN offers equivalent oncological control to open partial nephrectomy (OPN) and laparoscopic partial nephrectomy (LPN) while providing the additional benefit of shorter hospital stay, less intraoperative EBL and shorter warm ischaemia time (WIT).[27] In an analysis of over 100 RAPN and LPN cases, no significant difference was found in the rate of focal positive margins between the two modalities. [28] While it may be too early to assess long-term oncological control in this relatively new surgical technique, early results from a series of 100 RAPN showed no tumour recurrence at 12 months.[29] Intraoperative EBL during partial nephrectomy has been shown to be an accurate predictor of early and late recovery of kidney function,[30] and considering that 26% of patients undergoing partial or radical nephrectomy have some degree of renal impairment preoperatively, [31] RAPN holds the promise of better long-term nephron preservation. Studies also show that RAPN generally provides shorter WIT as compared to LPN. [27] This seems to hold true even in cases that require calyceal repair, have complex renal tumours or have multiple tumours.[27] New evidence reveals that RAPN has a relatively short learning curve with regard to parameters such as acceptable WIT and total operative time.[3] All the aforementioned advantages suggest, in our opinion, that RAPN will garner widespread acceptance as the minimally invasive treatment of choice for small renal masses. Figure 1 shows a stepwise demonstration of RAPN.
Figure 1.
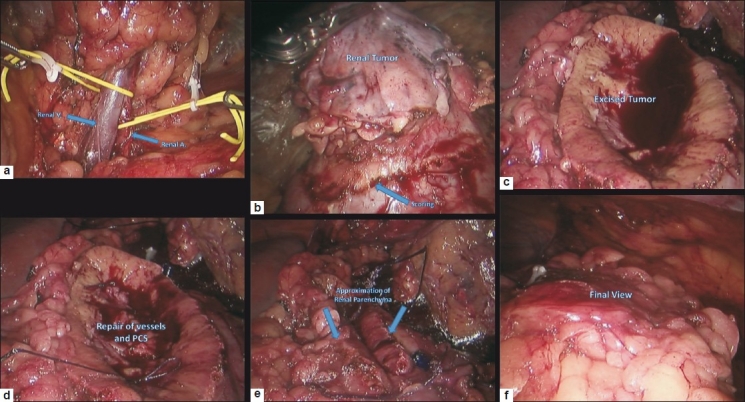
Stepwise demonstration of robot assisted partial nephrectomy; (a) Dissected renal hilum demonstrating renal artery (RA) and renal vein (RV); (b) Dissected renal tumor and renal scoring performed prior to clamping the RA; (c) View showing the renal parenchyma after tumor excision; (d) Repair of pelvicalyceal system (PCS) and small renal vessels; (e) Renal parenchyma reconstruction (renorrhaphy); and (f) final view showing closure of Gerota's fascia
Early results from trials of selective renal artery clamping in efforts to further decrease renal ischaemic damage have been promising and are especially attractive in patients with already compromised kidney function. [32] We recently compared the efficacy of clamping the renal artery alone versus both the renal artery and vein in 95 patients and found that clamping only the renal artery was associated with decreased EBL, decreased WIT, decreased operative time and less increase in serum creatinine (unpublished data). The feasibility of natural orifice translumenal endoscopic surgery (NOTES) for RAPN was recently assessed in a porcine model and while WIT was within acceptable standards, the great technical and surgical difficulty conferred with existing robotic instrumentation made the procedure especially laborious. [33] We believe that significant modifications in robotic design are necessary before such techniques are ready to enter the mainstream.
Robot-assisted pyeloplasty
Robot-assisted pyeloplasty (RAP) provides a viable alternative to the current gold standard open approach for the treatment of ureteropelvic junction (UPJ) obstruction.[34] Gettman et al. reported one of the earlier comparisons of RAP with the laparoscopic approach and found that the robotic method was associated with less operating time.[35] Gupta and colleagues reported their initial experience with 86 patients and found that RAP was associated with a mean operative time of 121 minutes (mean anastomosis time of 47 minutes), mean EBL of 45 ml and mean hospital stay of 2.5 days.[34] Most importantly, the success rate was found to be 97% at a mean follow-up of 13.6 months.[34] A nonrandomised comparison of 30 patients who underwent RAP versus 30 patients who had laparoscopic pyeloplasty showed that RAP had decreased average operating time (98.54 vs. 142.25 min; P < 0.001), shorter suturing and antegrade stenting time (33.21 vs. 57.11 min; P < 0.001) and less dissection time (33.11 vs. 51 min; P < 0.001).[36] RAP also provided less average EBL (40.36 vs. 101 ml; P = 0.035) and shorter mean hospital stay (2.5 vs. 5.5 days; P = 0.036).[36] It is important to note that all 60 procedures in the above study were performed by a single surgeon who was an expert in both robotic and laparoscopic modalities and had passed the learning curve for both procedures. The authors thought that the robotic approach correlated more than laparoscopy with ease of dissection, efficiency in the tailoring of pelvic flaps and elegance of suturing. [36] Recent reports have shown that RAP can be employed efficaciously in cases of complicated UPJ obstruction, which include horseshoe kidney, malrotated kidney, ectopic kidney and giant hydronephrosis, to name a few.[37] Gupta et al. also described a transmesocolic approach to RAP for left UPJ obstruction in 24 patients, which had a perfect success rate at a mean 1-year follow-up with no repeat obstructions.[38] We have experience of over 100 cases of RAP and believe that it is an effective surgical technique for correction of UPJ obstruction.
Robot-assisted nephroureterectomy with excision of the bladder cuff
Upper tract transitional cell carcinoma (TCC) is treated in the status quo with open nephroureterectomy with excision of the bladder cuff. Early feasibility studies show that robot-assisted nephroureterectomy with excision of the bladder cuff (RANUT) provides a viable treatment option for this long and technically challenging procedure.[39] Eandi and colleagues reported their initial experience with 11 patients who underwent RANUT for upper tract TCC and showed promising short-term intraoperative and postoperative outcomes with regard to oncological efficacy, hospital stay, EBL and operative time. [40] This was the first case series to utilise a completely robotic approach but required undocking and redocking of the da Vinci system during the procedure to allow for better surgical access. As can be inferred, this increased total operative time by 10-15 minutes. [40] We have recently assessed the feasibility of a new technique for RANUT with excision of the bladder cuff that does not require undocking of the robot. [41] In what comprised the largest series of RANUT till date, 15 patients underwent RANUT with excision of the bladder cuff for upper tract TCC.[41] The mean operative time was 184 minutes, average EBL was 103 ml and mean hospital stay was 2.7 days. [41] More importantly, there were no complications, no positive surgical margins and no cancer recurrence on short-term follow-up.[41] Compared to other series of RANUT, Hemal and colleagues reported that their technique was associated with less EBL, shorter operative time and less hospital stay.[41] They attribute this to the strategic placement of ports, which allowed for a seamless transition between the nephrectomy portion and excision of bladder cuff part of the case.[41] Additionally, the careful handling of the ureter and tactical bladder reconstruction were also instrumental in improved intraoperative and postoperative outcomes. [41]
Robotic management of urolithiasis
Percutaneous nephrolithotomy is the current treatment of choice for large renal stones but robot-assisted extended pyelolithotomy (REP) provides an appealing option in cases of staghorn calculi and in patients undergoing concurrent RAP.[42–43] Hemal and coworkers found that in six patients who underwent REP or robot assisted pyelolithotomy, the mean operative time was 106 minutes and EBL was less than 50 ml in all cases. [44] One patient required conversion to an open procedure because the renal calculus could not be localised. The study also assessed 29 cases of RAP with concomitant pyelolithotomy for UPJ obstruction with a secondary stone, and deemed the procedure to have a 97% symptomatic efficacy rate.[44]
FEMALE UROLOGY
Robotic management of urinary fistula
The robotic repair of primary vesicovaginal fistula was first described in five patients by Sundaram et al. in 2006 and was associated with acceptable postoperative outcomes.[45] In a matched comparative analysis of open versus robotic repair of recurrent vesicovaginal fistula, robot-assisted techniques were found to be more effective in regard to better morbidity related outcomes while providing similar postoperative success rates.[46] Hemal and coworkers presented the first report of robotic repair of complex vesicouterine fistula in three patients and established the procedure to be efficacious with or without concurrent robotic hysterectomy.[47] Feasibility studies have found complex ureterovaginal fistulas to be amenable to robotic ureteroneocystostomy with the robot conferring enhanced identification of relevant anatomical structures.[48]
URETER
Robot-assisted ureteral surgery
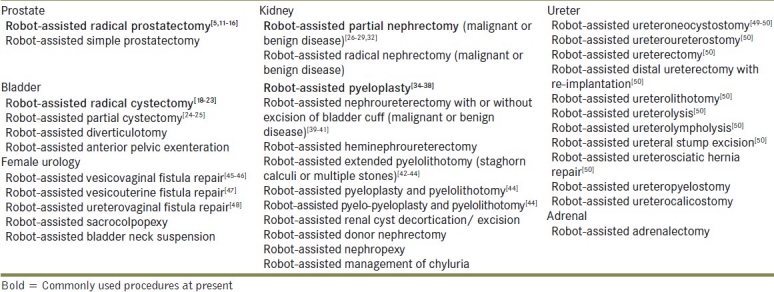
Robotic assistance is increasingly being utilised in a variety of urologic procedures and is furthering the applicability of this exciting technology. Hemal and colleagues recently reported the feasibility of robotic intracorporeal or extracorporeal ureteric tapering with ureteroneocystostomy for primary symptomatic obstructive megaureter.[49] Various ureteral pathologies seem to be especially amenable to robotic repair in the hands of an experienced surgeon with Hemal et al. demonstrating the feasibility of robotic ureteroneocystostomy, ureteroureterostomy, ureteral stump excision and ureterosciatic hernia repair.[50] Table 1 shows a variety of ureteral pathologies that have been managed with robotic assistance. While long-term data is obviously crucial in assessing the extended viability of all the procedures mentioned above, it seems that the dissemination of robotics into all aspects of urology has well and truly begun.
Table 1.
Current status of robot-assisted urologic surgery
CONCLUSION
Robotics has modernised the field of urology and has been crucial in providing patients and physicians with another surgical modality in the management of a vast array of urologic afflictions. The relatively short learning curve of robotic surgery is providing a comparative advantage over laparoscopic techniques and slowly making robotics the minimally invasive modality of choice. RARP signaled the commencement of the robotic revolution, and the past decade has been marked by tremendous progress in the use of minimally invasive surgery. Long-term data have recently started trickling down about the oncological outcomes provided by RARP and results indeed seem promising. While we await long-term follow-up of procedures such as RAPN and RARC, it appears that the short and intermediate term parameters of efficacy compare favourably to accepted standards and foreshadow the increased dissemination and utilisation of robot-assisted surgery in urology.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Hemal AK, Menon M. Laparoscopy, robot, telesurgery and urology: future perspective. J Postgrad Med. 2002;48:39–41. [PubMed] [Google Scholar]
- 2.Hemal AK, Menon M. Robotics in urology. Curr Opin Urol. 2004;14:89–93. doi: 10.1097/00042307-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Haseebuddin M, Benway BM, Cabello JM, Bhayani SB. Robot-assisted partial nephrectomy: evaluation of learning curve for an experienced renal surgeon. J Endourol. 2010;24:57–61. doi: 10.1089/end.2008.0601. [DOI] [PubMed] [Google Scholar]
- 4.Hemal AK, Eun D, Tewari A, Menon M. Nuances in the optimum placement of ports in pelvic and upper urinary tract surgery using the da Vinci robot. Urol Clin North Am. 2004;31:683–92. doi: 10.1016/j.ucl.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Menon M. Robot-assisted radical prostatectomy: Is the dust settling? Eur Urol. 2011;59:7–9. doi: 10.1016/j.eururo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Menon M, Hemal AK. Robotic urologic surgery: is this the way of the future? World J Urol. 2006;24:119. doi: 10.1007/s00345-006-0081-3. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Hemal AK. The ‘scrubbed surgeon’ in robotic surgery. World J Urol. 2006;24:144–7. doi: 10.1007/s00345-006-0068-0. [DOI] [PubMed] [Google Scholar]
- 8.Rogers CG, Laungani R, Bhandari A, Krane LS, Eun D, Patel MN, et al. Maximizing console surgeon independence during robot-assisted renal surgery by using the Fourth Arm and TilePro. J Endourol. 2009;23:115–21. doi: 10.1089/end.2008.0416. [DOI] [PubMed] [Google Scholar]
- 9.Autorino R, Cadeddu JA, Desai MM, Gettman M, Gill IS, Kavoussi LR, et al. Laparoendoscopic single-site and natural orifice transluminal endoscopic surgery in urology: A critical analysis of the literature. Eur Urol. 2011;59:26–45. doi: 10.1016/j.eururo.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Coelho RF, Rocco B, Patel MB, Orvieto MA, Chauhan S, Ficarra V, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A critical review of outcomes reported by high-volume centers. J Endourol. 2010;24:2003–15. doi: 10.1089/end.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon M, Hemal AK. Vattikuti Institute prostatectomy: a technique of robotic radical prostatectomy: experience in more than 1000 cases. J Endourol. 2004;18:611–9. doi: 10.1089/end.2004.18.611. [DOI] [PubMed] [Google Scholar]
- 12.Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, et al. Biochemical recurrence following robot-assisted radical prostatectomy: Analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010;58:838–46. doi: 10.1016/j.eururo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648–57. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 14.Patel VR, Coelho RF, Chauhan S, Orvieto MA, Palmer KJ, Rocco B, et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int. 2010;106:696–702. doi: 10.1111/j.1464-410X.2010.09541.x. [DOI] [PubMed] [Google Scholar]
- 15.Coelho RF, Palmer KJ, Rocco B, Moniz RR, Chauhan S, Orvieto MA, et al. Early complication rates in a single-surgeon series of 2500 robotic-assisted radical prostatectomies: report applying a standardized grading system. Eur Urol. 2010;57:945–52. doi: 10.1016/j.eururo.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945–9. doi: 10.1016/S0022-5347(05)64548-X. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Richards KA, Hemal AK, Kader AK, Pettus JA. Robot assisted laparoscopic pelvic lymphadenectomy at the time of radical cystectomy rivals that of open surgery: Single institution report. Urology. 2010;76:1400–4. doi: 10.1016/j.urology.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Hemal AK, Abol-Enein H, Tewari A, Shrivastava A, Shoma AM, Ghoneim MA, et al. Robotic radical cystectomy and urinary diversion in the management of bladder cancer. Urol Clin North Am. 2004;31:719–29. doi: 10.1016/j.ucl.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, Abol-Ein H, et al. Robot-assisted radical cystectomy and urinary diversion in female patients: technique with preservation of the uterus and vagina. J Am Coll Surg. 2004;198:386–93. doi: 10.1016/j.jamcollsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92:232–6. doi: 10.1046/j.1464-410x.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 22.Pruthi RS, Nielsen ME, Nix J, Smith A, Schultz H, Wallen EM. Robotic radical cystectomy for bladder cancer: surgical and pathological outcomes in 100 consecutive cases. J Urol. 2010;183:510–4. doi: 10.1016/j.juro.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Allaparthi S, Ramanathan R, Balaji KC. Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol. 2010;24:223–7. doi: 10.1089/end.2009.0367. [DOI] [PubMed] [Google Scholar]
- 25.Stanasel I, Richard J, Hemal AK. Robotic assisted laparoscopic partical cystectomy. Abstract 1562 presented at World Congress of Endourology and SWL. [Google Scholar]
- 26.Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004;64:914–8. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–72. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology. 2009;73:306–10. doi: 10.1016/j.urology.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 29.Scoll BJ, Uzzo RG, Chen DY, Boorjian SA, Kutikov A, Manley BJ, et al. Robot-assisted partial nephrectomy: a large single-institutional experience. Urology. 2010;75:1328–34. doi: 10.1016/j.urology.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colli J, Martin B, Purcell M, Kim YI, Busby EJ. Surgical factors affecting return of renal function after partial nephrectomy. Int Urol Nephrol. doi: 10.1007/s11255-010-9764-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viprakasit DP, Altamar HO, Miller NL, Herrell SD. Selective renal parenchymal clamping in robotic partial nephrectomy: initial experience. Urology. 2010;76:750–3. doi: 10.1016/j.urology.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 33.Haber GP, Crouzet S, Kamoi K, Berger A, Aron M, Goel R, et al. Robotic NOTES (Natural Orifice Translumenal Endoscopic Surgery) in reconstructive urology: initial laboratory experience. Urology. 2008;71:996–1000. doi: 10.1016/j.urology.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Gupta NP, Nayyar R, Hemal AK, Mukherjee S, Kumar R, Dogra PN. Outcome analysis of robotic pyeloplasty: a large single-centre experience. BJU Int. 2010;105:980–3. doi: 10.1111/j.1464-410X.2009.08983.x. [DOI] [PubMed] [Google Scholar]
- 35.Gettman MT, Peschel R, Neururer R, Bartsch G. A comparison of laparoscopic pyeloplasty performed with the daVinci robotic system versus standard laparoscopic techniques: initial clinical results. Eur Urol. 2002;42:453–7. doi: 10.1016/s0302-2838(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 36.Hemal AK, Mukherjee S, Singh K. Laparoscopic pyeloplasty versus robotic pyeloplasty for ureteropelvic junction obstruction: a series of 60 cases performed by a single surgeon. Can J Urol. 2010;17:5012–6. [PubMed] [Google Scholar]
- 37.Nayyar R, Gupta NP, Hemal AK. Robotic management of complicated ureteropelvic junction obstruction. World J Urol. 2010;28:599–602. doi: 10.1007/s00345-009-0469-y. [DOI] [PubMed] [Google Scholar]
- 38.Gupta NP, Mukherjee S, Nayyar R, Hemal AK, Kumar R. Transmesocolic robot-assisted pyeloplasty: single center experience. J Endourol. 2009;23:945–8. doi: 10.1089/end.2008.0430. [DOI] [PubMed] [Google Scholar]
- 39.Park SY, Jeong W, Ham WS, Kim WT, Rha KH. Initial experience of robotic nephroureterectomy: a hybrid-port technique. BJU Int. 2009;104:1718–21. doi: 10.1111/j.1464-410X.2009.08671.x. [DOI] [PubMed] [Google Scholar]
- 40.Eandi JA, Nelson RA, Wilson TG, Josephson DY. Oncologic outcomes for complete robot-assisted laparoscopic management of upper-tract transitional cell carcinoma. J Endourol. 2010;24:969–75. doi: 10.1089/end.2009.0340. [DOI] [PubMed] [Google Scholar]
- 41.Hemal AK, Stanasel I, Patel MN. Robotic assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning. Urology. 2011 doi: 10.1016/j.urology.2010.12.075. [In press] [DOI] [PubMed] [Google Scholar]
- 42.Badalato GM, Hemal AK, Menon M, Badani KK. Current role of robot-assisted pyelolithotomy for the management of large renal calculi: a contemporary analysis. J Endourol. 2009;23:1719–22. doi: 10.1089/end.2009.1540. [DOI] [PubMed] [Google Scholar]
- 43.Badani KK, Hemal AK, Fumo M, Kaul S, Shrivastava A, Rajendram AK, et al. Robotic extended pyelolithotomy for treatment of renal calculi: a feasibility study. World J Urol. 2006;24:198–201. doi: 10.1007/s00345-006-0099-6. [DOI] [PubMed] [Google Scholar]
- 44.Hemal AK, Nayyar R, Gupta NP, Dorairajan LN. Experience with robotic assisted laparoscopic surgery in upper tract urolithiasis. Can J Urol. 2010;17:5299–305. [PubMed] [Google Scholar]
- 45.Sundaram BM, Kalidasan G, Hemal AK. Robotic repair of vesicovaginal fistula: case series of five patients. Urology. 2006;67:970–3. doi: 10.1016/j.urology.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Gupta NP, Mishra S, Hemal AK, Mishra A, Seth A, Dogra PN. Comparative analysis of outcome between open and robotic surgical repair of recurrent supra-trigonal vesico-vaginal fistula. J Endourol. 2010;24:1779–82. doi: 10.1089/end.2010.0049. [DOI] [PubMed] [Google Scholar]
- 47.Hemal AK, Sharma N, Mukherjee S. Robotic repair of complex vesicouterine fistula with and without hysterectomy. Urol Int. 2009;82:411–5. doi: 10.1159/000218529. [DOI] [PubMed] [Google Scholar]
- 48.Laungani R, Patil N, Krane LS, Hemal AK, Raja S, Bhandari M, et al. Robotic-assisted ureterovaginal fistula repair: report of efficacy and feasiblity. J Laparoendosc Adv Surg Tech A. 2008;18:731–4. doi: 10.1089/lap.2008.0037. [DOI] [PubMed] [Google Scholar]
- 49.Hemal AK, Nayyar R, Rao R. Robotic repair of primary symptomatic obstructive megaureter with intracorporeal or extracorporeal ureteric tapering and ureteroneocystostomy. J Endourol. 2009;23:2041–6. doi: 10.1089/end.2009.0103. [DOI] [PubMed] [Google Scholar]
- 50.Hemal AK, Nayyar R, Gupta NP, Dorairajan LN. Experience with robot assisted laparoscopic surgery for upper and lower benign and malignant ureteral pathologies. Urology. 2010;76:1387–93. doi: 10.1016/j.urology.2010.01.044. [DOI] [PubMed] [Google Scholar]


