Abstract
Objectives:
To evaluate the effect of caffeine at the dose of 4.5 mg/kg on bladder function in overactive bladder (OAB) adults.
Materials and Methods:
Nine women and three men aged 21-40 years with OAB symptoms were included. Each subject drank 8 ml/kg of water with and without caffeine at two separate sessions. Cystometry and uroflowmetry were performed 30 minutes after each drink. The effects of caffeine on urodynamic parameters were compared.
Results:
After caffeine ingestion, the mean volume at bladder filling phase decreased at first desire to void and normal desire to void (P<0.05), compared to the mean volume after taking water (control) drink. The mean volume at strong desire to void, urgency and maximum cystometric capacity also tended to decrease. No change in the detrusor pressure at filling phase was found. At voiding phase, the maximal flow rate, average flow rate and voided volume were increased (P<0.05). The urine flow time and time to maximal flow rate were not changed.
Conclusion:
Caffeine at 4.5 mg/kg caused diuresis and decreased the threshold of sensation at filling phase, with an increase in flow rate and voided volume. So, caffeine can promote early urgency and frequency of urination. Individuals with lower urinary tract symptom should avoid or be cautious in consuming caffeine containing foodstuffs.
Keywords: Caffeine, overactive bladder, uroflowmetry
INTRODUCTION
Overactive bladder (OAB) is a common syndrome described by the International Continence Society (ICS) in 2002 as “urgency, with or without urge incontinence, usually with frequency and nocturia,” in the absence of infection or other proven pathology.[1] Quality of life is significantly impaired in OAB patients, especially in women.[2] Management includes excluding pathology and implementing behavioral changes such as caffeine reduction, bladder and pelvic floor training, as well as antimuscarinic drug therapy.[3] As the etiology in most cases is unknown, the treatment outcomes have until recently been unsatisfactory.[3] Treatment compliance, either in pharmacologic or nonpharmacologic therapy, is often problematic. Caffeine is often blamed to exacerbate OAB symptoms, so patients are usually advised to avoid caffeine consumption.[3–5] For those OAB patients who are coffee lovers, avoiding coffee and coffee products can equally hurt their quality of life, and they may wonder if caffeine actually has anything to do with their OAB symptoms at all.
This study aimed to evaluate the effect of caffeine at the dose of 4.5 mg/kg on bladder function in OAB adults.
MATERIALS AND METHODS
This study was reviewed and approved by the Siriraj Ethics Committee on Research Involving Human Subjects (No.114/2004). Twelve OAB patients (nine females and three males) were studied at the Urodynamic Laboratory, Division of Urology, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. They fulfilled the inclusion criteria that they were OAB patients (urgency, with or without urge incontinence, usually with frequency and nocturia), aged 18-40 years, regular caffeine consumers (more than three cups of coffee per week or equivalent), and routine urinalysis revealed no abnormalities (negative result for sugar, protein, bile and microscopic examination).
The exclusion criteria included diseases affecting urinary system such as diabetes mellitus, hypertension, neurological diseases, any urinary tract disorders such as benign prostatic hyperplasia (BPH), prostate or bladder cancer, urethral stricture, urinary tract stone, urinary retention, urinary tract infection (within 2 weeks before study), history of urinary tract surgery and females during menstrual period or pregnancy.
Each subject completed a previously validated, self-administered questionnaire to assess medical history and urinary symptoms. The questionnaire consisted of 11 questions covering frequency, nocturia, weak urinary stream, urgency, incomplete emptying, intermittency, hesitancy and straining modified from American Urological Association (AUA) symptom index to suit Thai cultures and used in Division of Urology, Siriraj Hospital.
Voiding diary and water intake were recorded. Subjects were asked to self-record 48-hour voiding diary and water intake. From this record, the total 24-hour urinary output, number of voids, voiding interval, diurnal distribution, timing and triggers for incontinence, and functional bladder capacity were determined.
On arrival in our urodynamics unit, the patients were asked to void in uroflowmetry machine (Dantec Urodyn 1000). The parameters recorded were voided volume (Vcomp), total voiding time (T100), time to maximum flowrate (TQmax), time of descending (Tdesc), voiding time for 90% voided volume, peak flow rate (Qmax), mean flow rate (Qave), maximum rate of increase of flow rate (dQ/dT).
Cystometry was performed with Dantec Manuet Multichannel clinical urodynamics investigation System (Dantec Electrons, Skovlunde, Danmark). It was set up and the method was performed according to “Good Urodynamics practice” as recommended by the International Continence Society.[6] A 10-Fr double lumen catheter was inserted into the bladder. The bladder was filled with 0.9% sodium chloride at room temperature at a rate of 50 ml/minute in sitting position. Intra-abdominal pressure was measured by inserting 8 Fr single lumen catheter into the rectum. Bladder sensations of first desire to void (FDV), normal desire to void (NDV), strong desire to void (SDV) and urgency were recorded toward the increase infiltrate volume. Maximum cystometric capacity (MCC) was recorded when the patients felt that they could no longer delay micturition. The following pressure parameters were recorded: abdominal pressure (Pabd), intravesical pressure (Pves), detrusor pressure (Pdet= Pves-Pabd), compliance (δV/δ P) and involuntary bladder contraction during filling the bladder.[7]
Two series of urodynamic study were performed on each subject at two different occasions by random, with at least 1 week interval. In one occasion, a control drink (8 ml/kg body weight of boiled water) was taken. In another occasion, a caffeine drink (caffeine 4.5 mg/kg of body weight in 8 ml/kg body weight of boiled water) was taken. According to many articles, caffeine content of beverages in the market ranges from a modest 50 mg to an alarming 505 mg per can or bottle. So, we aimed to test for caffeine effect at the dose of 200-250 mg or about 4.5 mg/kg.
All data were calculated in the computer using SPSS program version 12.0. All basic parameters including dPdetwere presented as mean and standard error of mean (SEM). Kolmogorov-Smirnor test was used to test the data distribution. Wilcoxon's signed-rank test was used to test the difference in variables that were not normal distribution. Two-sided P value of less than 0.05 was considered statistically significant.
RESULTS
The effects of caffeine on bladder functions were studied in 12 OAB subjects using cystometric and uroflowmetric techniques. The subjects′ characteristics are shown in (Table 1).
Table 1.
Subject characteristics and the amount of water and caffeine consumed (mean±SEM)

The temperature of the control and the caffeine drinks was about 24°C. The total amounts of water and caffeine consumed by the subjects are shown in Table 1.
The baseline data on bladder functions were obtained from the voiding diary. The mean frequency of urine voiding was 9.8±0.7 (range 6-16) times during the day and 3.0±0.6 (range 1-8) times during the night. Five subjects had incontinence episodes and nine subjects had urgency symptom.
The results of the control and the caffeine drinks on bladder sensation are shown in Figure 1. After caffeine drink, volumes of FDV and NDV decreased significantly compared to the volumes at control day. The filling volume at FDV after caffeine drink decreased in 10 subjects and increased in 2 subjects, while the filling volume at NDV after caffeine drink decreased in 9 subjects and increased in 3 subjects. The volumes of bladder filling at the SDV, urgency and MCC were all decreased slightly without statistical significance (P>0.05).
Figure 1.
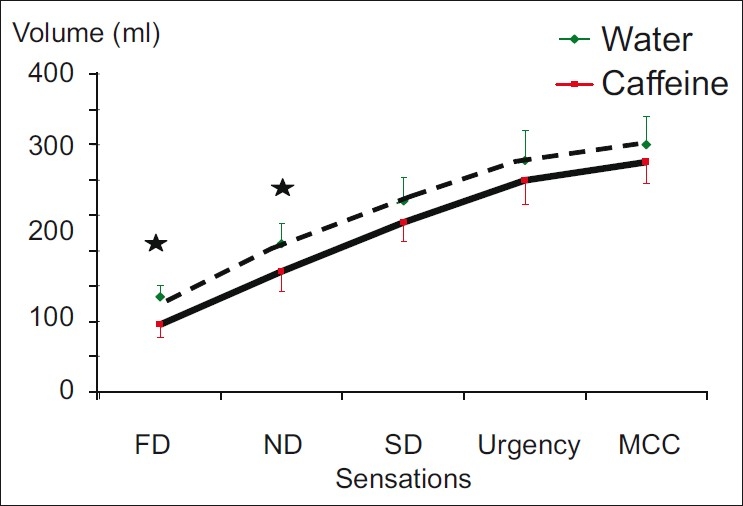
Sensation and volumes at bladder filling phases (mean±SE) in milliliters after water and caffeine ingestions (n=12) (FD=first desire to void, ND=normal desire to void, SD=strong desire to void, MCC=maximal cystometric capacity); * P≤0.05 on comparing the volumes after water and caffeine ingestions
After caffeine ingestion, the desire to void (FDV, NDV, and SDV) came earlier at lower Pdet compared to that found in those after drinking water, though no statistical significance was reached. This finding indicates a tendency to an increase in bladder sensitivity after caffeine ingestion.
The Pdet at urgency and MCC were higher after caffeine intake, though not statistically significant. This indicates a tendency to stronger detrusor contractions at urgency and MCC after caffeine ingestion. No involuntary detrusor contractions were found. The Pdet values are shown in Figure 2.
Figure 2.
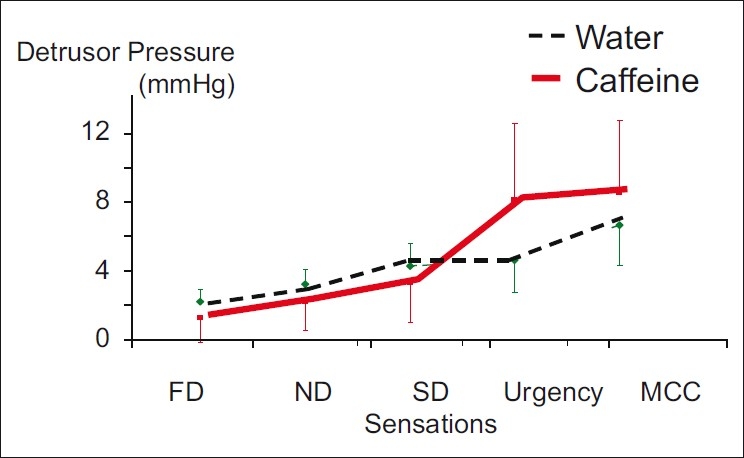
Detrusor pressure at filling phase (mean±SE) in mmHg after water and caffeine ingestions (FD=first desire to void, ND=normal desire to void, SD=strong desire to void, MCC=maximal cystometric capacity)
The uroflowmetric parameters examined were Vcomp, T100, TQmax, Qmax and Qave as presented in Figure 3.
Figure 3.
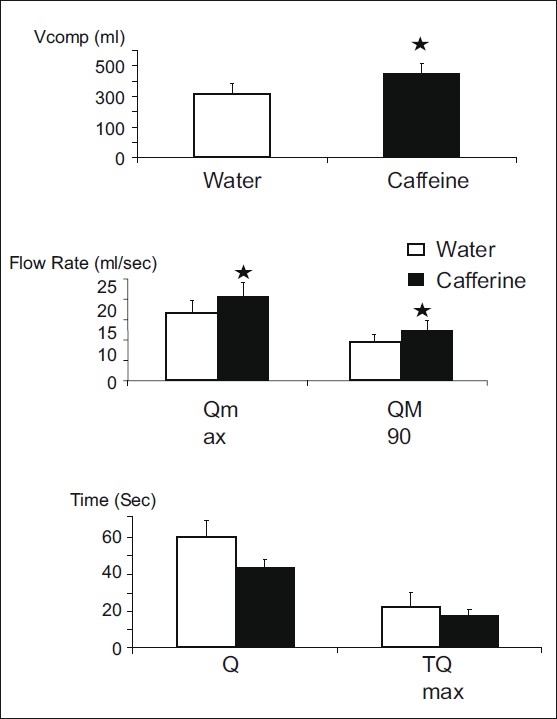
Uroflowmetric parameters (mean±SE) after water and caffeine ingestions (Q 100=flow time, TQmax=time to max flow rate, Qmax =maximal flow rate, QM90=average flow rate, Vcomp=voided volume); *P≤0.05 on comparing the values after water and caffeine ingestions
After caffeine consumption, the mean Vcomp increased significantly compared to that after water ingestion. The mean T100 tended to decrease after caffeine ingestion, though not significantly. T100 decreased in 10 subjects and increased in 2 subjects. TQmax after the caffeine ingestion was slightly decreased. Qmaxand the Qave increased significantly.
DISCUSSION
This study, using cystometric and uroflowmetric techniques, demonstrated that drinking caffeine at the dose of 4.5 mg/kg affected urinary bladder function in patients with OAB symptoms when compared to drinking plain water. The cardiovascular parameters recorded in this present study also indicated that caffeine at 4.5 mg/kg caused an increase in the systolic blood pressure and the diastolic blood pressure. This is most likely due to the action of caffeine in increasing cardiac contractility, and the slight decrease in heart rate is most likely due to the baroreceptor reflex.
Caffeine (chemical name 3,7-dihydro-1, 3, 7-trimethyl-1H-purine-2,6-dione) has four identifiable cellular actions in vitro.[8] Firstly, caffeine is able to significantly block adenosine effects on A2A and A1 receptors at the low concentrations achieved after a single cup of coffee. Secondly, caffeine inhibits cyclic nucleotide breakdown via inhibition of phosphodiesterase, for which 20 times higher concentrations are required. Thirdly, caffeine blocks GABAA receptors at 40 times higher concentrations. Fourthly, at 100 times higher concentrations, caffeine mobilizes intracellular calcium depots.
Based on these cellular actions, caffeine affects various organ functions in the human body, including the urinary system. In the present study, the urine volume was increased in the caffeine group, confirming the diuretic effect of caffeine. Nocturia was found to associate with caffeine consumption.[5]
The present study showed that Qmax and Qave were significantly increased in the caffeine group. Yi et al., in 2006, studied the effects of caffeine in streptozotocin-induced diabetic rats and found that caffeine improved the detrusor contractility.[9] Creighton et al. reported that after consuming 200 mg of caffeine, patients with detrusor instability showed an increase in detrusor pressure on bladder filling, while normal women showed no change in cystometric parameters.[10]
Caffeine at a high concentration is a calcium releaser, releasing calcium from its intracellular store.[11] This results in stronger muscular contraction. Caffeine at a high concentration also improves reaction time, increases tense arousal, including anxiety, nervousness and jitteriness.[12] This may result in an increase in perception of visceral sensation and interpretation.
Since the pathophysiology of OAB is quite complex and still not so clear,[13] the treatment is sometimes not satisfactory. It is suggested that various stimulations release many substances, including adenosine triphosphate, prostaglandins, nitric oxide, and acetylcholine, from urothelium, which contributes to pathophysiology of the increased bladder sensation, OAB symptoms, and detrusor overactivity.[14] The bladder sensory profiles displayed a more sensitive bladder in OAB patients compared with non-OAB subjects. OAB patients may have bladders that are not only overactive but also hypersensitive.[15] This study demonstrated that caffeine decreased the bladder volumes at the FDV and NDV, thereby making the bladder more sensitive to bladder filling. This finding agrees with the concept that coffee or caffeine aggravates the OAB symptoms and caffeine restriction can be beneficial.[16–20]
CONCLUSIONS
This study showed that caffeine at a dose of 4.5 mg/kg caused diuresis and decreased threshold of sensation at filling phase, with an increase in flow rate and voided volume. So, caffeine can promote early urgency and frequency of urination as well as nocturia symptoms. Individuals with lower urinary tract symptom should avoid or be cautious in consuming caffeine containing foodstuffs.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Tubaro A. Defining overactive bladder: Epidemiology and burden of disease. Urology. 2004;64:2–6. doi: 10.1016/j.urology.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RM, Adekanmi OA. Overactive bladder. Best Pract Res Clin Obstet Gynaecol. 2005;19:829–41. doi: 10.1016/j.bpobgyn.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Sussman DO. Overactive bladder: Treatment options in primary care medicine. J Am Osteopath Assoc. 2007;107:379–86. [PubMed] [Google Scholar]
- 5.Liao YM, Dougherty MC, Biemer PP, Liao CT, Palmer MH, Boyington AR, et al. Factors related to lower urinary tract symptoms among a sample of employed women in Taipei. Neurourol Urodyn. 2008;27:52–9. doi: 10.1002/nau.20457. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 7.Abram P. 2nd ed. London: Springer verlag London limited; 1997. Urodynamics; pp. 20–73. [Google Scholar]
- 8.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 9.Yi CR, Wei Zo, Deng XL, Sun ZY, Li XR, Tian CG. Effects of coffee and caffeine on bladder dysfunction in streptozotocin-induced diabetic rats. Acta Pharmacol Sci. 2006;27:1037–43. doi: 10.1111/j.1745-7254.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 10.Creighton SM, Stanton SL. Caffeine: Does it affect your bladder? Br J Urol. 1990;66:613–4. doi: 10.1111/j.1464-410x.1990.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Unno T, Matsuyama H, Kohda M, Masuda N, Nishimura M. Two types of cation channel activated by stimulation of muscarinic receptors in guinea-pig urinary bladder smooth muscle. J Pharmacol Sci. 2008;108:248–57. doi: 10.1254/jphs.08138fp. [DOI] [PubMed] [Google Scholar]
- 12.Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20:S85–94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- 13.Shafik A, El-Sibai O, Shafik AA, Ahmed I. The electrovesicogram in the overactive bladder: Role in determining pathogenesis and diagnostic significance. 2004;32:290–3. doi: 10.1007/s00240-004-0412-z. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Masunaga K, Nagata T, Yono M, Homma Y. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: Pathophysiology and pharmacotherapy of overactive bladder. J Pharmacol Sci. 2004;112:128–34. doi: 10.1254/jphs.09r12fm. [DOI] [PubMed] [Google Scholar]
- 15.Lee SR, Kim HJ, Kim A, Kim JH. Overactive bladder is not only overactive but also hypersensitive. Urology. 2010;75:1053–9. doi: 10.1016/j.urology.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: A survey of 374 patients. J Urol. 1993;149:465–9. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger MW, Goodman BM, Carnes M. Long-term efficacy of nonsurgical urinary incontinence treatment in elderly women. J Gerontol A Biol Sci Med Sci. 1999;54:M117–21. doi: 10.1093/gerona/54.3.m117. [DOI] [PubMed] [Google Scholar]
- 18.Shorter B, Lesser M, Moldwin RM, Kushner L. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145–52. doi: 10.1016/j.juro.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Bradley CS, Kennedy CM, Nygaard IE. Pelvic floor symptoms and lifestyle factors in older women. J Womens Health. 2005;14:128–36. doi: 10.1089/jwh.2005.14.128. [DOI] [PubMed] [Google Scholar]
- 20.Hashim H, Al Mousa R. Management of fluid intake in patients with overactive bladder. Curr Urol Rep. 2009;10:428–33. doi: 10.1007/s11934-009-0068-x. [DOI] [PubMed] [Google Scholar]


