Abstract
Aim:
To examine p27 (Kip 1) and MUC1 expression in specimens of papillary transitional cell carcinoma (PTCC) of the urinary bladder and to correlate their expression with the tumor grades,stages and outcome.
Patients and Methods:
Paraffin sections from previously diagnosed PTCC bladder were graded, staged and the patients were followed up for 5 years. Ten non-neoplastic urological lesions diagnosed as polypoid cystitis were taken as control. Three sections of 4 um thickness were obtained from every case. One was hematoxylin and eosin (H and E) stained for diagnosis, reviewing and confirmation. The other two sections were immunohistochemically stained for both p27and MUC1. The data of immunohistochemical results were correlated with the following conventional prognostic variables: tumor grade, stage, distant metastasis and 5 year survival.
Results:
The results showed a highly significant and an insignificant relationship between p27 expression and tumor grade and stage (P<0.01 and P>0.05), respectively. Correlating p27 expression with distant metastasis and overall survival showed a significant relationship with distant metastasis (P<0.05) and a highly significant one with overall survival (P<0.01). The results showed also a significant relationship between MUC1 expression and both tumor grade (P<0.01) and overall survival (P<0.05).
Conclusion:
p27 and MUC1 immunohistochemistry augment the classic histochemistry for the prognosis of PTCC of the bladder as well as improving the prediction of the patient outcome and survival.
Keywords: MUC1, p27, papillary transitional cell carcinoma, urinary bladder
INTRODUCTION
Urinary bladder cancer was responsible for more than 12,000 deaths in the United States in 1999. In Egypt, the urinary system malignancies represent a high incidence of 13.5% of total malignant tumors. The main bulk is dominated by the urinary bladder cancer (12.22%).[1,2] It is the second most common malignant tumor of the urinary system and the second most common cause of death of genitourinary system.[3] The highest incidence is in the six and seventh decades of life and men are more affected than women.[4]
The cycline dependant kinase (CDK) complexes are groups of intracellular enzymes that regulate and mediate the cell cycle regulatory proteins which are important indicators in the cell progression, either in normal cell cycle or invasive carcinoma.[5–7] The activity of the CDK is downregulated by CDK inhibitors that control entry of the cell into the DNA replicative S-phase.[6–8]
The p27 (Kip1) protein is a CDK inhibitor that blocks the activity of cyclin D1/CDK4,6 and cyclin E/CDK2. So, it is a very important determinator for G1/S phase transmission of cell cycle.[5,9] p27 is considered to be a tumor suppressor protein as its progressive downregulation is a common finding in different cancers.[7] It has been reported that decreased p27 protein level is a negative prognostic indicator in several human malignancies.[10,11]
Mucins (MUCs) are a family of heterogeneous high-molecular-weight glycolproteins with various roles in homeostasis and carcinogenesis, discovered for the first time in human milk.[12,13]
MUC1 is a signal transducer protein that can sense environmental changes and send messages to the cell, being expressed on the whole cell surface. The MUC1 expression pattern was investigated by immunohistochemical staining in different tissues such as invasive micropapillary carcinoma of breast, pancreas and gynecologic tract, and the expression was predominantly in the stroma-facing surface of the cell clusters (basal), accentuating the outlines of the micropapillary units by forming a distinct band on this surface. In conventional carcinoma, the labeling was mostly apical in areas with lumen formation, and intracytoplasmic and intercellular in the poorly differentiated areas. [13,14]
The high-molecular-weight glycoprotein MUC1 mucin is overexpressed in bladder tumors and represents a useful target for radioimmunoscintigraphy and radioimmunotherapy, and intended for intravesical radioimmunotherapy of superficial bladder cancer. Moreover, in previous studies, it was suggested that high-level MUC1 expression is correlated with prognosis of tumor patients. For example, MUC1 expression is closely related with prognosis of breast cancer sufferers and hepatocellular carcinoma patients. However, possible association of MUC1 expression levels in papillary transitional cell carcinoma (PTCC) of urinary bladder tissues with the prognosis still remains uncertain and is not well studied yet. [1,14,15]
PATIENTS AND METHODS
This study was a retrospective study of 50 cases of confirmed diagnosis of primary PTCC of the urinary bladder. Ten non-neoplastic urological lesions diagnosed as polypoid cystitis were taken as a control. The material included consecutive archival formalin-fixed, paraffin-embedded blocks processed in the Pathology Department of the National Cancer Institute, Benha University Hospital and Benha Faculty of Medicine, during the period between 1999 and 2001. Tumor specimens were obtained from 30 transurethral resections, 15 cystectomies and 5 transurethral biopsies. In all the cases, only samples in which the presence of detrusor muscle was identified histologically were used to ensure accuracy in performing pathological staging. None of the patients received radiation or chemotherapy before sampling. Eight tumors were graded as well differentiated (G1), 24 cases were moderately differentiated (GII) and 18 cases were poorly differentiated (GIII), according to the World Health Organization (WHO) classification criteria.[8] Patient's TNM staging was done according to American Joint Committee on Cancer (AJCC).[16] The patients were followed in this period to evaluate tumor recurrence, distant metastasis and 5-year overall patients survival.
Three sections of 4 μm thickness were obtained from every case. One was hematoxylin and eosin (H and E) stained for diagnosis, reviewing and confirmation, by fixation in 10% buffered formalin, embedding in paraffin and staining with HandE. The other two sections were immunohistochemically stained for p27 and MUC1 proteins.
Immunohistochemistry
Paraffin-embedded tissue sections were mounted on poly-Lysine coated slides and heated at 60°C for 30 minutes, then deparaffinized and rehydrated through a series of xylene and alcohol before staining. Antigen retrieval was done using microwave treatment in 10 mm citrate buffer (Neo-markers, cat′′AP-9003), pH 6, and endogenous peroxidase was blocked with 3% hydrogen peroxide for 20 minutes. These sections were washed three times with cold 0.01 phosphate buffered saline (PBS). After blocking with 10% normal rabbit serum, the sections were incubated with rabbit anticow polyclonal antibody against p27 (Santacruz biochemicals, Santacruz CA, USA; dilution 1:50) and with mouse antiswine monoclonal antibody against MUC1 (Decton Dickenson, San Jose, CA, USA; dilution 1:100). The immunohistochemical staining was performed using the avidin-peroxidase complex technique (ABC kit, Vector Laboratories, Burlinguine, CA, USA). The primary antibody was incubated overnight and for 2 hours for MUC1 proteins. The ABC reaction was developed in the presence of diamino benzidine (DAB) supplement with hydrogen peroxide. Lastly, the sections were counterstained with Mayer's hematoxylin.
Interpretation of immunohistochemical staining results
The stained slides were then microscopically examined by two observers using the following parameters and the semi-quantitative criteria for MUC1 expression.
MUC1 was present in all cases of TCC, and the pattern of expression was divided into three categories: luminal membrane staining only, luminal plus cytoplasmic staining of intermediate ±basal layers, or staining of only isolated cells or cell groups with cytoplasmic/membranous expression facing the tumor stroma.[3,13,17]
The degree of MUC1 expression was classified as:
0 = negative cases (no cells stained),
low expression = 1-10% of cells were stained,
moderate expression = 11-30% of cells were stained and
high expression ≥30% of cells were stained.[3,13,17]
Scoring of p27 expression was done and only nuclear expression was recorded. The number of distinctly positive tumor cell nuclei was counted under high power (×400). In total, 1000 tumor cells were assessed.[18]
The number of positive nuclei was expressed as a percentage of all tumor cell nuclei counted as follows: less than 50% of tumor cells show positive nuclear staining=low expression score and more than 50% of tumor cells show positive nuclear staining=high expression score.[18]
Statistical analysis
Data analysis was performed using the SPSS program (12.0 for windows) software package according to Pearson's correlation coefficient. Correlation between several variables and multivariate analysis was computed using Fisher's exact test. P<0.05 was considered significant.
RESULTS
In normal urothelium, MUC1 was limited predominantly to the apical membranes of the umbrella cell layer and p27 was expressed normally in nuclei of urothelial cells.
In Table 1, it is seen that positive p27 expression was shown by 35 out of 50 examined cases of PTCC (70%), GI tumors showed positivity in 100% of cases, GII showed positivity in 87.5% of cases and GIII showed positivity in 33.3% of cases examined which was statistically significant (P<0.01) [Figures 1 and 2]
Table 1.
Relationship between p27 expression and other clinicopathlogical variables in PTCC
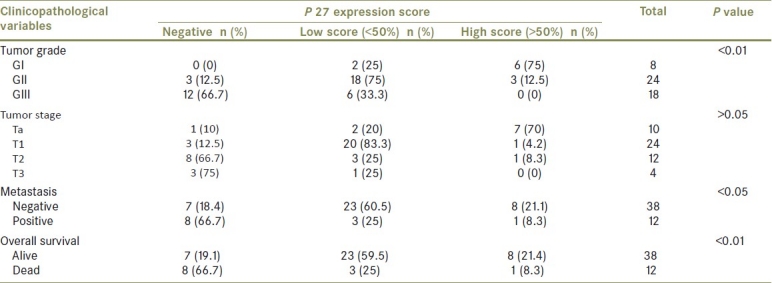
Figure 1.
Differentiated PTCC of the urinary bladder (GI) showing high expression of P27 (brown nuclear staining) in more than 50% of stained malignant cells (streptavidin-biotin DAB; ×400)
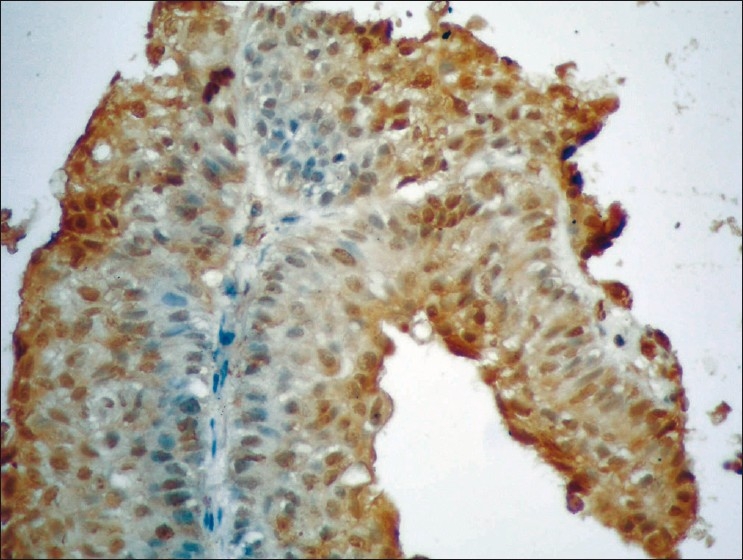
Figure 2.
Poorly differentiated PTCC of the urinary bladder (GIII) showing low expression of P27 (brown nuclear staining) in less than 50% of stained malignant cells (streptavidin-biotin DAB; ×400)
There was high p27 expression score in 70% of Ta stage cases, 4.2% of T1 stage cases, 8.3% of T2 stage cases and no p27 expression in T3 stage cases, but this difference was statistically not significant (P>0.05).
PTCC without metastasis showed low p27 expression score in 60.5% of cases and high p27 expression score in 21.1% of cases, while cases with distant metastasis showed low p27 expression score in 25% of cases and high p27 expression score in 8.3% of cases, this difference was statistically significant (P<0.05).
In alive cases, the low positve p27 expression was 59.5% and 21.4% of cases showed high expression, and in dead cases there was low positve p27 expression in 25% of cases and high expression in 8.3% of cases, this difference in expression was statistically significant (P<0.01).
In Table 2, it is seen that overall positive MUC1 expression was shown in 37 of 50 examined cases of PTCC (74%). According to the grade,37.5% of GI cases,75% of GII cases ,and 88.9% of GIII cases showed positive MUC1 expression and this difference in expression was statistically significant (P <0.01) [Figures 3–5]
Table 2.
Relationship between MUC1 expression and other clinicopathlogical variables in transitional cell carcinoma
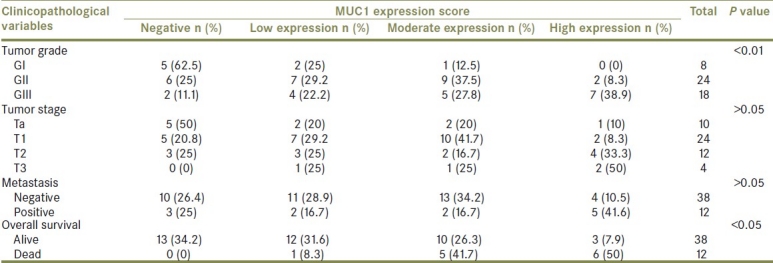
Figure 3.
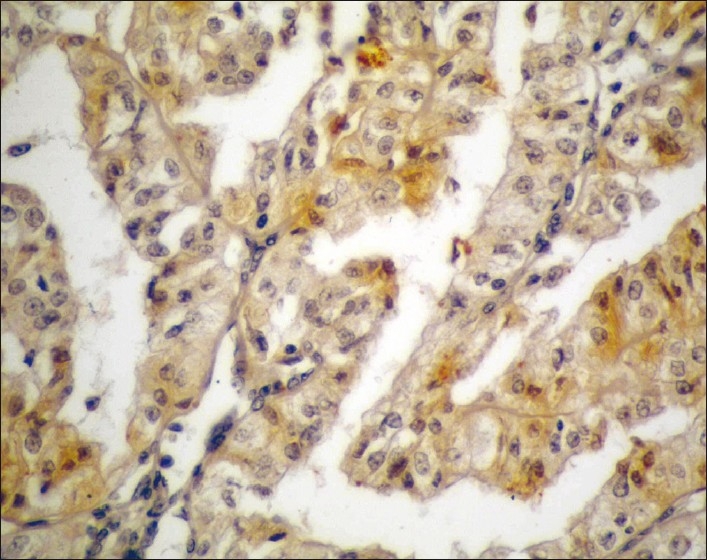
Well-differentiated PTCC urinary bladder (GI) showing low expression of MUC1 (brown cytoplasmic/membranous staining) in stained malignant cells (streptavidin-biotin DAB; ×400)
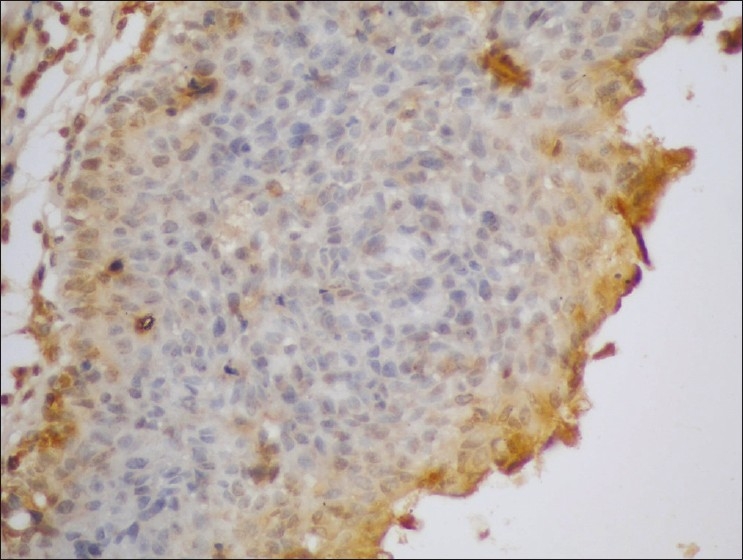
Figure 5.
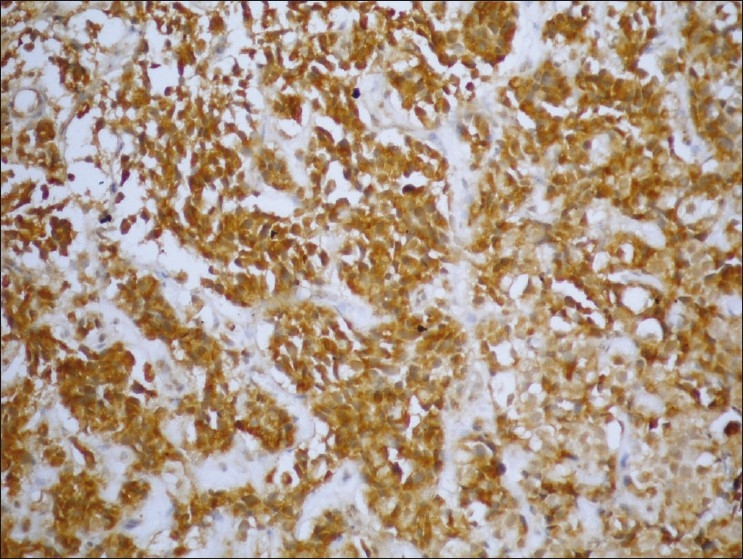
Poorly differentiated PTCC urinary bladder (GIII) showing high expression of MUC1 (brown cytoplasmic/membranous staining) in stained malignant cells (streptavidin-biotin DAB; ×200)
Figure 4.
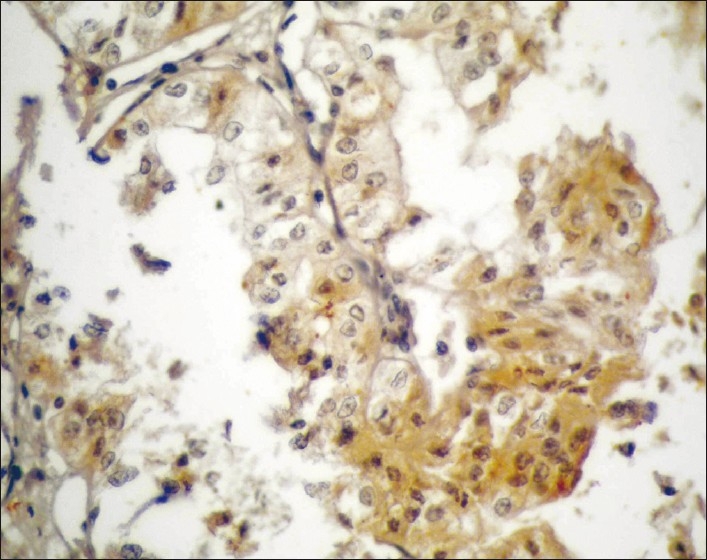
Moderately differentiated PTCC urinary bladder (GII) showing moderate expression of MUC1 (brown cytoplasmic/membranous staining) in stained malignant cells (streptavidin-biotin DAB; x400)
There was overall positive MUC1 expression in 50% of Ta stage cases in relation to positive expression in 79.2% of T1 stage cases, 75% of T2 stage cases and 100% in T3 stage cases, however this relation was statistically not significant (P>0.05) .
Examined cases without metastasis showed overall positive MUC1 expression in 73.6% of cases, while cases with distant metastasis showed overall positive MUC1 expression in 75% of cases, this difference was statistically not significant (P>0.05).
Alive cases showed overall positive MUC1 expression in 65.8% of cases, while all dead cases showed positive MUC1 expression (100%), which was statistically significant (P<0.05).
DISCUSSION
Downregulation of p27Kip1 is a potent mechanism that allows tumor cells to undergo uncontrolled proliferation. In normal and malignant tissues, concentrations of p27Kip1 and G1 cyclins are closely related to cell proliferation.[19] In the present study, we determined the nuclear expression of one of the key regulators of the G1-S transition, p27Kip in primary PTCC of urinary bladder. The expression of this cell cycle inhibitory protein was correlated with tumor grade, pathological stage, distant metastasis and patient's survival.[20,21]
Examined cases of PTCC showed positive p27 expression in 35 out of 50 cases examined (70%), GI tumors showed positivity in 100% of cases, GII showed positivity in 87.5% of cases, and GIII showed positivity in 33.3% of cases examined, this correlation between grade of PTCC and p27 expression was statistically highly significant (P<0.01). Similarly, PTCC without metastasis showed high score p27 expression in 21.1% of cases, while 8.3% of cases with distant metastasis showed high score p27 expression, this difference was statistically significant (P<0.05). So expression of p27 is an independent predictor of distant metastasis and, hence, poor outcome.These results are in agreement with the previously published data which concluded that expression of p27 in radical cystectomy specimens correlates inversely with biochemical high tumor grade and presence of distant metastasis.[22,23]
The data also showed that there was different expression of p27 between grades of PTCC, however this difference was statistically not significant (P>0.05). High p27 expression was seen in 21.4% of alive cases with PTCC examined, while only 1 dead case (8.3%) showed high expression, which was statistically significant (P<0.01). This was in agreement with the results of Zheng et al.[21] and Yang et al.,[23] who reported that downregulation of p21 and p27 expression was inversely correlated with tumor cell differentiation as well as patient's survival and consequently poor outcome, many studies also found that both p27Kip1 and cyclin D1 expressions were correlated with advancing tumor grade and depth of invasion, as well as were accompanied by increasing proliferation indices.[20,24,25]
In the papillary pattern of invasive carcinoma, the expression of MUC1 is largely limited to the basal surface of the cells in contrast to conventional carcinomas in which MUC1 is largely apical, intracytoplasmic or intercellular. This provides support for the reversal of cell orientation as an important factor of the morphogenesis and possibly the pathogenesis of invasive micropapillary carcinoma. Since MUC1 is known to have a role in lumen formation, and has an inhibitory role in the cell to stroma interaction, it is conceivable that it is a key factor in the detachment of cells from stroma, allowing for the dissection of the connective tissue and easing the spread of cell.[3,17] In the present work, MUC1 expression was present in 37 out of 50 cases of PTCC (74%), and the pattern of expression was divided into three categories: luminal membrane staining only, luminal plus cytoplasmic staining of intermediate±basal layers, or staining of only isolated cells or cell groups. Examined cases of PTCC showed positive MUC1 expression in 3 out of 8 cases (37.5%) of GI , 18 out of 24 (75%) cases of GII and 16 out of 18 cases (88.9%) of GIII, and this positive correlation was statistically significant (P<0.01).
Also there was overall positive MUC1 expression in 50% of cases of Ta stage, 79.2% of T1 stage cases, 75% of T2 stage cases and in all cases (100%) of T3 stage. Accordingly, it could be noted that there was a positive correlation between MUC1 expression and tumor stage; however, this correlation was statistically not significant (P>0.05). These results are consistent with the results of many studies which reported that high MUC1 expression in the examined cases of urinary bladder carcinoma was correlated with high tumor grade as well as high patient's TNM stage and, hence with a poor tumor outcome.[14,18,22]
Examined cases without metastasis showed positive MUC1 expression in 73.6% of cases, while cases with distant metastasis showed 75% positive MUC1 expression, indicating that there was a positive correlation between MUC1 expression and tumor distant spread but it was statistically not significant (P >0.05). Also, alive cases showed positive MUC1 expression in 65.8% of cases, while all dead cases (100%) showed positive MUC1 expression, denoting a statistically significant relation between MUC1 expression and poor patient's survival (P< 0.05). These records were in accordance with the results done by Samaratunga and Khoo[18] and Yang et al.,[23] who stated that MUC1 oncoprotein was positively correlated with both tumor grade and stage, and associated with poor survival. MUC1 expression is also related to poor prognosis in other cancers such as in cholangiocarcinoma in which MUC1 over expression was related to vascular invasion and poor prognosis and outcome of cholangiocarcinoma patients.[26] In a study by Li et al.,[27] they demonstrated that immunohistochemical detection of MUC1 is useful in diagnosis of pure form of invasive micropapillary carcinoma of the breast which is associated with higher incidences of lymphatic invasion and lymph node metastasis, and poor prognosis. Also in advanced gastric cancer, the examined cases with high MUC1 expression had higher incidences of lymphatic invasion and lymph node metastasis, and also showed a poor patient's survival.[28]
CONCLUSION
High expressions of MUC1 as well as loss of p27 oncoproteins are significantly correlated with tumor grade, presence of distant metastasis, and patient's survival. Thus, MUC1 and p27 could be considered as useful prognostic markers for poor outcome in urinary bladder PTCC patients. MUC1 and p27 immunohistochemistry can augment the classic histochemistry for prognosis of PTCC as well as prediction of the patient outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Murray A, Simms MS, Scholfield DP, Vincent RM, Denton G, Bishop MC, et al. Production and characterization of 188Re-C595 antibody for radioimmunotherapy of transitional cell bladder cancer. J Nucl Med. 2001;42:726–32. [PubMed] [Google Scholar]
- 2.Mokhtar N, Goda I, Adel I. Malignant urinary system tumors.Cancer pathology registry 2003-2004 and time trend analysis. Cairo, Egypt: Natl Cancer Inst, Cairo University; 2007:32. [Google Scholar]
- 3.Xiang ST, Zhou SW, Guan W, Liu JH, Ye ZQ. Expression of MUC1 and distribution of dendritic cells in human bladder transitional cell carcinoma. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:1114–8. [PubMed] [Google Scholar]
- 4.Reuter VE. The urothelial tract: Renal pelvis, ureter, urinary bladder, and urethra. In: Mills SE, Carter D, Greenson JK, Oberman HA, Reuter VE, Stoler MH, editors. Sternberg's Diagnostic Surgical Pathology. 4th ed. London: United Kingdom: Lippincott Williams & Wilkins;; 2004. [Google Scholar]
- 5.Santos LL, Amaro T, Pereira SA, Lameiras CR, Lopes P, Bento MJ, et al. Expression of cell cycle regulatory proteins and their prognostic value in superficial low-grade urothelial cell carcinoma of bladder. Eur J Surg Oncol. 2003;29:74–80. doi: 10.1053/ejso.2002.1371. [DOI] [PubMed] [Google Scholar]
- 6.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 7.Khan AA, Abel PD, Chaudhary KS, Gulzar Z, Stamp GW, Lalani EN. Inverse correlation between high level expression of cyclin E and proliferation index in transitional cell carcinoma of urinary bladder. Mol Pathol. 2003;56:353–61. doi: 10.1136/mp.56.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrier BP, Vriesema JL, Witjes JA, Kiemeney LA, Schalken JA. The predictive value of P53, p27 (Kip 1) and alpha catenin for progression in superficial bladder carcinoma. Eur Urol. 2006;50:76–82. doi: 10.1016/j.eururo.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Stavropoulos NE, Filiadis I, Ioachim E, Hastazeris K, Tsimaris I, Kalogeras D, et al. Prognostic significance of p53, bcl-2 and Ki67 in high - risk superficial bladder cancer. Anticaner Res. 2002;22:3759–64. [PubMed] [Google Scholar]
- 10.Kamai T, Takagi K, Asami H, Ito Y, Oshima H, Yoshida KI. Decreasing p27 (Kip1) and cyclin E protein levels are associated with progression from superficial into invasive bladder cancer. Br J Cancer. 2001;4(84):1242–51. doi: 10.1054/bjoc.2000.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybowski B, Kupryjanczyk J, Rembiszewska A, Pykalo R, Borkowski A. P27(Kip1) and Ki-67 expression analysis in transitional cell carcinoma of the bladder. Urol Res. 2003;31:397–401. doi: 10.1007/s00240-003-0356-8. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 13.Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456–62. doi: 10.1136/jcp.2003.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassar H, Pansare V, Zhang H, Che M, Sakr W, Ali-Fehmi R, et al. Pathogenesis of invasive micropapillary carcinoma: Role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045–50. doi: 10.1038/modpathol.3800166. [DOI] [PubMed] [Google Scholar]
- 15.Yuan SF, Li KZ, Wang L, Dou KF, Yan Z, Han W, et al. Expression of MUC1 and its significance in hepatocellular and cholangiocarcinoma tissue. World J Gastroenterol. 2005;11:4661–6. doi: 10.3748/wjg.v11.i30.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. 6th ed. New York: Springer-Verlag; 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 17.Walsh MD, Hohn BG, Thong W, Devine PL, Gardiner RA, Samaratunga ML, et al. Mucin expression by transitional cell carcinoma of the bladder. Br J Urol. 1994;73:256–62. doi: 10.1111/j.1464-410x.1994.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 18.Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder: A clinicopathological and immunohistochemical study. Mol Pathol. 2004;45:55–64. doi: 10.1111/j.1365-2559.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 19.Doganavsargil B, Simsir A, Boyacioglu H, Cal C, Hekimgil M. A comparison of p21 and p27 immunoexpression in benign glands, prostatic intraepithelial neoplasia and prostate adenocarcinoma. BJU Int. 2006;97:644–8. doi: 10.1111/j.1464-410X.2006.06054.x. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 21.Zheng JY, Wang WZ, Li KZ, Guan WX, Yan W. Effect of p27(KIP1) on cell cycle and apoptosis in gastric cancer cells. World J Gastroenterol. 2005;11:7072–7. doi: 10.3748/wjg.v11.i45.7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Said J. Biomarker discovery in urogenital cancer. Am J Cancer. 2005;10:S83–6. doi: 10.1080/13547500500215050. [DOI] [PubMed] [Google Scholar]
- 23.Yang FG, Zhang ZW, Xin DQ, Shi CJ, Wu JP, Guo YL, et al. Peroxisome proliferator-activated receptor gamma ligands induce cell cycle arrest and apoptosis in human renal carcinoma cell lines. Acta Pharmacol Sin. 2005;26:753–61. doi: 10.1111/j.1745-7254.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 24.Cardon-Cordon C. Mutations of cell cycle regulators: Biological and clinical implications for human neoplasia. Am J Pathol. 1995;147:545–60. [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, Cui YJ, Fang SM, Li Y, Wang N, Zhang JH. Association of polymorphisms of p21cip1 and p27kip1 genes with susceptibilities of esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma. Ai Zheng. 2006;25:194–9. [PubMed] [Google Scholar]
- 26.Boonla C, Sripa B, Thuwajit P, Cha-On U, Puapairoj A, Miwa M, et al. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:4939–46. doi: 10.3748/wjg.v11.i32.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YS, Kaneko M, Sakamoto DG, Takeshima Y, Inai K. The reversed apical pattern of MUC1 expression is characteristics of invasive micropapillary carcinoma of the breast. Breast Cancer. 2006;13:58–63. doi: 10.2325/jbcs.13.58. [DOI] [PubMed] [Google Scholar]
- 28.Ohno T, Aihara R, Kamiyama Y, Mochiki E, Asao T, Kuwano H. Prognostic significance of combined expression of MUC1 and adhesion molecules in advanced gastric cancer. Eur J Cancer. 2006;42:256–63. doi: 10.1016/j.ejca.2005.10.017. [DOI] [PubMed] [Google Scholar]


