Abstract
Vitamin D has been reported to lower blood pressure in vivo by regulating the renin-angiotensin system; however, there are limited clinical studies to support this finding in humans. We investigated the effect of vitamin D treatment on hypertension in a three-arm randomized placebo controlled pilot and feasibility study. We tested placebo with two forms of vitamin D: cholecalciferol (vitamin D3) and the active form of vitamin D, calcitriol. Subjects were recruited from the Atlanta Veterans Affairs Medical Center in Decatur, GA between April and August 2008. Subjects received 200,000 IU of vitamin D3 (n = 3) weekly for 3 weeks or matching placebo (n = 3) weekly for 3 weeks (n = 3) or 0.5 μg calcitriol (n = 2) taken twice daily for one week. Our primary endpoint was blood pressure measured by 24 h ambulatory blood pressure monitor. Subjects receiving calcitriol experienced a 9% decrease in mean systolic blood pressure (SBP) compared placebo (p < 0.001). One week after conclusion of calcitriol therapy SBP returned to pre-treatment levels. There was no reduction in blood pressure in the placebo or vitamin D3 groups. Results from this pilot study suggests that active vitamin D therapy may be an effective short-term intervention for reducing blood pressure and needs to be explored further in larger controlled studies.
Keywords: Vitamin D, Blood pressure treatment, Hypertension, Calcitriol, Cholecalciferol, Renin
1. Introduction
Inadequate blood pressure (BP) control predisposes an individual for increased risk of stroke, kidney disease, and heart disease [1,2]. Cross-sectional studies have demonstrated that higher levels of 25-hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D, are associated with lower BP [3,4] as is calcitriol, the short-acting active form of vitamin D [5,6]. Further, small randomized controlled studies suggest that vitamin D treatment lowers BP [7] and reduces inflammation in congestive heart failure [8]; however, these findings have not been replicated in larger studies [9]. Men with low vitamin D levels were six times more likely to develop hypertension in health professionals follow-up survey [10] and twice as likely to have a cardiac event in the Framingham Offspring Study [11].
The mechanism by which vitamin D may regulate BP is not firmly established. Initial studies by Li et al. suggested that vitamin D is a negative regulator of renin [12,13]. Vitamin D receptor (VDR) knockout mice have elevated BP and plasma renin activity which can be reversed with angiotensin converting enzyme inhibitor treatment. Furthermore, wild type mice given active vitamin D (1,25-dihydroxyvitamin D) have decreased mRNA expression of renin [12,13]. These early pre-clinical studies suggest that vitamin D may play a role in the regulation of BP. The purpose of our study was to examine whether vitamin D lowers BP in humans and to determine if vitamin D lowers plasma renin activity. We evaluated both the inactive form of vitamin D3 (cholecalciferol) and the active form of vitamin D (calcitriol) in a small pilot and feasibility study conducted over a three-week period.
2. Materials and methods
2.1. Participants
A total of 31 subjects were screened, 15 subjects were consented, 9 subjects were enrolled, and 7 subjects completed the study. In order to limit the heterogeneity of this study, we recruited subjects over the age of 30, self-defined as black or African American, with 25(OH)D levels between 25 and 75 nmol/L, and had systolic blood pressure (SBP) between 130 and 150 mm Hg. We excluded subjects who had history of hypercalciuria, hyperparathyroidism, current use of more than three anti-hypertensive medications, inability to understand the consent form, inability to return ambulatory blood pressure (ABP) monitor within 24–48 h after visit, history of alcohol abuse, current alcohol consumption of greater than two drinks per day, diagnosis of chronic kidney disease, diagnosis of HIV, history of heart disease, history of stroke, inability to comply with study protocol, current treatment for cancer, narcotic dependence, current use of vitamin D analog, and/or current use of greater than 2000 IU of vitamin D.
2.2. Study medication, anti-hypertensive medication use, and compliance
Subjects received calcitriol manufactured by Pliva, Inc. (East Hanover, NJ) or vitamin D3 or placebo manufactured by Tishcon (Westbury, NY). Vitamin D3 and placebo were taken orally and were identical in shape and color. Given the short half-life of calcitriol (~8 h), subjects took calcitriol twice daily for one week. In addition, subjects were requested to continue using prescription anti-hypertensive medication. Study personnel were blinded to vitamin D3 and placebo as were subjects; however, due to differences in dosing regimen study personnel were not blinded if subject was randomized to calcitriol. Seven subjects (vitamin D3 n = 2; calcitriol n = 2; placebo n = 3) completed all study requirements over a one-month period.
2.3. Blood collection, blood pressure assessment and quality control
Blood was collected at the screening visit, baseline, and final visits. Serum 25(OH)D was determined by ELISA (IDS Ltd, Fountain Hills, AZ). Plasma renin activity (PRA) was determined by RIA (Quest Diagnostics, San Jose, CA). Urinary creatinine, potassium, calcium and sodium levels were measured by standard hospital methods. BP was measured at screening with the subject sitting quietly in the examination room for 5 min. For baseline and follow-up visits, subjects were requested to wear an ambulatory blood pressure (ABP) monitor (Spacelabs 90207) for 24 h. In order to consider the data usable, we required at least 75% of measures to be present. We calibrated the monitor with a stationary monitor at the clinic before sending it with the subject and upon return of the monitor.
2.4. Statistical analysis
Data was analyzed using mixed linear models to account for correlation of repeated measures within the same subject. Microsoft Excel and SAS 9.1 (SAS Institute, Cary, NC) were used for all data analyses. Data from the ABP monitors was downloaded using software provided by the manufacturer and was then transferred to Excel using an encrypted spreadsheet. BP averages were obtained using Microsoft Excel and then transferred into a SAS data set. Data was analyzed using PROC MIXED with a REPEATED statement to account for colinearity. We also used PROC GLM to calculate adjusted means controlling for initial BP. Average waking BP was used as the main outcome variable. We also examined changes in biomarkers between treatment and control groups and examined ratio of daytime to nighttime BP changes. All analysis was done using intent to treat.
3. Results
3.1. Evaluation of the effects of cholecalciferol, calcitriol, or placebo on blood pressure
We randomized nine subjects to receive cholecalciferol, calcitriol, or placebo; seven subjects completed the study requirements. All study subjects were using anti-hypertensive medications (Table 1). All three groups had different initial BP and vitamin D levels (ANOVA p < 0.01) but were balanced in terms of starting plasma renin activity, age, and use of medication. Subjects randomized to calcitriol had a significant decrease in SBP compared to placebo. In contrast, there was no significant change in SBP in subjects randomized to vitamin D3 compared to placebo. The calcitriol group experienced a 9% reduction in SBP compared to placebo (p < 0.001). In subjects randomized to calcitriol, there were no significant reductions in diastolic BP; however, there was a significant reduction in heart rate (85 ± 9.3 vs 78 ± 7.6, p = 0.004). The reduction in heart rate observed in the calcitriol group was significant when compared with placebo group (p < 0.001).
Table 1.
Baseline characteristics.
| Placebo | Calcitriol | D3 | p | |
|---|---|---|---|---|
| n | 3 | 3 | 3 | |
| Age | 46.7 | 46.5 | 42.3 | 0.67 |
| Number of anti-HT medications | 1.7 | 2.5 | 1.5 | 0.83 |
| Blood pressure | 141/84 | 141/76 | 125/70 | 0.02 |
| Vitamin D (nmol/dL) | 27 | 46 | 24 | 0.05 |
| Plasma renin activity (ng/mL/h) | 0.5 | 0.7 | 0.7 | 0.35 |
| Urinary CaCR (mg/mg) | 0.1 | 0.1 | 0.04 | 0.28 |
| Urinary ACR (mg/g) | 45.8 | 57.9 | 48.9 | 0.33 |
3.2. Calcitriol follow-up
We further evaluated whether decreased SBP was sustained after one week of calcitriol therapy. The subjects randomized to calcitriol wore the ABP monitor one week after conclusion of treatment (Fig. 1). One week after subjects discontinued use of calcitriol, SBP returned to pre-treatment levels.
Fig. 1.
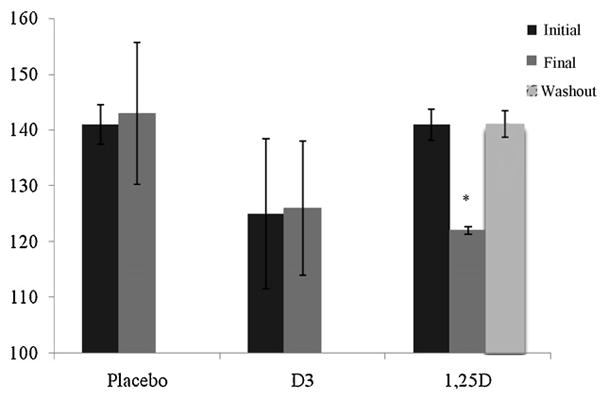
Mean 24 h systolic blood pressure measures in three treatment groups. Blood pressure decreased significantly in those subjects receiving 1,25D when compared with vitamin D3 and placebo.
p < 0.05 when compared to initial and extra measure.
3.3. Biomarkers
We did not observe a significant change in any of the serum biomarkers: glucose, calcium, parathyroid hormone, creatinine, phosphorous, cholesterol, and triglycerides. All remained unchanged throughout the course of the study. Calcium excretion increased significantly in the group supplemented with calcitriol. Urinary excretion of sodium was not different across the three groups nor was plasma renin activity (PRA).
4. Discussion
This pilot and feasibility study demonstrates that calcitriol lowers BP in the short term with no significant change in BP with cholecalciferol. We did not observe any adverse events in any of the three groups. These encouraging results support the findings of Li et al. who suggest vitamin D may lower BP by acting as a negative regulator of renin [12,13] and one case study in which calcitriol suppressed PRA and lowered BP from 145/96 mm Hg to 128/85 after two weeks of calcitriol therapy [14]. In addition to direct effects on renin [12,13] calcitriol has been shown to modify the vascular endothelium [15–17] and reduce inflammatory cytokines from activated T-cells [18]. Activated T-cells have been shown to increase oxidative stress and subsequently increase angiotensin-II production, thus suggesting numerous mechanisms through which calcitriol may act to reduce blood pressure [19]. Unfortunately, our study was not powered to detect any changes in PRA or other surrogate biomarkers of cardiovascular risk or inflammation.
Our study was limited due to small sample size. In addition, the Vitamin D3 supplement arm started with significantly lower blood pressures than the placebo and calcitriol group. This could be why no difference in blood pressure was observed. We were under-powered to examine reduction in PRA and mechanistic effects of vitamin D3; however, we did observe significant reduction in BP in one subject with high PRA and was treated with vitamin D3. This suggests that vitamin D3 therapy may need to be targeted specifically to those with high PRA in future studies. Evidence exists indicating vitamin D therapy may be a complementary treatment strategy in the management of cardiovascular and renal disease. These data should be interpreted with caution as calcitriol is a risky anti-hypertensive therapy. We have demonstrated in a small pilot study that BP can be reduced with calcitriol though future studies with larger numbers of participants would be needed to examine the safety and efficacy of calcitriol. Large-scale studies examining the role of vitamin D in regulating blood pressure seem warranted.
Footnotes
Special issue selected article from the 14th Vitamin D Workshop held at Brugge, Belgium on October 4–8, 2009.
References
- 1.Kannel WB. Risk stratification in hypertension: new insights from the Framingham study. Am J Hypertens. 2000;13:03S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 2.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham study. Am J Epidemiol. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 3.Scragg R, Sowers M, Bell C, et al. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 4.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutrition. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 5.Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:894–901. doi: 10.1016/0895-7061(95)00154-H. [DOI] [PubMed] [Google Scholar]
- 6.Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension. 1997;30:1289–1294. doi: 10.1161/01.hyp.30.5.1289. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 8.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutrition. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 9.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 10.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y, Kawamura M, Owada M, et al. Effectiveness of 1,25-dihydroxyvitamin D supplementation on blood pressure reduction in a pseudohypoparathyroidism patient with high renin activity. Intern Med. 1999;38:31–35. doi: 10.2169/internalmedicine.38.31. [DOI] [PubMed] [Google Scholar]
- 15.Bukoski RD, DeWan P, McCarron DA. 1,25(OH) Vitamin D3 modifies growth and contractile function of vascular smooth muscle of spontaneously hypertensive rats. Am J Hypertens. 1989;2:553–556. doi: 10.1093/ajh/2.7.553. [DOI] [PubMed] [Google Scholar]
- 16.Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-Dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87:1889. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somjen D, Weisman Y, Kohen F, et al. 25-Hydroxyvitamin D3-1a-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Am Heart Assoc. 2005:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 18.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res Clin Pract. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiot Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]


