Abstract
Our laboratory has investigated two hypotheses regarding the effects of fructose consumption: 1) The endocrine effects of fructose consumption favor a positive energy balance, and 2) Fructose consumption promotes the development of an atherogenic lipid profile. In previous short- and long-term studies, we demonstrated that consumption of fructose-sweetened beverages with 3 meals results in lower 24-hour plasma concentrations of glucose, insulin, and leptin in humans compared with consumption of glucose-sweetened beverages. We have also tested whether prolonged consumption of high-fructose diets could lead to increased caloric intake or decreased energy expenditure, thereby contributing to weight gain and obesity. Results from a study conducted in rhesus monkeys produced equivocal results. Carefully controlled and adequately powered long-term studies are needed to address these hypotheses. In both short- and long-term studies we demonstrated that consumption of fructose-sweetened beverages substantially increases postprandial triacylglycerol concentrations compared with glucose-sweetened beverages. In the long-term studies, apolipoproteinB concentrations were also increased in subjects consuming fructose, but not those consuming glucose. Data from a short-term study comparing consumption of beverages sweetened with fructose, glucose, high fructose corn syrup (HFCS) and sucrose, suggest that HFCS and sucrose increase postprandial triacylglycerol to an extent comparable to that induced by 100% fructose alone. Increased consumption of fructose-sweetened beverages along with increased prevalence of obesity, metabolic syndrome, and type 2 diabetes underscore the importance of investigating the metabolic consequences fructose consumption in carefully controlled experiments.
Keywords: Fructose, glucose, insulin, leptin, lipids, triacylglycerol, apolipoprotein-B
INTRODUCTION
Some investigators have proposed that increased fructose consumption may be related with the current epidemics of obesity and metabolic syndrome (1–4). The purpose of this paper is to review some of our recent work investigating two hypotheses: 1) Fructose consumption promotes a state of positive energy balance, and 2) Fructose consumption favors the development of an atherogenic lipoprotein profile.
FRUCTOSE CONSUMPTION AND ENERGY BALANCE
Consumption of dietary fructose has increased in conjunction with rising intake of fructose-containing sugars, largely in the form of sugar-sweetened beverages. Malik et al conducted a systematic review of the relationship between sugar-sweetened beverage consumption and risk of weight gain and concluded that the evidence indicates that increased consumption of sugar-sweetened beverages is associated with weight gain (5). We have hypothesized that fructose consumption could promote weight gain because it does not stimulate insulin secretion or leptin production by adipose tissue (2, 3). Since leptin production is regulated by insulin-mediated glucose metabolism (6–8) and ingestion of fructose does not result in meal-related increases of plasma glucose or insulin concentrations, we hypothesized that meals accompanied with fructose-sweetened beverages would result in reduced circulating leptin concentrations when compared with glucose-sweetened beverages. We compared leptin concentrations over two separate 24-h periods in 12 normal-weight young women who consumed fructose- or glucose-sweetened beverages with meals. Consumption of fructose-sweetened beverages at 30% of energy requirements with 3 meals resulted in lower 24-h circulating glucose, insulin, and leptin concentrations, and resulted in less postprandial suppression of ghrelin after each meal compared with consumption of glucose-sweetened beverages (9). In a second short-term study comparing fructose- and glucose-sweetened beverages (30% of energy requirements), meal-induced insulin secretion was attenuated and 24-hour circulating leptin profiles were reduced in both overweight/obese men and overweight/obese women (10). Fructose-sweetened beverage consumption also reduced the percent (proportional) change of leptin concentrations between the morning nadir and the late night peak (9, 10). Results from a clinical study investigating the weight/body fat loss during an ad libitum low-fat, high carbohydrate diet suggest an association between the amplitude of the diurnal leptin pattern and long-term energy balance (11).
In long-term comparisons of fructose- and glucose-sweetened beverages (25% of energy requirements consumed with meals), fructose consumption resulted in significant reductions in the 24-h areas under the curve (AUC) for glucose, insulin, and leptin (12), whereas consumption of glucose did not. These results indicate that reductions of insulin secretion and attenuated 24-hour leptin profiles observed in the short-term studies are not transient, but are maintained during long-term fructose consumption.
Insulin and leptin function as key endocrine signals to the central nervous system in the long-term regulation of energy balance (13, 14). Therefore, prolonged consumption of diets high in energy from fructose could lead to increased caloric intake or decreased caloric expenditure, contributing to weight gain and obesity as a result of reduced insulin and leptin signaling in the brain (3). However, obtaining definitive evidence in support of this hypothesis in human subjects would be extremely difficult. It would require that subjects be provided and restricted to ad libitum consumption of a high fructose or high glucose diet that has been designed to achieve a comparable and controlled macronutrient distribution in all subjects, regardless of quantities consumed. It would also require that the intervention last at least 12 months, since a difference in body weight change as small as 0.5 kg/year between groups would be a clinically relevant finding. The costs, as well at the compliance and retention issues, involved in conducting such a study would likely prove to be prohibitive.
FRUCTOSE AND LONG-TERM ENERGY BALANCE IN RHESUS MONKEYS
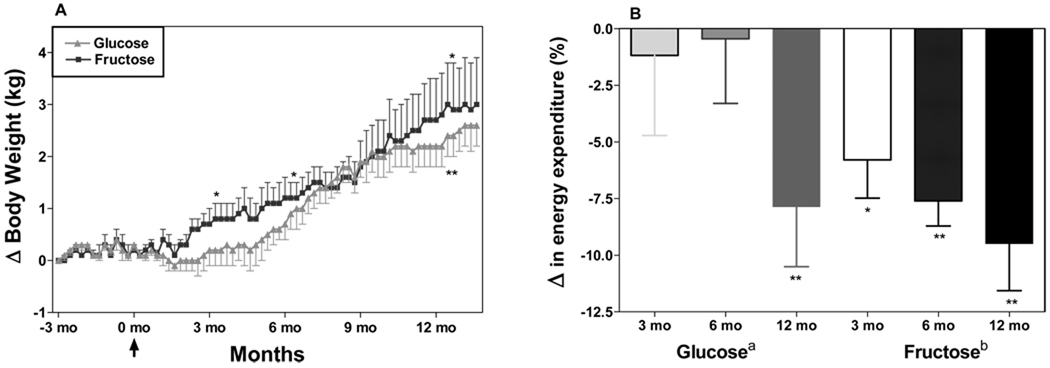
We conducted a 12-month study in 16 adult male rhesus monkeys (Macaca mulatta) to determine if prolonged consumption of a diet high in energy from fructose would lead to greater weight gain via increased caloric intake and/or decreased energy expenditure compared with a diet high in glucose. Monkeys (n=8/group) were fed an ad libitum standard chow diet supplemented with either glucose- or fructose-sweetened beverages (100 g sugar/day). The two groups of monkeys consumed an average 43.8 ± 4.1% and 41.5 ± 2.7% of total energy as glucose and fructose respectively during the 12-month intervention period. Monkeys fed fructose beverages gained significant amounts of weight at 3 and 6 months compared to their baseline weights, while the animals consuming glucose did not. However, weight gain was not significantly different between the two groups by the end of the study at 12 months (Figure 1A).
Figure 1.
Changes of body weight from baseline in male rhesus monkeys consuming 380 kcal/d of fructose- or glucose-sweetened beverage for one year (n=8/group). Baseline body weights were not significantly different. Data are mean ± SEM (analysis described in Figure 2). Response to each sugar over time was analyzed by repeated measures ANOVA (ap<0.01; bp<0.05 using SAS 9.1 (Cary, NC)), with contrasts comparing weights at 3, 6, and 12 months to baseline weights (*p<0.05, **p<0.01). Comparison of fructose and glucose response analyzed by 2-factor repeated measures ANOVA: Sugar×Time interaction = NS. Data are mean ± SEM.
B. Percent change of energy expenditure (measured by indirect calorimetry) from baseline in rhesus monkeys after 3, 6 and 12 months of consuming 380 kcal/d of fructose- or glucose-sweetened beverages. Baseline energy expenditure was not significantly different between groups. Response to each sugar over time analyzed by repeated measures one-factor ANOVA (ap<0.05; bp<0.01 with Greenhouse-Geisser Epsilon correction using SAS 9.13 (Cary, NC)), with contrasts comparing energy expenditure at 3, 6, and 12 months to baseline energy expenditure (*p<0.05, **p<0.01). Comparison of fructose and glucose response analyzed by two-factor repeated measure ANOVA: Sugar×Time interaction = NS. n=6/group. Data are mean ± SEM.
Food and beverage intake was measured daily, and differences in energy intake did not account for the weight gain in monkeys fed fructose-sweetened beverages during the first 6 months of the study. Differences in energy expenditure between the two groups of animals may explain the early differences in body weight gain (Figure 1B). Energy expenditure was measured by indirect calorimetry at baseline and at 3, 6, and 12 months. We monitored the monkeys for three 24-hour periods at each time point, calculated postprandial energy expenditure (from 1700h to 0100h), and averaged the results from the 2 closest measurements.
The energy expenditure profiles at baseline were comparable for both groups (Glucose: 0.205 ± 0.004; Fructose: 0.202 ± 0.007 kJ/min/kg BW.75). At 3 and 6 months, energy expenditure during the postprandial period in the fructose-fed monkeys was significantly decreased when compared with baseline, whereas energy expenditure in monkeys consuming glucose was unchanged (Figure 1B). However, at 12 months, the energy expenditure of the monkeys consuming glucose was significantly reduced compared with the earlier time points, and more comparable to the profiles of the monkeys consuming fructose. Thus, the timing of changes in body weight of both the animals consuming fructose and those consuming glucose appear to be more closely related to changes of energy expenditure than to changes of energy intake. These equivocal results from a year-long study in nonhuman primates indicate that additional carefully controlled studies will be required to determine whether fructose consumption preferentially promotes positive energy balance compared with consumption of glucose.
FRUCTOSE AND LIPID METABOLISM
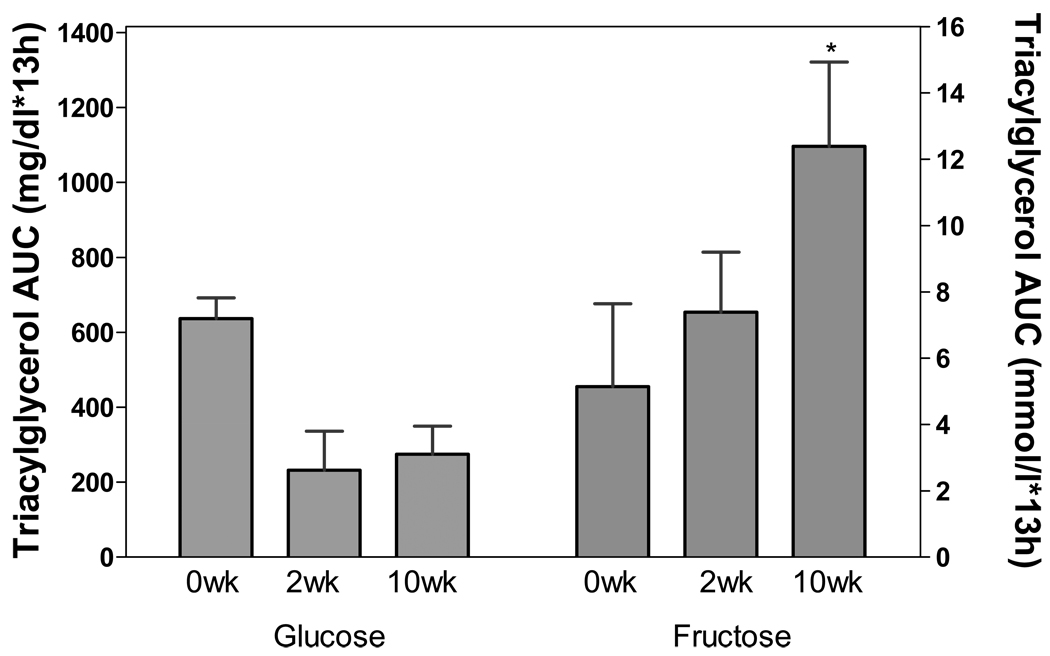
Other important differences in the metabolic consequences of fructose and glucose consumption warrant investigation. Both of our short-term studies (in normal weight women and overweight men and women) demonstrated that consumption of fructose-sweetened beverages with meals increased 24-h circulating plasma triacylglycerol (TG) concentrations compared with consumption of glucose-sweetened beverages (9, 10). Results from a long-term study indicate that consuming fructose-sweetened beverages at 25% of energy requirements for 10 weeks increased 24-h TG exposure by 140% in overweight women (12). In contrast, in subjects consuming the same amount of glucose-sweetened beverages for 10 weeks, the 24-h TG AUC tended to decrease (Figure 2) (15). These findings are consistent with our current long-term study in a larger number of overweight/obese men and women (16).
Figure 2.
14-h area under the curve (AUC) for plasma TG at baseline and at 2 and 10 weeks of dietary intervention in women consuming 25% of energy as glucose-sweetened beverages (n=3) or fructose-sweetened beverages (n=5). Comparison of fructose and glucose response analyzed by two-factor repeated measures ANOVA using GraphPad Prism (version 4.03; San Diego, CA) with Bonferroni posttests. Sugar×Time interaction: P = 0.017; *P < 0.05 vs 10 wk glucose. Data are mean ± SEM.
Previous studies indicate that hepatic de novo lipogenesis (DNL) increases during fructose ingestion (17, 18). Fructose consumption may promote hepatic lipogenesis via several mechanisms: the liver is the main site of fructose metabolism (19); fructose enters glycolysis via fructose-1-phosphate, bypassing the main rate-controlling step of glucose metabolism through glycolysis catalyzed by phosphofructokinase, thus, providing unregulated amounts of the lipogenic substrates acetyl-CoA and glycerol-3-phosphate (19); fructose upregulates sterol receptor element binding protein-1c (SREBP-1c) independently of insulin, thus, activating genes involved in DNL, eg, fatty acid synthase and acetyl coA carboxylase (20, 21).
Growing evidence links postprandial lipemia with proatherogenic conditions (22–25). The relationship between TG-rich lipoprotein and atherogenesis is most likely mediated by the effects of postprandial hypertriacylglycerolemia, which promotes lipoprotein remodeling to a more atherogenic lipid profile consisting of increased concentrations of TG rich lipoprotein remnants and small dense LDL-C (25–27). This mechanism is consistent with our long-term results showing increased concentrations of fasting and postprandial apolipoprotein B100 (ApoB), (12, 16) with fructose consumption. ApoB concentrations were increased in the absence of comparable increases in LDL-C, which suggests that fructose consumption increased the number of total LDL-C particles (28) while decreasing particle size (29). As LDL-C particles become smaller, conformational changes occur in ApoB that increase its affinity for arterial wall proteoglycans (30). Thus, ApoB is a clinically important apolipoprotein that assembles atherogenic lipoproteins and promotes the development of atherosclerosis (30). Therefore, long-term consumption of diets containing 25% of energy from fructose produces a lipoprotein profile that has been associated with the development of atherosclerosis.
In these studies, we have compared the metabolic effects of beverages sweetened with fructose and glucose alone, however, pure fructose and pure glucose are not commonly employed as sweeteners. Until a few decades ago, most foods and beverages in the U.S. were sweetened with the disaccharide sucrose, which is composed of 50% glucose and 50% fructose. In 1970, the enzymatic process to convert corn sugar (composed of glucose) into high fructose corn syrup (HFCS) was developed. Since then, HFCS, primarily 55% fructose and 45% glucose (HFCS-55), has replaced sucrose as the predominant sweetener in soft drinks and represents approximately 40% of the sweeteners added to foods consumed in the U.S. (31).
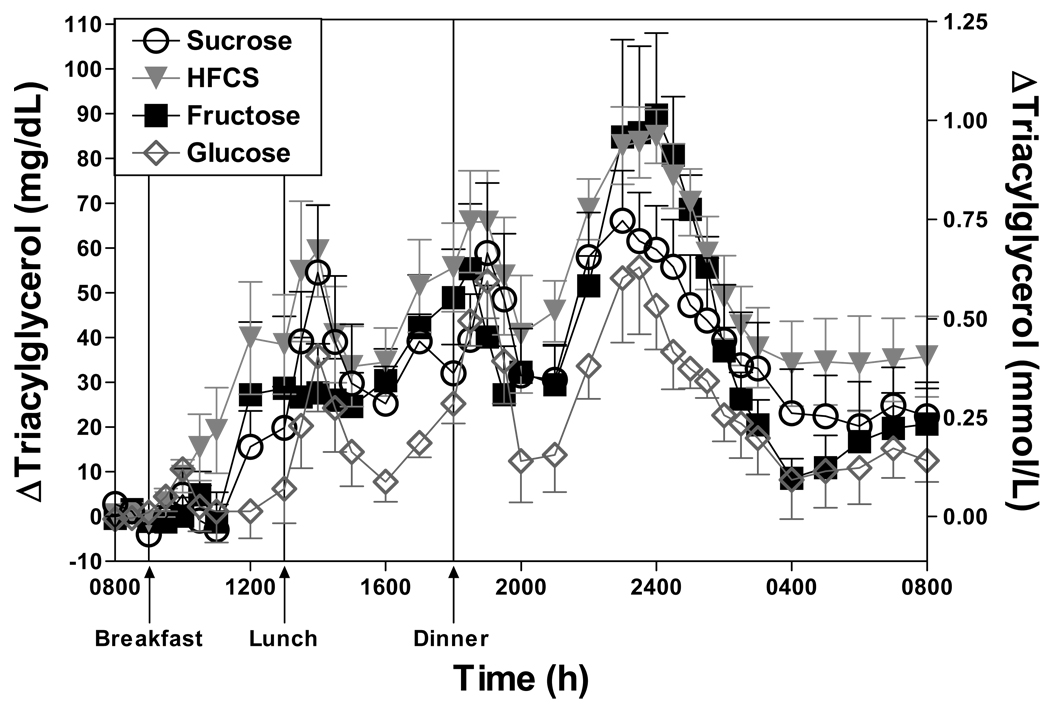
It is reasonable to hypothesize that the endocrine/metabolic effects of HFCS and sucrose would be similar to each other and that both would produce responses intermediate between those of pure fructose and glucose, and we have investigated this hypothesis (32). In a short-term study comparing the effects of consuming beverages sweetened with HFCS, sucrose, fructose, and glucose (25% of energy) with meals in male subjects, consumption of either HFCS- or sucrose-sweetened beverages produced postprandial glucose, insulin and leptin profiles that were intermediate to responses induced by pure fructose and pure glucose. However, unexpectedly postprandial triacylglycerol responses to consumption of sucrose and HFCS were comparable to 100% fructose in both peak concentrations and integrated 24-h areas under the curve (Figure 3) (32). Long-term studies are needed to confirm these results and we are currently initiating a dose-response study to compare the effects of consuming diets containing three different levels of HFCS or fructose.
Figure 3.
Change of plasma TG concentrations from mean baseline levels (0800–0900 h) during four separate 24-h periods (0800–0800 h) in 7 men consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages at 25% of calculated energy requirements with each meal. Baseline TG concentrations were not different on the four study days. The effects of the 4 sugars on 24-h TG AUC were significantly different (p=0.007: repeated measures one-factor ANOVA) and the 24-h AUC during HFCS was significantly higher than during glucose consumption (p<0.01, Tukey’s post-test). Data are mean ± SEM.
FRUCTOSE AND ADDED SUGAR CONSUMPTION
If prolonged consumption of 25% of energy from HFCS or sucrose increases postprandial TG, Apo-B, and small dense-LDL to a comparable degree as fructose alone, this finding is likely to have important public health implications. The Institute of Medicine of the National Academies in the 2002 Dietary References Intakes (DRI) concluded that there was insufficient evidence to set an upper intake level for added sugars since there were not specific adverse health outcomes associated with excessive intake (33). Therefore, they suggested a maximal intake level of 25% of energy intake from added sugars.
The estimated mean intake of added sugars by Americans is 15.8%, however this value is based on consumption data from the 1994–1996 Continuing Survey of Food Intakes by Individuals (CSFII) (34). Recent data demonstrate that these intake rates may significantly underestimate actual sugar and sugar-sweetened beverage consumption by children and young adults. It was reported that the mean energy intake from sugar-sweetened beverages by 265 college students was 543 kcal/d (35), representing more than 20% of energy in a 2,500 kcal/d diet and over 25% of a 2,000 kcal/d diet. The mean intakes of sugar-sweetened beverages in 172 boys and 211 girls (age 13 years) were 809 ml/d and 674 ml/d, respectively (36). Assuming a 2,500 kcal/d and 2,000 kcal/d energy intake for the boys and girls, respectively, these adolescents consumed approximately 15% of energy as sugar-sweetened beverages. Mundt et al followed 208 boys and girls (aged 8–19) for an average of 5 years and found that sugar-sweetened beverage consumption increased with age, while physical activity declined (37). By the final year of the study, both males and females were consuming over 16% of energy as sugar-sweetened beverages. Similar results were reported for 2,371 girls followed from ages 9–15 (38). Sugar-sweetened beverage consumption increased with age and averaged 14%–16% of total energy intake during the final study year. A recent analysis of energy consumed as beverages in the U.S. population (using 1999–2002 National Health and Nutrition Examination Survey data) (39) reported that the percent of energy consumed from soft drinks, fruit drinks, and juices averaged 18.5% for males and 13.5% for females (20–39 years of age). These data suggests that the proportion of energy intake consumed from sugar-sweetened beverages by adolescents, college students, and adults up to 39 years of age approaches or exceeds 15.8% (the current estimate for the mean intake of total added sugar), without accounting for any other dietary sources of sugars. The large standard deviations in several of these reports (35–37) suggest that at least 16% of the studied populations were consuming greater than two times the mean intake, and therefore well over 25% of daily energy requirements from sugar-sweetened beverages. Based on these more recent intake data (35–39) and the current DRI guideline for maximal added sugar intake (33), as well as our short-term results suggesting 25% of energy HFCS or sucrose increases postprandial triacylglycerol concentrations comparably to fructose alone (32), long-term dose-response studies investigating the metabolic effects of consuming HFCS and/or sucrose consumption up to the level of 25% of energy are needed.
CONCLUSIONS
Results from both short-term and long-term studies demonstrate that fructose consumption results in decreased circulating levels of insulin and leptin when compared with glucose. Since insulin and leptin function as key signals to the CNS in the long-term regulation of energy balance, prolonged consumption of diets high in energy from fructose could lead to increased caloric intake or decreased caloric expenditure, thereby contributing to weight gain and obesity. Results from a 1-year study in nonhuman primates were equivocal, and testing this hypothesis in human subjects is likely to be difficult and costly.
In both short- and long-term studies we have demonstrated that fructose consumption substantially increases postprandial TG concentrations (9, 10, 12, 16). In long-term studies, plasma apoB concentrations and small-dense LDL are increased in subjects who consumed fructose-sweetened, but not glucose-sweetened, beverages for 10 weeks (12, 16). Results from a short-term study suggest that consuming HFCS- and sucrose-sweetened beverages increases postprandial triacylglycerol concentrations to the same degree as fructose alone (32). Further long-term studies are needed to investigate the effects of fructose, sucrose, and HFCS not only on lipid metabolism, but on glucose tolerance, insulin sensitivity, visceral adiposity and hepatic triacylglycerol content. These studies should include populations that differ in age, gender, and metabolic status, as well as dose-response studies to determine the amounts of dietary fructose, HFCS and sucrose that result in potentially adverse effects on lipid and carbohydrate metabolism.
ACKNOWLEDGMENTS
The authors thank and acknowledge James Graham for his essential technical support of the studies presented in the manuscript. Kimber L. Stanhope was responsible for implementing the studies presented in the manuscript, organization and analysis of data, and primary preparation of the manuscript. Peter J. Havel was responsible for the conception and design of the studies, obtaining funding, and preparation of the manuscript. Both authors read and approved the final submitted manuscript.
Sources of Support
This work was supported in part with research funding from The American Diabetes Association, the United States Department of Agriculture, and the National Institutes of Health (HL-HL075675, HL-091333, AT-002599, AT-002993, and AT-003545, and DK-58108). This work was supported in part with funding from Pepsico, Inc., Purchase NY. This research also received support from the California National Primate Research Center (RR-00169) and the U.C., Davis Clinical and Translational Science Center (Grant Number UL1 RR024146) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The content of this manuscript is solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp."
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in The American Journal of Clinical Nutrition, copyright American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://www.ajcn.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclaimers, if any
None
The authors have no conflicts of interest.
REFERENCES
- 1.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 3.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 4.Le KA, Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006;9:469–475. doi: 10.1097/01.mco.0000232910.61612.4d. [DOI] [PubMed] [Google Scholar]
- 5.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller WM, Gregoire FM, Stanhope KL, et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139:551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 7.Mueller WM, Stanhope KL, Gregoire F, Evans JL, Havel PJ. Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res. 2000;8:530–539. doi: 10.1038/oby.2000.66. [DOI] [PubMed] [Google Scholar]
- 8.Wellhoener P, Fruehwald-Schultes B, Kern W, et al. Glucose metabolism rather than insulin is a main determinant of leptin secretion in humans. J Clin Endocrinol Metab. 2008;85:1267–1271. doi: 10.1210/jcem.85.3.6483. [DOI] [PubMed] [Google Scholar]
- 9.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 10.Teff KL, Keim NL, Townsend RR, Havel PJ. Fructose-sweetened beverages decrease circulating leptin levels and increase postprandial triglycerides in obese men and women. Diabetes. 2005;54:A385. (abstract) [Google Scholar]
- 11.Weigle DS, Cummings DE, Newby PD, et al. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab. 2003;88:1577–1586. doi: 10.1210/jc.2002-021262. [DOI] [PubMed] [Google Scholar]
- 12.Swarbrick MM, Stanhope KL, Elliott SS, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008:1–6. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood) 2001;226:963–977. doi: 10.1177/153537020122601102. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 15.Havel PJ, Elliott S, Keim NL, Rader D, Krauss R, Teff K. Short-term and long-term consmption of high fructose, but not high glucose, diets increases postprandial triglycerides and apo-lipoprotein-B in women. J Invest Med. 2003;51:S163. (abstract) [Google Scholar]
- 16.Stanhope KL, Griffen S, Krauss RM, et al. Consumption of Fructose-, but not Glucose- Sweetened Beverages Produces an Atherogenic Lipid Profile in Overweight/Obese Men and Women. Diabetes. 2007;56:A16. (abstract) [Google Scholar]
- 17.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz JM, Neese RA, Schakleton C, Hellerstein MK. De novo lipogenesis during fasting and oral fructose ingestion in lean and obese hyperinsulinemic subjects. Diabetes. 1993;42:A39. (abstract) [Google Scholar]
- 19.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaka T, Shimano H, Yahagi N, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–569. doi: 10.2337/diabetes.53.3.560. [DOI] [PubMed] [Google Scholar]
- 21.Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:E1180–E1190. doi: 10.1152/ajpendo.00471.2001. [DOI] [PubMed] [Google Scholar]
- 22.Hyson D, Rutledge JC, Berglund L. Postprandial lipemia and cardiovascular disease. Curr Atheroscler Rep. 2003;5:437–444. doi: 10.1007/s11883-003-0033-y. [DOI] [PubMed] [Google Scholar]
- 23.Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–355. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Miranda J, Perez-Martinez P, Marin C, Moreno JA, Gomez P, Perez-Jimenez F. Postprandial lipoprotein metabolism, genes and risk of cardiovascular disease. Curr Opin Lipidol. 2006;17:132–138. doi: 10.1097/01.mol.0000217894.85370.c2. [DOI] [PubMed] [Google Scholar]
- 25.Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low- density lipoprotein. Biochem Soc Trans. 2003;31:1066–1069. doi: 10.1042/bst0311066. [DOI] [PubMed] [Google Scholar]
- 26.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437–446. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 28.Lamarche B, Lemieux I, Despres JP. The small, dense LDL phenotype and the risk of coronary heart disease: epidemiology, patho-physiology and therapeutic aspects. Diabetes Metab. 1999;25:199–211. [PubMed] [Google Scholar]
- 29.Brunzell JD. Increased ApoB in small dense LDL particles predicts premature coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:474–475. doi: 10.1161/01.ATV.0000156537.78366.1d. [DOI] [PubMed] [Google Scholar]
- 30.Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med. 2005;258:395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 31.Fields S. The fat of the land: do agricultural subsidies foster poor health? Environ Health Perspect. 2004;112:A820–A823. doi: 10.1289/ehp.112-a820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four- hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Academies IoMotN. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, D.C.: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51. doi: 10.1016/S0002-8223(00)00018-3. quiz 49-50. [DOI] [PubMed] [Google Scholar]
- 35.West DS, Bursac Z, Quimby D, et al. Self-reported sugar-sweetened beverage intake among college students. Obesity (Silver Spring) 2006;14:1825–1831. doi: 10.1038/oby.2006.210. [DOI] [PubMed] [Google Scholar]
- 36.van der Horst K, Kremers S, Ferreira I, Singh A, Oenema A, Brug J. Perceived parenting style and practices and the consumption of sugar-sweetened beverages by adolescents. Health Educ Res. 2007;22:295–304. doi: 10.1093/her/cyl080. [DOI] [PubMed] [Google Scholar]
- 37.Mundt CA, Baxter-Jones AD, Whiting SJ, Bailey DA, Faulkner RA, Mirwald RL. Relationships of activity and sugar drink intake on fat mass development in youths. Med Sci Sports Exerc. 2006;38:1245–1254. doi: 10.1249/01.mss.0000227309.18902.fe. [DOI] [PubMed] [Google Scholar]
- 38.Striegel-Moore RH, Thompson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148:183–187. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106:1992–2000. doi: 10.1016/j.jada.2006.09.009. [DOI] [PubMed] [Google Scholar]