Abstract
Objective
We assessed whether vasomotor symptoms (VMS) were associated with coronary artery calcium (CAC) and how hormone therapy may influence this association.
Methods
Subjects were a subset of women aged 50 to 59 and a history of hysterectomy that enrolled in the Women’s Health Initiative (WHI) clinical trial of estrogen alone and underwent a computed tomography scan of the chest at the end of the trial to determine CAC. Participants provided information about VMS (hot flashes and/or night sweats), as well as HT use, on self-administered questionnaires at trial baseline.
Results
The sample consisted of 918 women with a mean (SD) age of 55.1 (2.8) years at WHI randomization and 64.8 (2.9) years at CAC ascertainment. The prevalence of a CAC score > 0 was 46%, while the prevalence of a CAC score ≥ 10 and > 100 was 39 and 19%, respectively. At randomization, 77% reported a history of any VMS at any time prior to or at enrollment in the WHI while 20% reported any VMS only present at enrollment. Compared to those without a history of any VMS and after adjustment for potential confounders, a history of any VMS at any time up to and including WHI enrollment was associated with a significantly reduced odds for CAC > 0 (Odds Ratio 0.66, 95% CI 0.45 – 0.98). Moreover, as duration of HT increased the inverse association between any VMS and CAC moved toward the null.
Conclusion
A history of any VMS was significantly associated with a reduced odds for CAC independent of traditional CVD risk factors and other relevant covariates. This association appears to be influenced by duration of hormone therapy.
Keywords: calcium, coronary, vasomotor symptoms, women, menopause, atherosclerosis
INTRODUCTION
Vasomotor symptoms (VMS), including hot flashes and night sweats, are relatively common and cause significant morbidity in women.1 The prevalence of these symptoms varies in relation to the menopausal transition with the lowest levels typically being found in premenopausal women (14 – 51%), and higher levels in both perimenopausal (35 – 50%) and postmenopausal women (30 – 80%).2 Although VMS have been shown to be responsive to hormone therapy,3, 4 their specific etiology is uncertain. In this regard, VMS in women of middle age have been associated with lower serum estradiol and inhibin levels but higher FSH concentrations.5 Since this pattern of reproductive hormones has been shown to be associated with higher levels of CVD risk factors6 we hypothesize that women with vasomotor symptoms would be at increased risk for coronary artery disease.
In a regulated process similar to skeletal bone formation,7 calcium is deposited in atherosclerotic plaques.8 With the advent of computed tomography, these calcified atheromatous plaques can be detected throughout the vasculature,9 including the coronary arteries.10 The extent of coronary artery calcium (CAC) is highly correlated with both the total atheromatous plaque burden11 and the percent stenosis in that vascular bed.12 Moreover, several studies have shown CAC to be a strong and independent predictor of incident coronary heart disease (CHD) events in both men and women.13, 14
Use of CAC as marker of coronary atherosclerosis is increasingly advocated as a component of individual cardiovascular disease (CVD) risk stratification procedures.15 Accordingly, the aim of this study was to determine the magnitude of the associations between VMS and CAC in women with a history of hysterectomy that were between the ages of 50 and 59 years at the time of their enrollment in the Women’s Health Initiative (WHI).
METHODS
Subjects
This study examined a subset of participants in the WHI Conjugated Equine Estrogen trial (ET). These women were postmenopausal and aged 50 to 79 years at randomization with a history of hysterectomy prior to enrollment. The women were randomized (N = 10,739) to receive CEE, 0.625 mg/d (Premarin; Wyeth Pharmaceuticals, St Davids, PA) or a matching placebo. Women enrolled in the ET agreed to not take hormone therapy, beyond that provided in the clinical trial. Methods for data collection, management, and quality assurance have been published previously.16 The ET trial was ended after an average of 6.8 years follow-up.
The Women’s Health Initiative Coronary Artery Calcium Study (WHI-CACS) was a substudy of the WHI ET trial, conducted approximately 1.3 years after its completion (8.7 years after the baseline WHI visit). All WHI clinical sites were asked to participate in this substudy and 28 agreed. Reasons for site non-participation included lack of suitable equipment and logistical concerns. Women who were between 50 to 59 years old at the time of their randomization into the ET trial were invited to enroll. Invitations were mailed to these women (N=1742) requesting them to undergo a one-time cardiac computed tomography (CT) scan to determine CAC by electron beam or multidetector-row computed tomography. Exclusion criteria were a last measured or reported weight of 300 lbs or higher (due to technical and equipment-related restrictions), participant request for no further contact or clinic visits, or participant lost to follow-up or deceased since randomization (30.4% of participants were excluded for one or more of these reasons). Informed consent for WHI-CACS was provided by 1,079 women (61.6% of those eligible at the 28 clinical centers).
The Human Subjects Review Committee at each participating institution approved the WHI study protocols. In accordance with institutional guidelines, all subjects provided written informed consent.
Data Collection
Trial participants provided data on a wide range of factors at the ET study baseline clinic visit. Ethnicity was determined by self-report with the following categories: non-Hispanic white, African-American/black (non-Hispanic), Hispanic, Asian/Pacific Islander, American Indian/Alaska Native, or unknown (women who indicated “other” ethnicity or did not answer the question). Education and income were ascertained by self-report from a range of categories. The presence of high cholesterol or diabetes was identified by self-reported use of a medication for these conditions. Smoking was categorized as current, former or none. Use of postmenopausal hormone therapy before WHI CEE trial enrollment was ascertained via an in-person interview at the baseline clinic visit.
Vasomotor symptom data was collected at baseline by a questionnaire specifically designed to ascertain the participant’s reproductive history. In this questionnaire, participants were asked their age at the time of their last regular menstrual period, their age at time they had their last menstrual bleeding of any kind and for a history of any oophorectomy and their age at the time of this procedure. The questionnaire included items on menopausal symptoms (i.e. “Have you ever had menopausal symptoms, such as hot flashes or night sweats?”), and, if these had occurred, participants were asked to provide their age when these symptoms started (i.e. “How old were you when you first had symptoms such as hot flashes or night sweats?”) and ended (i.e. “How old were you when you last had symptoms such as hot flashes or night sweats? If you are still having symptoms such as hot flashes or night sweats, enter your current age.”) Presence of vasomotor symptoms at baseline, as well as other menopausal symptoms, was assessed by self report at baseline on a different questionnaire.
Anthropomorphic measurements were obtained at baseline. Body mass index was calculated as weight (kg) / height (m2). Waist and hip circumferences (in cm) were obtained using a standardized measuring tape. After a 5-minute rest and using a conventional mercury sphygmomanometer and appropriately sized cuffs, blood pressure was measured twice at baseline and then at each clinic visit until the end of the CEE trial.
Coronary Artery Calcified Plaque Measurements
A standardized protocol was developed based on prior multi-center experience with cardiac CT17. Phantom scan and test images were obtained from each CT system to verify technical parameters and CT system performance. Analyses of the measurements were performed by certified staff at the central reading center at Wake Forest University who were masked to participants’ treatment assignment. The Agatston score was calculated on a computer workstation (TeraRecon Inc, San Mateo, CA) by experienced image analysts using established parameters (lesion size of >1 mm2, adjustment for slice thickness, and threshold of 130 HU).18 After the scan was scored, the participants were provided with a letter documenting their calcium score, which could be reviewed with their health care provider (if desired).
Women with a history of coronary revascularization (CABG or PTCA) prior to randomization were excluded from the analysis. Also, the reading protocol specified exclusion of coronary stents, pacemakers, metallic clips, and other surgical remnants from the analysis process. Three women with incomplete scans were excluded. After excluding these women and accounting for missing data, the final dataset included 918 participants. If participant had missing age at menopause data, we used age at oophorectomy (as applicable or available) or age at hysterectomy as a surrogate.
Statistical Analyses
The primary outcome variable was a CAC score > 0 while CAC severity (CAC = 0, 0 < CAC ≤ 100 and CAC > 100) was treated as a secondary outcome. The primary exposure variable was a history of ever having vasomotor symptoms. Secondary analysis investigated duration of vasomotor symptoms and the interaction between self-report of HT use and VMS (yes/no).
Using baseline measurements, hypertension was defined as a systolic blood pressure ≥ 140 or a diastolic blood pressure ≥ 90 or use of a blood pressure lowering medication. Diabetes and hypercholesterolemia were defined as physician prescribed current use of medications for these conditions. Cigarette smoking covariate adjustment was by pack years.
Baseline characteristics were compared between participants with a CAC score of zero versus greater than zero. Differences between the groups were assessed using age adjusted logistic regression models. For the main analysis, logistic regression models were used to evaluate the association between the presence of any CAC and the exposure variables a) VMS ever (yes/no) and b) VMS timing (prior to WHI enrollment/prior to and present at WHI enrollment/no VMS). Nominal multinomial logistic regression models were used to evaluate the association between the primary exposure variables and the severity of CAC. To control for potential confounding, we adjusted for baseline values of age (categorical and linear), race/ethnicity (White/Black/Hispanic/Other), diabetes, dyslipidemia, smoking, BMI (categorical and linear), systolic blood pressure, diastolic blood pressure (linear and quadratic), family history of CHD, education, income, pre-enrollment HT use, bilateral oophorectomy and CEE randomization assignment; unless specified categorical variables were coded as shown in Table 2 and continuous variables were fit linearly. A two-sided p-value < 0.05 was considered statistically significant.
TABLE 2.
Distribution of Study Variables by the Presence/Absence of Vasomotor Symptoms
| Continuous Variables | VMS (Yes) | VMS (No) | p-value* |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age at screening (years) | 55.1 (2.9) | 55.3 (2.8) | 0.35 |
| Body-mass index (kg/m2) | 30.4 (6.1) | 31.4 (6.6) | 0.04 |
| Waist circumference (cm) | 91.1 (14.1) | 93.3 (14.9) | 0.05 |
| Hip circumference (cm) | 111.3 (13.2) | 112.8 (12.9) | 0.15 |
| Systolic BP (mm Hg) | 124.8 (15.5) | 123.4 (16.6) | 0.21 |
| Diastolic BP (mm Hg) | 77.7(8.7) | 77.1 (9.6) | 0.35 |
| Pack years of smoking | 10.6 (17.8) | 9.7 (16.1) | 0.5 |
| Total energy expenditure/wk (MET-hours) | 10.0 (12.3) | 9.9 (15.5) | 0.96 |
| Categorical Variables | VMS (Yes) | VMS (No) | p-value* |
| % (N) | % (N) | ||
| Agatston score > 0 | 54.9 (385) | 49.5 (103) | 0.21 |
| Race/ethnicity | 0.009 | ||
| - White | 73.8 (517) | 76.9 (160) | |
| - Black | 18.8 (132) | 10.6 (22 | |
| - Hispanic | 5.4 (38) | 9.6 (20) | |
| - American Indian | 0.9 (6) | 1.0 (2) | |
| - Asian/Pacific Islander | 0.3 (2) | 0.0 (0) | |
| - Unknown | 0.9 (6) | 1.9 (4) | |
| Baseline income (< $35,000) | 38.8 (262) | 39.5 (77) | 0.86 |
| Education | 0.59 | ||
| - High school/GED or less | 26.2 (182) | 24.2 (50) | |
| - School after high school | 43.5 (302) | 47.3 (98) | |
| - College degree or higher | 30.4 (211) | 28.5 (59) | |
| HRT arm (CEE group) | 50.2 (352) | 51.9 (108) | 0.67 |
| HRT use status | <0.01 | ||
| - Never used | 42.1 (295) | 60.6 (126) | |
| - Past user | 34.5 (242) | 21.6 (45) | |
| - Current user | 23.4 (164) | 17.8 (37) | |
| Bilateral oophorectomy | 38 (257) | 39.3 (75) | 0.79 |
| Alcohol consumption | 0.32 | ||
| - Non Drinker | 45.1 (315) | 51.0 (106) | |
| - <= 1 drink/day | 45.6 (318) | 40.4 (84) | |
| - > 1 drink/day | 9.3 (65) | 8.7 (18) | |
| Antihypertensive medication use at Baseline | 26.0 (182) | 20.7 (43) | 0.11 |
| Baseline hypertension | 0.4 | ||
| - Normotensive: < 120/80 and no meds | 28.7 (201) | 33.2 (69) | |
| - Prehypertensive: [120,140)/[80,90) and no meds | 33.4 (234) | 32.7 (68) | |
| - Hypertensive: >= 140/90 or on medication | 37.9 (266) | 34.1 (71) | |
| History high cholesterol or taking lipid-lowering med | 9.8 (69) | 11.1 (23) | 0.66 |
| Antihyperlipidemic use (including statins) | 4.9 (34) | 6.3 (13) | 0.47 |
| History treated diabetes or taking anti-diabetic med | 5.0 (35) | 4.3 (9) | 0.66 |
| Family History of MI | 47.2 (317) | 47.4 (93) | 0.98 |
Adjusted for age
We also investigated the interaction of VMS ever (yes/no) with a) HT duration (self-report of hormone therapy use prior to WHI enrollment) and b) timing of HT initiation (<= 2 years after menopause, > 2 years after menopause). For b) HT use includes HT use prior to WHI enrollment, as well as CEE use in the WHI clinical trial. Statistical significance of this subgroup analyses was based on Wald tests of the multiplicative interactions. The main statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
The characteristics of the cohort stratified by the presence and absence of CAC are presented in Table 1. Those who had any CAC were significantly older. After adjustment for age, the CAC positive group had significantly higher levels of body mass index, hip and waist circumference, hypertension, cholesterol medication use, diabetes medication use and pack-years cigarette smoking while having significantly lower levels of physical activity, annual income and education. In this analysis, a history of any VMS, VMS severity and VMS duration were not significantly different by CAC status.
TABLE 1.
Cohort Characteristics by Coronary Calcium Status
| Characteristic | CAC > 0 (n = 423) | CAC = 0 (n = 495) | P-value* |
|---|---|---|---|
| Age at screening† (years) | 55.6 (2.7) | 54.8 (2.9) | <0.01 |
| Age at menopause† (years) | 43.4 (7.7) | 43.8 (7.2) | 0.30 |
| Body Mass Index (kg/m2) | 31.6 (6.2) | 29.8 (6.1) | <0.01 |
| Waist circumference† (cm) | 95.1 (13.7) | 88.7 (14.2) | <0.01 |
| Hip circumference† (cm) | 113.3 (13.0) | 110.4 (13.1) | <0.01 |
| Pack years of smoking† | 13.6 (19.5) | 7.5 (14.7) | <0.01 |
| Hypertension‡ | 44.4 | 30.5 | <0.01 |
| Dyslipidemia‡ | 14.2 | 6.7 | <0.01 |
| Diabetes‡ | 6.9 | 3.0 | 0.01 |
| Family history of CHD‡ | 48.7 | 46.0 | 0.52 |
| Physical Activity† (MET-hours/week) | 8.9 (12.2) | 11.0 (13.8) | 0.04 |
| History of any VMS† | 51.5 | 56.7 | 0.24 |
| VMS severity at baseline | 0.75 | ||
| - None | 41.4 | 39.2 | |
| - Mild | 33.6 | 36.1 | |
| - Moderate/Severe | 25.0 | 24.7 | |
| Duration of VMS‡ | 0.87 | ||
| - < 5 years | 18.2 | 22.7 | |
| - 5 to <10 years | 25.5 | 25.9 | |
| - 10 to < 15 years | 20.8 | 21.1 | |
| - ≥ 15 years | 35.5 | 30.3 | |
| HRT use status‡ | 1.00 | ||
| - Never used | 45.4 | 47.3 | |
| - Past user | 32.6 | 30.5 | |
| - Current user | 22.0 | 22.2 | |
| Bilateral oophorectomy‡ | 39.8 | 37.2 | 0.72 |
Adjusted for age;
Mean (SD),
Percent (Frequency)
Seven-hundred and one women had any VMS
Table 2 shows the distributions of the cohort characteristics stratified by the presence/absence of ever having vasomotor symptoms. Compared to those with no history of VMS, women with a history of any VMS had a significantly lower body mass index, waist circumference and were more likely to report current or former use of hormone therapy. Those with any VMS were also more likely to be African American and less likely to be Hispanic or non-Hispanic White. The prevalence of any CAC in those with a history of any VMS was 45.1% while the prevalence in those without a history of VMS was 50.5% (p = 0.17). Adjustment for age at enrollment in the WHI did not change the significance of the associations listed above.
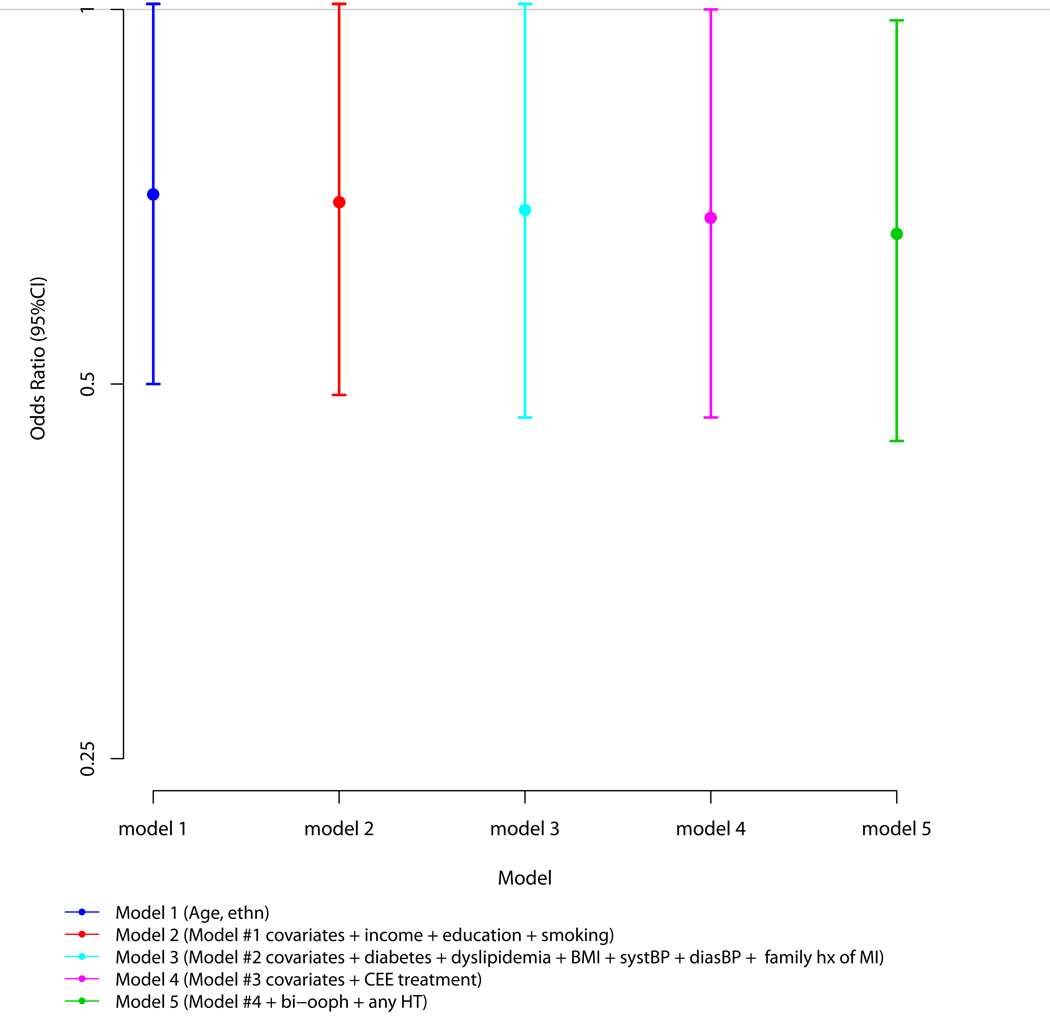
The results of multivariable logistic regression analyses for the association between a history of any VMS and the presence of a CAC score greater than zero are presented in Figure 1. With adjustment for age and ethnicity and compared to those without a history of VMS, women with a history of any VMS had a 29% lower odds for any CAC (p = 0.06). Additional adjustment for income, education and smoking (model 2), these variables plus diabetes, dyslipidemia, BMI, systolic blood pressure, diastolic blood pressure and family history of myocardial infarction (model 3), and then model 3 plus CEE randomization status (model 4) minimally accentuated the magnitudes of the associations (OR: 0.70, 95% CI: 0.49 – 1.01, p = 0.06; 0.69, 0.47 – 1.01, p = 0.05; 0.68, 0.47 – 1.00, p = 0.05; respectively). Addition of both a history of bilateral oophorectomy and any hormone therapy use prior to WHI enrollment to the multivariable models (model 5) further accentuated the association (0.66, 0.45 – 0.98, p=0.04). Addition of 1) time from the baseline WHI visit to the time of CT scan and 2) physical activity did not change these results. Moreover, the results did not differ by severity of CAC. Specifically and after complete multivariable adjustment, the OR for the presence of severe CAC (>100) was 0.67 (0.41 – 1.12) compared to 0.66 (0.43 – 1.02) for moderate CAC (<= 100).
Figure 1. Multivariable Associations between the History of Any Vasomotor Symptoms and the Presence of Coronary Artery Calcium.
316 (45.1%) of participants with a history of any VMS had a CAC score > 0.
105(50.5%) of participants with no history of any VMS had a CAC score > 0.
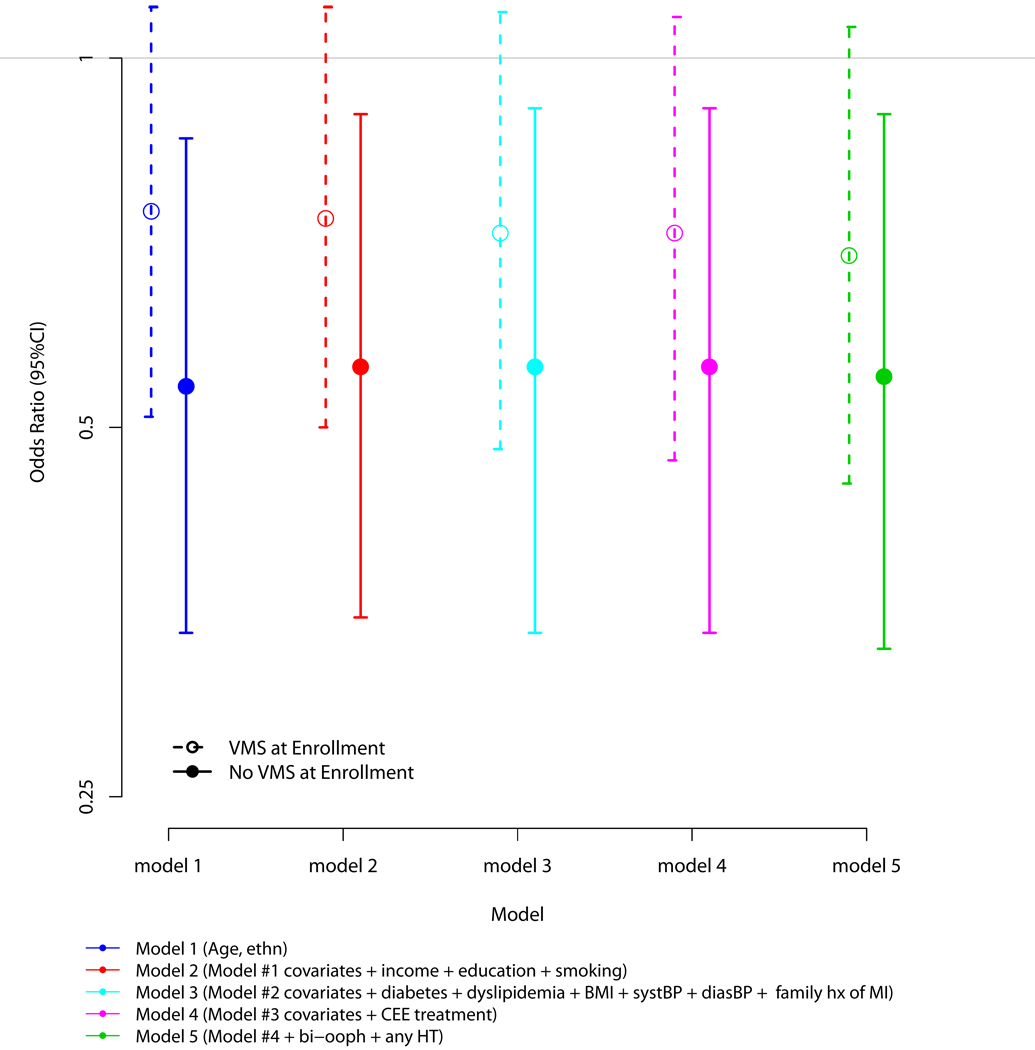
Since the variable “any VMS” is rather crude and duration of VMS may be relevant to the presence of CAC, we conducted analyses to determine if there were differences in the odds for CAC between those who had a history of any VMS, but did not report VMS at WHI enrollment (i.e. VMS were absent at enrollment), and those who had a history of any VMS and did report VMS at enrollment (i.e. VMS were present at enrollment). Twenty percent of the sample had a history of any VMS present at WHI enrollment. Utilizing the same analytic strategy employed in the previous paragraph and using the group with no history of VMS as the reference category, those with a history of any VMS, but that were absent at baseline, had a lower odds for the presence of any CAC than those with a history of any VMS that were also present at WHI enrollment (p=0.06, Figure 2).
Figure 2. Multivariable Associations between History of Any Vasomotor Symptoms for Women with and without Vasomotor Symptoms at Enrollment and the Presence of Any Coronary Artery Calcium.
246 (45.2%) of participants with a history of any VMS and did report VMS at enrollment had a CAC score > 0.
80 (43.7%) of participants with a history of any VMS and reported no VMS at enrollment had a CAC score > 0.
92 (51.1%) of participants with no history of any VMS and reported no VMS at enrollment had a CAC score > 0.
Note that 28 participants reported VMS at enrollment had either missing(n=5) or reported "no" to ever having VMS (n=25). For this analysis, these women were coded as a history of any VMS and reporting VMS at enrollment
When we examined the association between severity of VMS and CAC, there was no significant associations found. Specifically, compared to those with no VMS, the odds of CAC among those with moderate to severe VMS was 0.99 (0.65 – 1.50) while the odds were 0.95 (0.66 – 1.37) among those with mild VMS.
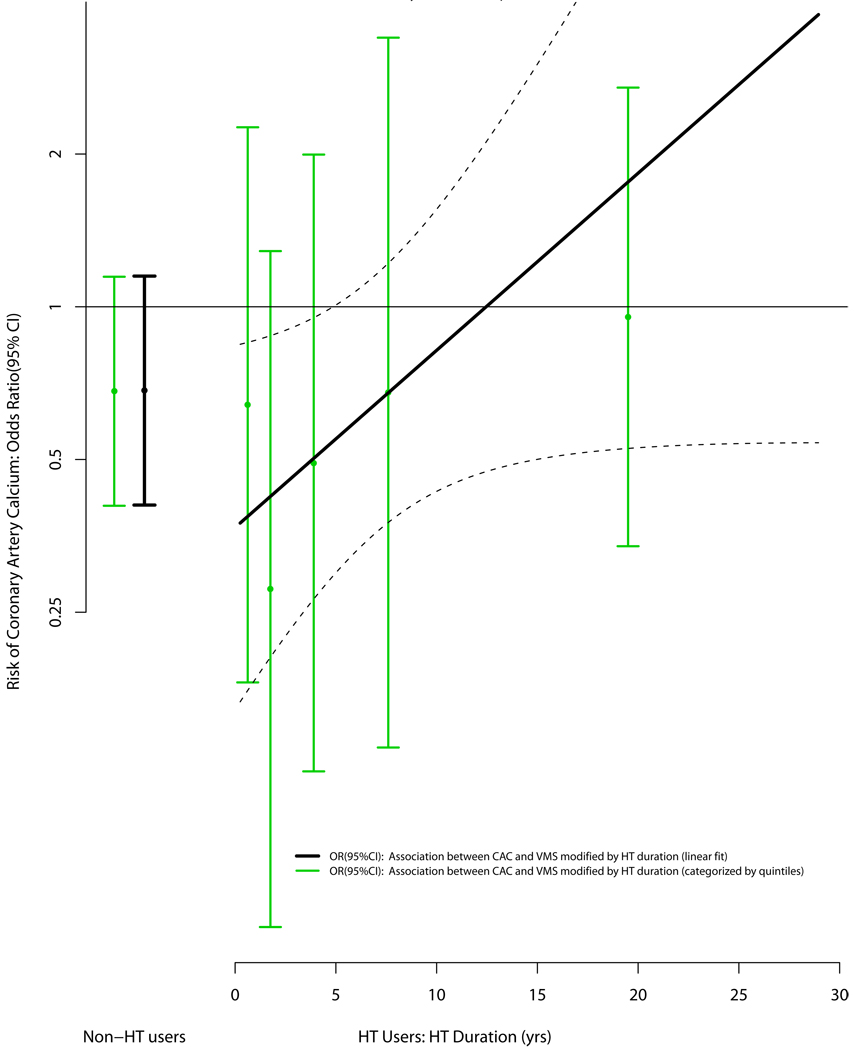
As HT has been used to treat VMS, and HT may be associated with CAC in women of the age group studied,19 we postulated that use of HT may modify the association between VMS and CAC. Accordingly, we conducted analyses testing the association between CAC and VMS, with the latter being contingent on the reported duration of HT use. In these analyses, as duration of HT increased the inverse association between VMS and CAC moved toward the null (Figure 3). A test for interaction between VMS and HT (as a linear function of time) for CAC was of borderline significance (p = 0.06) and the results were unchanged when “age at starting HT” was included in the models. Model diagnostics suggested that the linear fit of HT was reasonable and the association was not influenced by any extreme values.
Figure 3. Multivariable Association between the Presence of Any Vasomotor Symptoms and the Presence of Coronary Artery Calcium (Modified by Hormone Therapy Duration).
Adjusted for age, ethnicity, ethnicity, income, education, smoking, diabetes, dyslipidemia, BMI, SBP, DBP, family history of MI, CEE treatment, bilateral oophorectomy and hormone therapy use
129 (43.7%) of participants with a history of any VMS and no prior HT use had a CAC score > 0.
62 (49.2%) of participants with no history of any VMS and no prior HT use had a CAC score > 0.
187 (46.1%) of participants with a history of any VMS and prior HT use had a CAC score > 0.
43(52.4%) of participants with no history of any VMS and prior HT use had a CAC score > 0.
We then explored whether the timing of starting HT, relative to menopause, modified the association between VMS and CAC. Specifically, we created 3 HT groups (no HT use, HT use starting within 2 years of menopause and HT use starting 2 or more years after menopause) and tested the interaction of this categorization with VMS and CAC. In this analysis and compared to no VMS, the presence of any VMS among those who reported no history of taking HT or who started HT within 2 years of menopause resulted in the same odds for any CAC (0.48, 0.23 – 1.03 and 0.48, 0.26 – 0.89; respectively). Conversely, the odds for any CAC among those with any VMS and who started HT 2 or more years after menopause was 1.40 (0.71 – 2.75). The p-value for interaction was significant (0.04) indicating a significant difference in odds for CAC by when HT use was initiated relative to menopause. Similar results were found when the HT use variable was defined by 5 years after menopause [results not shown].
We also conducted analyses where the type of VMS was based on self-report of bilateral oophorectomy. Here, VMS was coded as "none", "Natural VMS" (no self-report of bilateral oophorectomy or VMS occurred before bilateral-oophorectomy) or "Other VMS" (VMS occurred after bilateral oophorectomy). After multivariable adjustment, the results did not demonstrate differences in the magnitudes of the associations for the presence of any CAC by type of VMS. That is, with adjustment for the covariates listed in model 5 of figures 1, 2 or 3, the odds (95% CI) for the presence of any CAC were 0.73 (0.50 – 1.08) for those with a history of Natural VMS and 0.76 (0.47 – 1.21) for those with a history of Other VMS. As before, the odds ratios did not differ by severity of CAC.
DISCUSSION
In this study of women with a history of hysterectomy and between the age of 50 and 59 at enrollment in the WHI, a history of vasomotor symptoms was associated with a reduced odds for the presence and extent of coronary artery calcium independent of the traditional CVD risk factors and other relevant covariates. This association was stronger among those who did not have VMS that persisted until enrollment in the WHI suggesting that shorter duration of symptoms is associated with a lower odds for having any CAC. The association between VMS and CAC may be influenced by hormone therapy use such that longer HT use may attenuate the inverse association between VMS and CAC. Moreover, the timing of when HT use is started appears to modify this association in that those who either did not take HT or took it within 2 years of menopause had a lower odds for any CAC while those who took HT more than 2 years after menopause had a higher odds for any CAC. Taken together, the results presented indicate complex relationships between vasomotor symptoms, hormone therapy and calcification of atherosclerotic plaques.
Atherosclerosis is a systemic20 inflammatory process21, 22 that is typically initiated at arterial branch points.23 Calcium is actively deposited in the lipid core of atherosclerotic plaques via an organized and regulated process24–26 analogous to embryonic cortical bone formation.7, 27 In this regard, previous studies have shown different phases of bone formation in these plaques to include acellular matrix, calcified matrix, osteoid and mature skeletal bone.7 Since previous studies have demonstrated either very weak or null associations between plasma lipid levels and calcified atherosclerosis,28, 29 it seems plausible that the formation of skeletal bone in atherosclerotic plaques may be regulated by metabolic processes relatively distinct from the endothelial function, lipid deposition and smooth muscle infiltration characteristic of the atherosclerotic process.24 If true, pathophysiologic processes (such as VMS) could have differential effects on calcified atherosclerotic plaques depending on which ‘component’ (i.e. atherosclerosis or calcification) is influenced by that process at the stage of disease that exists when the process occurs.
The pathophysiology of VMS is not clearly delineated. Previous studies are mixed on the association between estradiol levels and the presence of hot flashes. Similarly, luteinizing hormone (LH) levels have not consistently been shown to be associated with VMS such that symptomatic women can have normal LH levels.30 Interestingly, recent research suggests that frequent vasomotor symptoms and bone loss is linked to follicle stimulating hormone (FSH) levels and not necessarily estradiol levels.31, 32 In animal models, higher FSH levels result in increased osteoclast formation and activity.33 Clinical studies extend these findings by demonstrating increased bone turnover with higher FSH levels.34 In this context, estrogen supplementation would reduce the level of FSH resulting in lower osteoclast activity and thereby lower bone turnover resulting in a slower loss of bone (i.e. more bone relative to those who don’t supplement with HT).
Using data from the Eindhoven Perimenopausal Osteoporosis study, Gast and colleagues examined the association between VMS (flushing and/or night sweats) and bone mineral density (BMD) of the lumbar spine by dual energy x-ray absorptiometry (DEXA). In this study, women with a higher frequency of VMS symptoms were found to have significantly lower BMD values independent of age, body mass index, menopause status, smoking, education, exercise and hormone therapy use.32 Similarly, results from the Study of Women’s Health Across the Nation (SWAN) indicate a lower BMD by DEXA for women with VMS regardless of whether the VMS occurred pre-, peri- or postmenopausally.31 These results support the thesis of either lower bone formation or higher levels of bone resorption among those with VMS. However, results from the Rancho Bernardo Study (RBS) did not find a significant association between VMS and BMD.35 Importantly, the women in the RBS were significantly older (mean age: 73 years) than in our study (as well as those cited above) and had a higher prevalence of HT use, which was on average for over 12 years.
Contrary to their findings on VMS and BMD, the SWAN published results showing current (i.e. within 2 weeks) VMS being associated with higher levels of both coronary and aortic calcified atherosclerosis, with the latter association being independent of CVD risk factors and estradiol levels.36 From this, the authors suggest that hot flashes may mark adverse underlying vascular changes among midlife women. Of note, women enrolled in the SWAN were younger (mean age: ~47 years) and over half were either pre- or perimenopausal. Also, night sweats was not part of the definition of VMS. Since our definition of VMS was based on ever having VMS and included night sweats, and our population was composed entirely of postemenopausal women with a history of hysterectomy, direct comparisons of our results to those from the SWAN are difficult, as they are likely confounded by the issues described above.
The lack of association between VMS and BMD in the RBS raises the question of whether HT use could modify the association between VMS and calcium deposits in atherosclerotic plaques. Estrogen is important for maintaining higher levels of BMD in both men37 and women.38 Clinically, trial data from the WHI indicate a significantly lower risk for incident fractures among women without a uterus who received estrogen alone39, as well as women with a uterus who received estrogen plus progestin,40 further substantiating the association between HT and higher levels of BMD. The results from our study are in agreement with these findings in that women with VMS who reported either no HT use or a shorter duration of HT use had significantly lower odds of having any calcified atherosclerotic plaques.
The finding of an attenuation of the odds between VMS and CAC with longer HT use appears to be disparate from previous findings in the WHI-CACS cohort of lower CAC among those who received estrogen alone.19 In this regard, although the previous and current analyses utilized the WHI-CACS cohort, it is important to note that the results presented from the current analysis are within the context of VMS, whereas the previous analysis were conducted independently of VMS. Also, the duration of HT use may be relevant. In the WHI-CACS trial of ET, the mean duration of estrogen alone treatment was 7.4 years, which corresponded to a 22% lower odds of a CAC score greater than zero. Odds of CAC for shorter durations of treatment are not available. In our study, the odds for a CAC score greater than zero were 38% lower for those who took HT for 6 years and 27% lower for those who took HT for 8 years. Clearly, these results are similar to those for the WHI-CACS trial and suggest that among women of the same relative age (50 – 59 years), use of HT for less than 10 years is likely to be associated with a reduced odds for CAC.
Results from our study also indicate that the timing of starting HT may result in differences in the magnitudes (and direction) of the association between VMS and CAC. That is, women with VMS who started HT relatively shortly after menopause (i.e. < 2 years) had a reduced odds for having any CAC; however, their odds for any CAC did not differ from those who did not take HT within 2 years. On the other hand, those with VMS who start HT later than 2 years had a significantly higher odds for any CAC. These results are in agreement with previous studies that tend to have fewer incident CHD events among those who begin HT shortly after menopause while those who take HT later are at higher risk for incident CHD, especially if they also reported vasomotor symptoms at baseline.41 Because inflammatory changes are believed to precede the development of atheroma, and calcification of atherosclerotic plaques (i.e. CAC) is a relatively late development of this process42, 43, the lack of association in women with VMS who started HT within 2 years of menopause may reflect protection from this early process, limiting or delaying the subsequent stages leading to calcification.
Vasomotor symptoms in women of middle age have been associated with lower serum estradiol and inhibin levels but higher FSH.5 This pattern of reproductive hormones has been shown to be associated with higher levels of CVD risk factors.6 In this regard, the results from the SWAN study (discussed above) suggest that VMS are associated with an increased risk for CVD. However, a recent report from the RBS demonstrated a reduced risk for incident CVD among women with a history of VMS.44 This, combined with the results we present, are contrary to those from the SWAN and indicate the need for further study on the association between VMS and risk of cardiovascular disease.
Although the WHI-CACS study was conducted using women enrolled in a randomized clinical trial, the current analysis was observational and cross-sectional in nature and conducted using a subset of women in the WHI ET clinical trial. Therefore, there could be residual confounding or bias affecting the results. We have attempted to address this issue by considering as many potential confounding variables as possible in the analysis. Also, as the measures for coronary calcium were obtained at the end of the WHI-ET trial, there is the possibility of survival bias. As the presence and extent of coronary calcium is significantly associated with incident morbidity and mortality, the bias in this case would result in an attenuation of the magnitude of the effect. Also, since the average age of the cohort at baseline and time of CT scan was less than 65 years, the likelihood for a significant number of incident CHD deaths in this study population is relatively small. History of VMS was ascertained retrospectively by questionnaire, which may result in recall bias for this exposure. Similarly, self-report of oophorectomy may have resulted in some misclassification. Finally, the results of this study are limited to those women who are recently postmenopausal and with a history of a hysterectomy.
CONCLUSION
The presence of any VMS was associated with a lower odds for the presence of calcified coronary atherosclerosis. This association appears to differ by the duration of VMS, as well as the duration and timing of HT use, with the latter associations indicating shorter duration and more immediate use after menopause being associated with a reduced odds for CAC.
ACKNOWLEDGEMENTS
We gratefully acknowledge the dedication of investigators and staff at the WHI clinical centers, the WHI-CACS centers, the WHI Clinical Coordinating Center (CCC), and the NHLBI Program Office. Most importantly, we are indebted to the WHI participants for their extraordinary commitment to women’s health research. The National Heart, Lung, and Blood Institute and U.S. Department of Health and Human Services funds the WHI program and provided support for the WHI-CACS ancillary study. Wyeth provided study pills (active and placebo) for the WHI-CEE trial but had no other role in the study. This work was also supported in part by a grant from the American Heart Association (Allison). The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources: Support for this research was provided by contracts with the NIH and a grant from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES
None
REFERENCES
- 1.Porter M, Penney GC, Russell D, Russell E, Templeton A. A population based survey of women's experience of the menopause. BJOG: An International Journal of Obstetrics & Gynaecology. 1996;103:1025–1028. doi: 10.1111/j.1471-0528.1996.tb09555.x. [DOI] [PubMed] [Google Scholar]
- 2.N. I. H. State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Management of Menopause-Related Symptoms. Ann Intern Med. 2005;142:1003–1013. [PubMed] [Google Scholar]
- 3.Barnabei VM, Grady D, Stovall DW, et al. Menopausal Symptoms in Older Women and the Effects of Treatment With Hormone Therapy. Obstet Gynecol. 2002;100:1209–1218. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 4.Simon J, Klaiber E, Wiita B, Bowen AH-MY. Differential Effects of Estrogen-Androgen and Estrogen-Only Therapy on Vasomotor Symptoms, Gonadotropin Secretion, and Endogenous Androgen Bioavailability in Postmenopausal Women. Menopause (New York, NY. 1999;6:138–146. [PubMed] [Google Scholar]
- 5.Guthrie JR, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol. 1996;88:437–442. doi: 10.1016/0029-7844(96)00196-2. [DOI] [PubMed] [Google Scholar]
- 6.Matthews KA, Wing RR, Kuller LH, Meilahn ENPP. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154:2349–2355. [PubMed] [Google Scholar]
- 7.Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–1743. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 8.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89 Suppl 2:28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 9.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 10.Budoff M. Comparison of spiral and electron beam tomography in the evaluation of coronary calcification in asymptomatic persons. Int J Cardiol. 2002;82:299. doi: 10.1016/s0167-5273(00)00449-6. [DOI] [PubMed] [Google Scholar]
- 11.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 12.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Shaw LJ, Liu ST, et al. Long-Term Prognosis Associated With Coronary Calcification: Observations From a Registry of 25,253 Patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 14.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-Beam Tomography Coronary Artery Calcium and Cardiac Events: A 37-Month Follow-Up of 5635 Initially Asymptomatic Low- to Intermediate-Risk Adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 15.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 Clinical Expert Consensus Document on Coronary Artery Calcium Scoring By Computed Tomography in Global Cardiovascular Risk Assessment and in Evaluation of Patients With Chest Pain: A Report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Developed in Collaboration With the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Allison MA, Rossouw JE, et al. Estrogen Therapy and Coronary-Artery Calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 20.Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80:1429–1433. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 21.Fazio S, Linton MF. The inflamed plaque: cytokine production and cellular cholesterol balance in the vessel wall. Am J Cardiol. 2001;88:12E–15E. doi: 10.1016/s0002-9149(01)01717-9. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 23.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg. 1985;201:115–131. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostrom K. Insights into the mechanism of vascular calcification. Am J Cardiol. 2001;88:20E–22E. doi: 10.1016/s0002-9149(01)01718-0. [DOI] [PubMed] [Google Scholar]
- 25.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 26.Engelse MA, Neele JM, Bronckers AL, Pannekoek H, de Vries CJ. Vascular calcification: expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc Res. 2001;52:281–289. doi: 10.1016/s0008-6363(01)00375-3. [DOI] [PubMed] [Google Scholar]
- 27.Mody N, Tintut Y, Radcliff K, Demer LL. Vascular calcification and its relation to bone calcification: possible underlying mechanisms. J Nucl Cardiol. 2003;10:177–183. doi: 10.1067/mnc.2003.0012. [DOI] [PubMed] [Google Scholar]
- 28.Allison MA, Wright CM. Age and gender are the strongest clinical correlates of prevalent coronary calcification (R1) Int J Cardiol. 2005;98:325–330. doi: 10.1016/j.ijcard.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Bild DE, Detrano R, Peterson D, et al. Ethnic Differences in Coronary Calcification: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 30.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 31.Crandall CJ, Zheng Y, Crawford SL, et al. Presence of vasomotor symptoms is associated with lower bone mineral density: a longitudinal analysis. Menopause (New York, NY. 2009;16:239–246. doi: 10.1097/gme.0b013e3181857964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gast GC, Grobbee DE, Pop VJ, et al. Vasomotor symptoms are associated with a lower bone mineral density. Menopause (New York, NY. 2009;16:231–238. doi: 10.1097/gme.0b013e318185e25b. [DOI] [PubMed] [Google Scholar]
- 33.Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 35.von Muhlen DG, Soroko S, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms are not associated with reduced bone mass in postmenopausal women: the Rancho Bernardo Study. J Womens Health Gend Based Med. 2000;9:505–511. doi: 10.1089/15246090050073585. [DOI] [PubMed] [Google Scholar]
- 36.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Napoli N, Faccio R, Shrestha V, Bucchieri S, Rini GB, Armamento-Villareal R. Estrogen metabolism modulates bone density in men. Calcif Tissue Int. 2007;80:227–232. doi: 10.1007/s00223-007-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsay R. The Role of Estrogen in the Prevention of Osteoporosis. Endocrinology & Metabolism Clinics of North America. 1998;27:399–409. doi: 10.1016/s0889-8529(05)70012-8. [DOI] [PubMed] [Google Scholar]
- 39.The Women's Health Initiative Steering C. Effects of Conjugated Equine Estrogen in Postmenopausal Women With Hysterectomy: The Women's Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 40.Writing Group for the Women's Health Initiative I. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 42.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 43.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 44.Svartberg J, von Mühlen D, Kritz-Silverstein DEB-C. Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause (New York, NY. 2009;16:888–891. doi: 10.1097/gme.0b013e3181a4866b. [DOI] [PubMed] [Google Scholar]