Abstract
Ca2+ transfer from endoplasmic reticulum (ER) to mitochondria at contact sites between the organelles can induce mitochondrial dysfunction and programmed cell death after stress. The ER-localized chaperone glucose-regulated protein 78 kDa (GRP78/BiP) protects neurons against excitotoxicity and apoptosis. Here we show that overexpressing GRP78 protects astrocytes against ischemic injury, reduces net flux of Ca2+ from ER to mitochondria, increases Ca2+ uptake capacity in isolated mitochondria, reduces free radical production, and preserves respiratory activity and mitochondrial membrane potential after stress. We conclude that GRP78 influences ER-mitochondrial Ca2+ crosstalk to maintain mitochondrial function and protect astrocytes from ischemic injury.
Keywords: astrocytes, endoplasmic reticulum, 78 kDa glucose-regulated protein (GRP78/HSPA5/BiP), ischemia, mitochondria
1. Introduction
GRP78, also referred to as immunoglobulin heavy chain binding protein (BiP/HSPA5), is an important ER chaperone that binds hydrophobic stretches in newly synthesized polypeptides, while also playing a central role in signaling the unfolded protein response. Structurally, GRP78 is a member of the heat shock protein 70 (HSP70) family of chaperones. The presence of HSP70 homologues in organisms from bacteria to man, reflects the central roles it plays in protein homeostasis and cell survival. In mammals the HSP70 family includes members with different primary intracellular localization; GRP78 is mainly localized to ER, GRP75 to mitochondria and HSP72 to cytosol.
Several studies suggest that GRP78 plays a role in the regulation of cell death, including apoptotic Purkinje cell death in the cerebellum (Wang et al., 2010) and autophagy (Li et al., 2008), both relevant for brain cell loss following ischemia. Two reports show that prior induction of increased levels of GRP78 with a pharmacological inducer reduces neuronal loss in both forebrain (Oida et al., 2008a) and focal cerebral ischemia (Kudo et al., 2008), and translocation of GRP78 to mitochondria following ER stress has also been observed (Sun et al., 2006). This led us to postulate that GRP78 might influence calcium handling, a critical determinant of outcome following ischemic insults, by modulating the interaction between the ER and mitochondria. While these prior studies focused on GRP78 in neurons, our previous studies demonstrated that selective dysfunction of astrocytes occurs much earlier than delayed neuronal death (Ouyang et al., 2007) and overexpressing protective genes selectively in astrocytes can protect neighboring neurons (Xu et al., 2010). In this study we therefore used astrocytes as our target cells to investigate the protective effect of GRP78 as well as the mechanism involved.
Calcium plays a major role in intracellular signaling mechanisms after brain ischemia. The ER is a multifunctional organelle central to Ca2+ homeostasis, protein synthesis, protein trafficking and secretion, and the regulation of apoptosis. Mitochondria are the site of oxidative phosphorylation dependent ATP generation, integrate and transduce apoptotic signals, and also help regulate intracellular Ca2+. Recent structural and functional studies revealed zones of close contact between ER and mitochondria called MAM (mitochondria associated membranes) (Giorgi et al., 2009). MAM enables highly efficient transmission of Ca2+ from ER to mitochondria and molecular chaperones such as calnexin, calreticulin, ERp44, ERp57, GRP75 and the sigma-1 receptor coexist at the MAM (Hayashi et al., 2009). Signaling from the ER to mitochondria can be critical in the induction of mitochondrial dependent cell death pathways (Scorrano et al., 2003; Deniaud et al., 2007; Hetz, 2007; Hom et al., 2007). Post-conditioning protects cardiomyocytes from ischemia/reperfusion injury through inhibiting ER-mitochondria Ca2+ crosstalk (Dong et al., 2010). Despite some studies involving GRP78 in stress-induced apoptosis, the connection of this protein with the ER-mitochondria Ca2+ crosstalk during ischemic stress remains poorly understood. In this study we found that GRP78 overexpression protects primary astrocytes against ischemic injury in vitro and preserves mitochondrial function. GRP78 slows the increase of Ca2+ in mitochondria after stress and reduces free radical generation.
2. Materials and Methods
All the fluorescent dyes: TMRE, Hydroethidine (HEt), Mag-Fura-2-AM, Calcium Green-5N, Rohod-2-AM and Fluo-4-AM were from Molecular Probes, Inc. (Eugene, OR, USA). All the other commonly used chemicals were from Sigma (St Louis, MO, USA).
2.1. Astrocyte culture and GRP78 expression construct transfection
Primary mouse astrocyte cultures were prepared as previously described (Ouyang et al., 2006) in accordance with a protocol approved by the Stanford University animal care and use committee. The construction of the plasmid pcDNA3-His-GRP78 expressing hamster GRP78 was described (Zeng et al., 2004). Three day old astrocyte cultures were co-transfected with equimolar amounts of pcDNA3-GRP78 encoding wild type GRP78 and pAcGFP1-Mito (Clontech, CA, USA) to identify transfected cells using FuGeneHD (Roche, New Jersey, USA) according to the manufacturer’s instructions, at a ratio of 2µl FuGeneHD to 0.5µg DNA per well of a 24-well culture plate. Control transfections were done using pAcGFP1-Mito.
2.2. Injury paradigms and cell viability assays
Glucose deprivation (GD) and oxygen-glucose deprivation (OGD) were used as injury paradigms as described previously (Ouyang et al., 2006; Ouyang et al., 2007). Cell injury was quantified after GD or OGD by microscopic evaluation and cell counting of Hoechst 33342 (5 µM) and propidium iodide (PI, 5 µM) labeled cells. PI readily penetrates cells with compromised plasma membranes (dead cells) but does not cross intact plasma membranes. Hoechst is a cell-permeant nucleic acid stain that labels both live and dead nuclei.
2.3. Immunocytochemistry and immunoblotting
Fluorescence immunocytochemistry was performed on cell cultures in 24-well plates as described before (Ouyang et al., 2007). Briefly after washing and blocking buffer, the cells were incubated with anti-GRP78 (PA1-37806, Affinity BioReagents, Golden, CO, USA) or anti-AFT4 (ab50546, Abcam Inc., Cambridge, MA, USA) primary antibody in blocking buffer overnight at 4°C, and then washed and incubated with FITC- or rhodamine-conjugated secondary antibody at 1:100 dilutions. Fluorescence was visualized with an epifluorescence microscope (Zeiss Axiovert 200M, Carl Zeiss, Göttingen, Germany), and images were obtained on a Macintosh computer using Openlab software from Improvision Inc (Lexington, MA, USA).
Immunoblotting was performed as previously described (Ouyang et al., 2007). Briefly equal amounts (50 µg) of protein were loaded and separated on a polyacrylamide gel (BIO-RAD, Hercules, CA), and electrotransferred to Immobilon polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA). Membranes were blocked and incubated overnight with primary antibody against GRP-78 (PA1-37806, Affinity BioReagents, Golden, CO, USA), washed and incubated with 1:2000 anti-rabbit antibody. Immunoreactive bands were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ) according to the manufacturer’s protocol.
2.4. Mitochondrial, ER, and cytosolic calcium measurements
To detect changes in mitochondrial calcium astrocytes were incubated with 5 µM Rhod-2-acetyl ester (Rhod-2-AM), an indicator of mitochondrial calcium, for 60 min at 37°C. Before making fluorescence measurements, cells were washed in indicator-free medium to remove any dye that is nonspecifically associated with the cell surface, and then incubated for a further 30 minutes to allow complete de-esterification of AM esters. Prolonged incubation (18–24 hrs) after washing eliminates cytosolic staining produced by Rhod-2 AM, where as mitochondrial staining is retained. Changes in fluorescence related to mitochondria were quantified by selecting mitochondria-rich perinuclear regions in each cell, and all subsequent fluorescence measurements were normalized to the baseline fluorescence (F0) for the same cell at the start of the experiment.
ER Ca2+ measurements in astrocytes were performed according to a procedure previously described for other cells with some modification (Tu et al., 2006). Briefly, cells were loaded with 2 µM low affinity Ca2+ imaging dye Mag-Fura-2-AM in buffer containing 120 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 2 mM CaCl2, 15 mM glucose, and 20 mM HEPES, pH 7.3 for 45 min at 37°C. The plasma membranes of astrocytes loaded with Mag-Fura-2 were selectively permeabilized by application of 10 µM Digitonin in intracellular buffer (125 mM KCl, 25 mM NaCl, 10 mM HEPES, 0.1 mM MgCl2, pH 7.3) containing 170 nM free Ca2+ (clamped by 5 mM EGTA) and 3 mM ATP. The Mag-Fura-2 signals were collected as 340/380 ratios for the duration of the experiment at RT.
Cytosol calcium was measured using 2 µM Fluo-4-AM according to manufacturer’s instructions.
2.5. Measurement of mitochondrial Ca2+ uptake capacity
Extramitochondrial free Ca2+ was measured according to the methods described by Murphy et al. (Murphy et al., 1996). Mitochondria were isolated by Percoll density gradient centrifugation as described before (Ouyang et al., 1997). Isolated mitochondria (0.25 mg·ml−1) were resuspended in 2 ml of buffer containing 125 mM KCl, 2 mM K2HPO4, 1 mM MgCl2, 1 mM EGTA, 20 mM Tris (pH 7.2 at room temperature), 5 mM glutamate, and 5 mM malate. Free Ca2+ was monitored using the hexapotassium salt of Calcium Green-5N (0.1 µM). Fluorescence was recorded continuously in a stirred cuvette at RT using a Perkin–Elmer LS-50B fluorescence spectrometer with excitation and emission wavelengths of 506 and 532 nm, respectively. Ca2+ uptake was prevented by the addition of 5 µM rotenone plus 0.5µM ruthenium red (RR). The Y axis of the curve is Ca2+ per milligram of protein. Quantitation of partial uptake of a pulse of Ca2+ was done by determining the quantity of each pulse that the mitochondria were unable to sequester. The maximal quantity of Ca2+ that can be sequestered by mitochondria can be measured by monitoring the disappearance of extramitochondrial free Ca2+ from media following the addition of quantified pulses of CaCl2.
2.6. Mitochondrial membrane potential and reactive oxygen species (ROS) determination
Live cell imaging studies were performed as previously described (Ouyang et al., 2006). Briefly, for assessment of changes in mitochondrial membrane potential, astrocytes were incubated for 30 min with tetramethylrhodamine ethyl ester (TMRE) (50 nM) at 37°C, and the same concentration of TMRE was maintained in all bathing solutions throughout the experiments. Cells were excited at 535 nm, and fluorescence emission was monitored at 590 nm at RT.
Oxygen radical production was monitored using hydroethidine (HEt). Cultures were loaded with 5 µM HEt for 30 min at 37°C, and the same concentration of HEt was maintained in the bath throughout each experiment. Cells were excited at 495 nm, and fluorescence emission monitored at 530 nm at RT. Fluorescence measurements for each cell (Ft) were normalized to the initial fluorescence intensity (F0) for that cell.
2.7 Measurements of mitochondrial complex activity
Activity assay of rotenone-sensitive NADH-decylubiquinone oxidoreductase (complex I) was performed at 340 nm using the acceptor coenzyme Q0 and NADH as a donor (Pandey et al., 2008). The reaction was monitored spectrophotometrically using a microplate reader at 340 nm for 3 min at 37°C in a solution containing 40–50 µg of the submitochondrial particles, 35 mmol/L potassium phosphate buffer, pH 7.4, 2.65 mmol/L NaN3, 1 mmol/L EDTA, 5 mmol/L MgCl2, 200 µmol/L NADH, and 100 µmol/L coenzyme Q0.
The activity of cytochrome c oxidase (complex IV) was measured using a Cytochrome c Oxidase Assay kit (CYTOX-OX1, Sigma), using reduced cytochrome c as the donor. The oxidation of cytochrome c was monitored as the decrease in absorbance at 550 nm using a microplate reader and the initial rate of cytochrome c reduction was used for the calculation of activity. The mitochondrial complex-I and IV activities were normalized by dividing them by protein concentration and expressed as a ratio to control.
2.8. Statistics
All data reported represent at least 3 independent experiments for n=3–6 cultures in each experiment. Data reported are means ± SD. Statistical difference was determined using ANOVA followed by Scheffé test with P < 0.05 considered significant, using StatView software (SAS Institute, Cary, NC, U.S.A.).
3. Results
3.1. Overexpression of GRP78 in primary cultured astrocytes
We co-transfected astrocytes with an expression plasmid for hamster GRP78 and the marker plasmid encoding mitochondrially localized eGFP, pAcGFP1-Mito (Fig. 1). We observed about 70% transfection efficiency by eGFP fluorescence, and confirmed that these represent double transfected cells by immunostaining for GRP78. Under fluorescence microscopy GRP78 (red) demonstrated perinuclear expression with higher fluorescence intensity in eGFP (green) positive (double transfected) cells (Fig. 1a). Increased levels of GRP78 protein expression after transfection were confirmed by Western blotting using antibody against GRP78 (Fig. 1b). To check whether transfection induced ER stress we assessed levels of activating transcription factor 4 (ATF4), a marker for the ER stress response. In unstressed transfected astrocytes, ATF4 protein levels are the same in control and GRP78 transfected astrocytes (Fig. 1c) indicating the absence of the ER stress response. This is consistent with our previous observation that overexpression of GRP78 did not cause ER stress as indicated by lack of induction of CHOP (Zhang et al.).
Fig. 1.
Overexpression of GRP78 in primary cultured astrocytes after co-transfection of GRP78 and eGFP-Mito. eGFP shows the typical mitochondrial distribution (green in a). The great majority of eGFP positive cells are also GRP78 over-expressing ones by immunostaining using antibody against GRP78 (red in a). GRP78 overexpression is confirmed by immunoblotting (b). ATF4 (red) does not change after GRP78 transfection (c). Ctrl: control. Transfected: GRP78 transfected * P < 0.05 statistically different from control group. Scale bars in (a) and (c), 25 µm.
3.2. Overexpressed GRP78 protects primary cultured astrocytes against ischemic injury
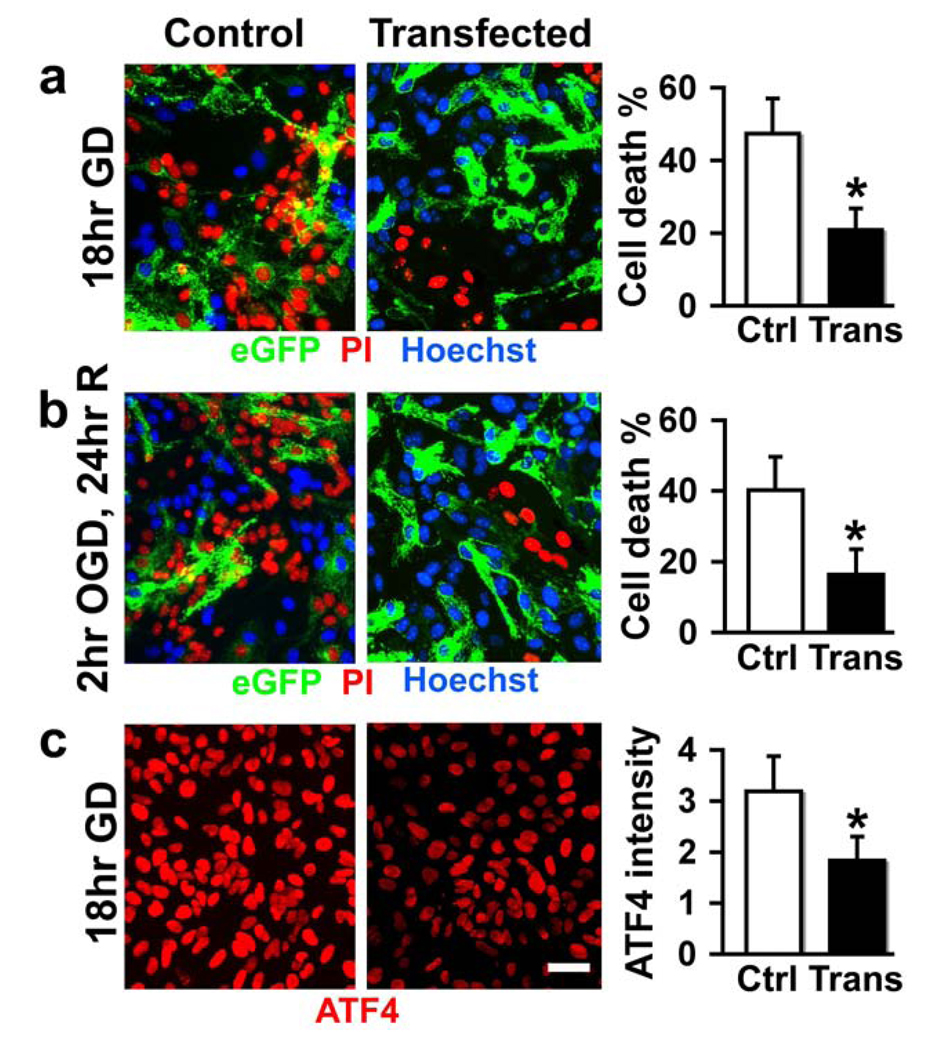
Astrocyte cultures were subjected to 18 hr GD and cell damage was quantified by cell counting after staining with propidium iodide (red for dead cells) and Hoechst dye (blue for intact cells) (Fig. 2a). To better approximate ischemia in vitro, cultures were subjected to 2 hr OGD followed by 24 hr recovery (Fig. 2b). There were fewer red nuclei in GRP78 overexpressing cultures compared to control groups under both stress conditions. It is interesting to notice that in the GRP78 overexpressing cultures (Fig. 2a, b), the green cells (transfected) nearly always have blue nuclei (representing intact cells) and only rarely stain with PI indicating damage. Bar graphs show the protective effect of overexpressing GRP78 against cell death in astrocytes induced by GD or OGD (Fig. 2a, b). Astrocyte cultures subjected to 18 hr GD showed about 47% cell death in controls transfected only with pAcGFP-1mito. Injury was reduced to 21% in GRP78 overexpressing cultures. Similar protection was observed against OGD (Fig. 2b).
Fig. 2.
GRP78 overexpression protects primary cultured astrocytes against ischemic injury in vitro. Astrocyte cultures were subjected to 18 hr GD (a) or 2 hr OGD followed by 24 hr recovery (b). Induction of ATF4 was reduced in cells overexpressing GRP78 after GD (c). Bar graphs show the significant protective effect of overexpressing GRP78 against cell damage in astrocytes under both stress conditions (a and b). * P < 0.05 statistically different from control (Ctrl) group. Trans: GRP78 transfected. Scale bars, 25 µm.
Cerebral ischemia induces an ER stress response and both GRP78 and ATF4 are induced in response to ER stress (Oida et al., 2008b). While control unstressed cultures showed no change in levels of ATF4 (Fig. 1c), GD causes ER stress as shown by induction of ATF4, but this induction was reduced in cells overexpressing GRP78 prior to the stress (Fig. 2c).
3.3. Effect of overexpressing GRP78 on mitochondrial free calcium during GD
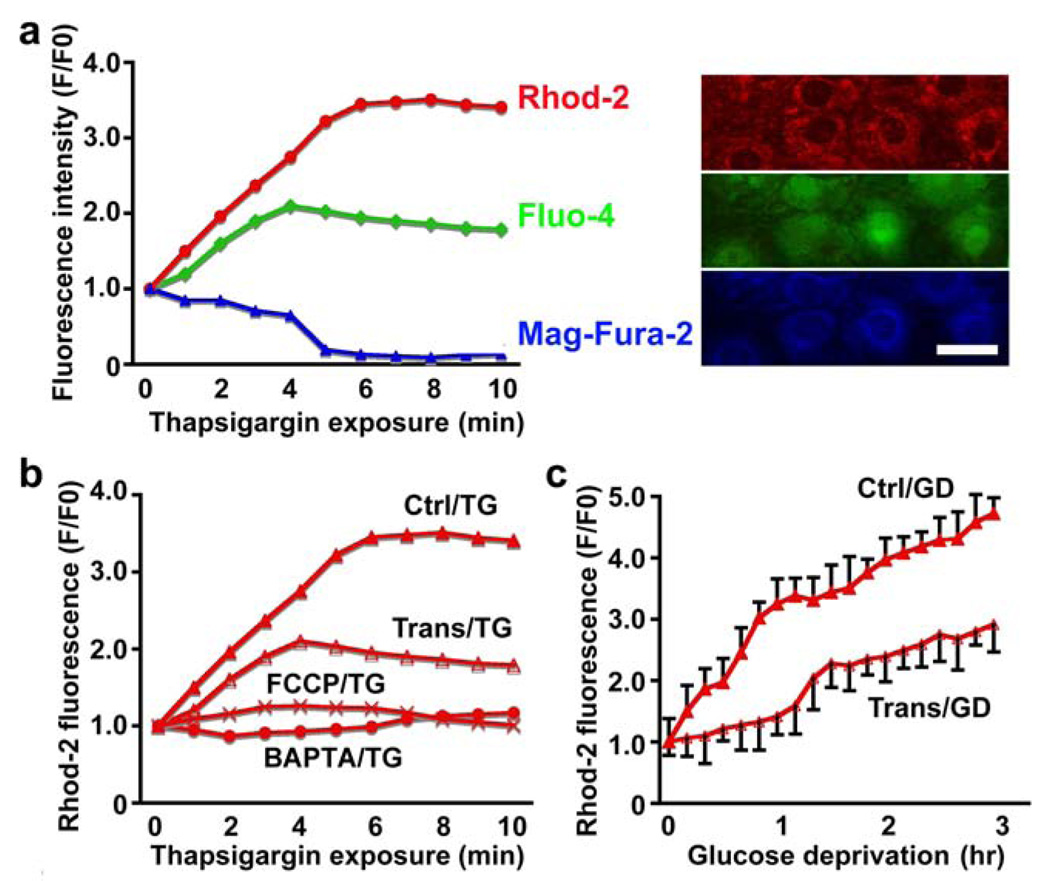
To assess calcium in mitochondria, cytosol, and ER, fluorescent dyes Rhod-2 (red), Fluo-4 (green) and Mag-Fura-2 (blue) were used respectively (Fig. 3a, right panel). To assess the importance of calcium release from intracellular stores we applied thapsigargin (TG, 1 µM) in the absence of extracellular calcium. As expected this caused mitochondrial and cytosolic Ca2+ increases and a decrease in ER Ca2+ (Fig. 3a, left panel). The increase in mitochondrial calcium provoked by TG could be blocked by depolarizing mitochondria with FCCP (1 µM) or chelating intracellular calcium with BAPTA-AM (2 µM). Overexpression of GRP78 also markedly decreased mitochondrial Ca2+ increase upon TG treatment (Fig. 3b). GD performed in normal extracellular medium containing calcium but lacking glucose also increased the mitochondrial Ca2+ level, again with less change observed when GRP78 was overexpressed (Fig. 3c).
Fig. 3.
Effect of overexpressing GRP78 on mitochondrial free calcium during GD. Calcium from mitochondria, cytosolic and ER vesicles were labeled with Rhod-2 (red), Fluo-4 (green) and Mag-Fura-2 (blue) respectively (a, right panel). Scale bar, 25 µm. When applying thapsigargin (TG, 1 µM) mitochondrial and cytosolic Ca2+ increased and ER Ca2+ decreased (a, left). GRP78, FCCP (1 µM) and BAPTA-AM (2 µM) markedly decreased mitochondrial Ca2+ uptake upon TG treatment (b). Glucose deprivation (GD) slowly increased the mitochondrial Ca2+ level, with less change in GRP78 group than control (c). Ctrl: control. Trans: GRP78 transfected.
3.4. Effect of overexpressing GRP78 on isolated mitochondrial Ca2+ uptake capacity after GD
Calcium green-5N fluorescence indicates the extramitochondrial calcium concentration which increases transiently with each pulse of CaCl2 (40 nmol/mg protein) as indicated by a rapid rise in dye fluorescence, followed by mitochondrial sequestration of Ca2+ from the medium causing a decrease. The Ca2+ uptake capacity of mitochondria isolated from unstressed astrocytes is shown in Fig. 4a. Calcium pulses were repeated every 3 min until there was no further evidence of Ca2+ uptake. The maximum calcium uptake was similar whether the mitochondria were isolated from cells overexpressing GRP78 or control transfected. We then isolated mitochondria from cells subjected to GD for 3 hr, which does not kill any of the cells. It is evident in Fig. 4b that mitochondria isolated from GRP78 overexpressing astrocytes following stress sequester a greater number of pulses and total amount of Ca2+ uptake is greater than those from control transfected cells subjected to the same duration of GD. It is also clear that mitochondria are impaired as total cell uptake is reduced compared to unstressed cells (Fig. 4b, bar graph compare to Fig 4a bar graph).
Fig. 4.
Effect of overexpressing GRP78 on Ca2+ uptake by isolated mitochondria from astrocytes after GD. Pulses of CaCl2 (40 nmol of Ca2+ /mg of protein) were added to the medium (arrows) every 3 min until there was no evidence of further uptake. (a) A typical Calcium green-5N fluorescence tracing measuring the extra mitochondrial Ca2+ of mitochondria isolated from control astrocyte. Insert: maximal capacity for Ca2+ accumulation in control and mitochondria isolated from GRP78 transfected cells with 40 nmol (10 µM final conc.) Ca2+ added. Maximal Ca2+ sequestration by mitochondria from control and GRP78 astrocytes after 3 hr GD (b) is shown in the insert. * P < 0.05 statistically different from control (Ctrl) group.
3.5. Overexpression of GRP78 preserves mitochondrial function under in vitro ischemic stress
With stress, calcium loading of mitochondria can increase ROS generation and impair electron transport and oxidative phosphorylation. Overloading mitochondria with Ca2+ will trigger opening of the inner membrane permeability transition pore resulting in the loss of mitochondrial membrane potential, release of proapoptotic factors into cytosol and initiation of the cell death cascade.
3.5.1. Free radicals (ROS)
Astrocyte cultures were co-transfected with pcDNA3-GRP78 and pAcGFP1-Mito and loaded with HEt (red) to assess production of ROS. Transfected cells were identified by green fluorescence and the same field is shown before and after 1 hr GD. As shown in Fig. 5a (left panel), three of 4 transfected green cells show red HEt fluorescence that remains relatively dim (arrows), indicating less ROS after 1 hr GD than the non-transfected cells. The time course curve shows that astrocytes overexpressing GRP78 and subjected to GD show a smaller increase in HEt fluorescence than control transfected cells with GD (Fig. 5a, graph right panel).
Fig. 5.
GRP78 overexpression preserves mitochondrial function under in vitro ischemic stress. Transfected cells fluoresce green. a. TMRE (red) for assessing mitochondrial membrane potential. b. Mitochondrial electron respiration chain complex I and IV activity. c. HEt (red) for assessing free radical production. GD means glucose deprivation. Scale bars in (a) and (c), 25 µm. Values are expressed as mean±SD. * P<0.05 statistically different compared with 0 hr under same condition; $P<0.05 statistically different compared with the vector control at the same time by ANOVA followed by Scheffe’s test.
3.5.2. Mitochondrial respiratory complex activity
Because the activities of complexes I and/or IV of the electron transport chain were reduced in our in vitro OGD (Xu et al., 2010) and in in vivo focal ischemia (Xu et al., 2009), we tested whether overexpressing GRP78 could protect complex activity in astrocytes subjected to GD. After 3 hr GD, the activity of complex I decreased significantly (Fig. 5b) while by 5 hr GD, both complex I and IV activities decreased significantly (Fig. 5b, c). Overexpressing GRP78 preserved complex activities at both time points.
3.5.3. Mitochondrial membrane potential
Astrocytes were co-transfected as above then loaded with TMRE (red) to assess mitochondrial membrane potential. Transfected cells fluoresce green and preserve their mitochondrial membrane potential after 1 hr GD (arrows) better than surrounding untransfected cells (Fig. 5d, left panel). Normal mitochondrial membrane potential is indicated by red fluorescence, with loss of potential shown by decreased fluorescence. The same field is shown before and after 1 hr GD. In examining the time-course of change in mitochondrial membrane potential we observe a delay in the time at which potential decreases when GRP78 was overexpressed (Fig. 5d, right panel). Therefore mitochondrial membrane potential is better preserved with GRP78 overexpression.
4. Discussion
Although the many aspects of GRP78 function relevant to neural death are not fully established, levels of GRP78 are markedly increased in response to cerebral ischemia (Wang et al., 1993). ER stress and GRP78 is involved in the CA1-selective neuronal cell death observed in a gerbil transient forebrain ischemia model (Oida et al., 2008a) and also in the periphery of the middle cerebral artery (MCA) territory after MCA occlusion in mice (Morimoto et al., 2007). Pretreatment of neuroblastoma cells with BIX, a GRP78 inducer, reduced cell death and intracerebroventricular pretreatment with BIX reduced the area of infarction due to focal cerebral ischemia in mice (Kudo et al., 2008) and prevented hippocampal CA1 neurons from delayed neuronal death due to transient forebrain ischemia in gerbil (Oida et al., 2008a). We transfected astrocytes with a plasmid encoding GRP78 with high transfection efficiency and found that GRP78 protects primary astrocyte cultures from ischemia-like injury in vitro.
Little information is available concerning the mechanisms of GRP78 protection against ischemic injury. Earlier studies with GRP78 antisense led to the suggestion that GRP78 protects cultured hippocampal neurons against glutamate, Fe2+ and Aβ insults by a mechanism involving suppression of oxidative stress and stabilization of calcium homeostasis (Yu et al., 1999), since pretreatment with GRP78 antisense resulted in increased levels of reactive oxygen species and intracellular calcium with these insults. They further observed that a blocker of ER calcium release and an antioxidant protected neurons against the death-enhancing action of GRP78 antisense. Our results are consistent with these observations and extend them. We used specific calcium indicators for mitochondria, cytosol, and ER simultaneously in intact astrocytes and also assessed calcium uptake into isolated astrocyte mitochondria using Calcium green-5N. We found that overexpression of GRP78 not only markedly reduced mitochondrial Ca2+ increase in cells but also increased calcium uptake capacity of isolated mitochondria after stress. We further demonstrated that overexpressing GRP78 preserves mitochondrial membrane potential and respiratory activity after stress.
The mechanism of GRP78 regulation of ER-mitochondria Ca2+ crosstalk and preservation of mitochondrial function is not known. The highly efficient Ca2+ transmission structure MAM (Giorgi et al., 2009) coexists with many chaperones (Hayashi et al., 2009). Future studies should test the interaction of GRP78 with MAM. Another possibility for the influence of GRP78 on ER-mitochondria Ca2+ crosstalk is via GRP78 translocating to mitochondria after stress and locally influencing mitochondrial Ca2+ or other mitochondrial functions that affect their ability to sequester Ca2+. It has been shown that GRP78 can be found in mitochondria, on cell surfaces, as well as in ER in a number of cells (Misra et al., 2005; Sun et al., 2006), suggesting that it plays different roles in distinct cell locations. GRP78 was observed to translocate to mitochondria, mainly located in the intermembrane space, inner membrane and matrix, but was not associated with the outer mitochondrial membrane under the unfolded protein response (Sun et al., 2006). In contrast with the observation in the study of Sun et al (Sun et al., 2006), the majority of GRP78 was located in the outer mitochondrial membrane in H460 cells after similar stress (Shu et al., 2008). These observations indicate that GRP78 is found in different intracellular locations depending on cell type and type of stress. Further work is warranted on the role of mitochondrial localization and function of GRP78 in astrocytes after ischemia- like stress. Interestingly, GRP78 has recently been implicated in regulation of mitochondria energy balance and modulation of PCGa and GRP75 expression. In a mouse model, GRP78 haploinsufficiency ameliorates high-fat diet induced obesity and type 2 diabetes, as a consequence of activated adaptive unfolded protein response and increased insulin sensitivity in white adipose tissue (Ye et al.).
In summary we demonstrate that overexpressing the ER-resident chaperone GRP78 protect astrocytes from ischemic injury, reduces the net flux of Ca2+ from ER to mitochondria, increases Ca2+ uptake capacity in mitochondria isolated from stressed cells, reduces free radical production, and preserves mitochondrial function during stress. These findings suggest that increasing levels of GRP78 could be used to protect mitochondrial function and promote astrocyte survival of acute ischemia, as well as in other neurodegenerative settings that involve mitochondrial and ER dysfunction.
Acknowledgements
This work was supported in part by grant NIH GM 49831 to RGG and NIH CA27607 and DK 079999 to ASL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Deniaud A, Sharaf El Dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2007 doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Dong S, Teng Z, Lu FH, Zhao YJ, Li H, Ren H, Chen H, Pan ZW, Lv YJ, Yang BF, Tian Y, Xu CQ, Zhang WH. Post-conditioning protects cardiomyocytes from apoptosis via PKC(epsilon)-interacting with calcium-sensing receptors to inhibit endo(sarco)plasmic reticulum-mitochondria crosstalk. Mol Cell Biochem. 2010;341:195–206. doi: 10.1007/s11010-010-0450-5. [DOI] [PubMed] [Google Scholar]
- Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz CA. ER Stress Signaling and the BCL-2 Family of Proteins: From Adaptation to Irreversible Cellular Damage. Antioxid Redox Signal. 2007 doi: 10.1089/ars.2007.1793. [DOI] [PubMed] [Google Scholar]
- Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y. Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol. 2007;212:498–508. doi: 10.1002/jcp.21051. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Sharma T, Pizzo SV. Ligation of cell surface-associated glucose-regulated protein 78 by receptor-recognized forms of alpha 2-macroglobulin: activation of p21-activated protein kinase-2-dependent signaling in murine peritoneal macrophages. J Immunol. 2005;175:2525–2533. doi: 10.4049/jimmunol.175.4.2525. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Oida Y, Shimazawa M, Miura M, Kudo T, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience. 2007;147:957–967. doi: 10.1016/j.neuroscience.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc.Natl.Acad.Sci.U.S.A. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida Y, Izuta H, Oyagi A, Shimazawa M, Kudo T, Imaizumi K, Hara H. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008a;1208:217–224. doi: 10.1016/j.brainres.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Oida Y, Shimazawa M, Imaizumi K, Hara H. Involvement of endoplasmic reticulum stress in the neuronal death induced by transient forebrain ischemia in gerbil. Neuroscience. 2008b;151:111–119. doi: 10.1016/j.neuroscience.2007.10.047. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Kuroda S, Kristian T, Siesjo BK. Release of mitochondrial aspartate aminotransferase (mAST) following transient focal cerebral ischemia suggests the opening of a mitochondrial permeability transition pore. Neuroscience Research Communications. 1997;20:167–173. [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–186. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M, Varghese M, Sindhu KM, Sreetama S, Navneet AK, Mohanakumar KP, Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington's disease. J Neurochem. 2008;104:420–434. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shu CW, Sun FC, Cho JH, Lin CC, Liu PF, Chen PY, Chang MD, Fu HW, Lai YK. GRP78 and Raf-1 cooperatively confer resistance to endoplasmic reticulum stress-induced apoptosis. J Cell Physiol. 2008;215:627–635. doi: 10.1002/jcp.21340. [DOI] [PubMed] [Google Scholar]
- Sun FC, Wei S, Li CW, Chang YS, Chao CC, Lai YK. Localization of GRP78 to mitochondria under the unfolded protein response. Biochem J. 2006;396:31–39. doi: 10.1042/BJ20051916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ye R, Barron E, Baumeister P, Mao C, Luo S, Fu Y, Luo B, Dubeau L, Hinton DR, Lee AS. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010;17:488–498. doi: 10.1038/cdd.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Longo FM, Chen J, Butman M, Graham SH, Haglid KG, Sharp FR. Induction of glucose regulated protein (grp78) and inducible heat shock protein (hsp70) mRNAs in rat brain after kainic acid seizures and focal ischemia. Neurochem Int. 1993;23:575–582. doi: 10.1016/0197-0186(93)90106-f. [DOI] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Zeng L, Lu M, Mori K, Luo S, Lee AS, Zhu Y, Shyy JY. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004;23:950–958. doi: 10.1038/sj.emboj.7600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]