Abstract
Propylene glycol and glycol ether (PGE) in indoor air have recently been associated with asthma and allergies as well as sensitization in children. In this follow-up report, sources of the PGEs in indoor air were investigated in 390 homes of pre-school age children in Sweden. Professional building inspectors examined each home for water damages, mold odour, building’s structural characteristics, indoor temperature, absolute humidity and air exchange rate. They also collected air and dust samples. The samples were analyzed for four groups of volatile organic compounds (VOCs) and semi-VOCs (SVOCs), including summed concentrations of 16 PGEs, 8 terpene hydrocarbons, 2 Texanols, and the phthalates n-butyl benzyl phthalate (BBzP), and di(2-ethylhexyl)phthalate (DEHP). Home cleaning with water and mop ≥ once/month, repainting ≥ one room prior to or following the child’s birth, and “newest” surface material in the child’s bedroom explained largest portion of total variability in PGE concentrations. High excess indoor humidity (g/m3) additionally contributed to a sustained PGE levels in indoor air far beyond several months following the paint application. No behavioral or building structural factors, except for water-based cleaning, predicted an elevated terpene level in air. No significant predictor of Texanols emerged from our analysis. Overall disparate sources and low correlations among the PGEs, terpenes, Texanols, and the phthalates further confirm the lack of confounding in the analysis reporting the associations of the PGE and the diagnoses of asthma, rhinitis, and eczema, respectively.
Keywords: indoor, volatile organic compound, glycol ether, asthma, solvent, children
1. Introduction
Inhalation exposure to evaporated components of the consumer products, building structural materials as well as their secondary reactive products at home has been associated with increased risks of asthma-like symptoms, asthma diagnosis, as well as other allergic symptoms in both adults [1] and children [2,3]. In particular, occupational and non-occupational exposure to volatilized components of water-based paint, water-based cleaning products, glass cleaning, oven cleaning, dish-washing, and the use of chlorine bleach significantly increases the risks of self-reported asthma, clinically diagnosed asthma, and other acute respiratory symptoms [1,4–13]. However, specific compounds underlying these illnesses due to such consumer product uses have remained overall unclear [13,14]. In addition, the mechanisms by which volatile organic compounds (VOCs) or semi-VOCs (SVOCs) act as sensitizers or respiratory irritants are not obvious [13].
In our recent case-control investigation of the children between the ages 3 and 8, those within the top 25 % for the level of the summed 16 PGEs in indoor air had a 130 % higher likelihood of multiple allergic symptoms (i.e., parental report and clinically validated asthma, rhinitis and eczema) (95% CI, 20–370%), a 100% higher likelihood of asthma diagnosis (95% CI, −10–340%), a 320% higher likelihood of rhinitis diagnosis (95% CI, 70–930%), a 150% higher likelihood of eczema diagnosis (95% CI, 10–430%), and a 120% higher likelihood of being IgE-sensitized among the cases only (95% CI, −10–480%), accounting for gender, secondhand smoke, allergies in both parents, wet cleaning with chemical agents, residence construction period, limonene, cat and dog allergens, butyl benzyl phthalate (BBzP), and di(2-ethylhexyl)phthalate (DEHP) [15].
Water-based cleaning products either applied with an applicator (e.g., mop) or sprayed as solution can release a number of glycol ethers, terpenes, and terpene alcohols to air [16]. Major volatile organic compounds emitted from the water-based paint include propylene glycol, other glycols, glycol ethers, and Texanols (also known as TXIB™ or 2,2,4-trimethyl 1,3-pentanediol monoisobutyrate) [17]. The water-based paint also emits smaller amounts of isobutanol, toluene, xylene, trimethylbenzenes, n-nonane, n-decane, and n-undecane [17]. Due to their effectiveness as solvents, propylene glycol and glycol ethers (PGEs) are also used in pharmaceuticals, pesticides, cosmetics, varnishes, inks, pigments, adhesives, detergents, agrochemicals, and processed foods [16,18]. For other purposes, they are used in polyvinyl chloride (PVC) pipes [19], hydraulic and brake fluids, de-icing fluids for aircrafts, and artificial theatrical smoke [20].
It has been known for more than three decades that inhalation of several vaporized PGE compounds, including α- and β- isomers of propylene glycol monomethyl ether (PGME), induces airway and ocular irritation of humans [17,21,22] and rats [23]. Several experimental investigations of healthy adult volunteers have demonstrated that an administration of propylene glycol, and a mixed vapour of glycol ethers and Texanol, could respectively induce acute eye, nose, throat irritation and dyspnea [7,17,22,24]. In a prospective cohort study of house-painters, an exposure to water-based paint led to a significantly higher incidence of chest tightness/wheezing, airway irritation, bronchial hyper-responsiveness, and shortness of breath [6]. Within a non-occupational setting, greater likelihood of asthma symptoms have been observed in adults exposed to a newly painted wood or kitchen surfaces [25], and/or synthetic material-based furniture [26,27].
However, one of the considerable challenges regarding the assessment of human health risk of PGEs stems from the correlated nature of the VOC compounds emitted from multiple sources within the indoor environment. Furthermore, the role of other indoor environmental conditions (e.g., humidity, ventilation, or temperature) on the emission and/or retention of these PGEs are poorly understood [28]. Human exposure scenarios at home are likely to be different from the emission characteristics within controlled experimental setting. For example, glycol ethers emitted from completely assembled flooring materials within a completely built structure was > 100-fold higher than the levels emitted by the individual components in a laboratory chamber [29].
Here, we examined the sources and interactions of four VOC groups commonly found in water-based paints and/or cleaning products in homes, including PGEs (16 compounds), terpenes (8 compounds), Texanols (2 compounds), and phthalates (2 compounds). The PGEs, Texanols [15], and phthalates [2,30] have recently been identified as potential contributors of asthma and allergies. In addition, terpene hydrocarbons and their alcohols are investigated here because they represent common scenting agents in cleaning products [31,32]. Their reaction with ozone could induce upper airway and eye irritation [31,32]. Specific goals were to: a) examine the human activities and sources that predict the PGE levels in air; and b) investigate correlation of home indoor PGEs with other compounds emitted from cleaning and wood based materials (e.g., terpenes), home structural material and consumer products (e.g., phthalates), and paints (e.g., Texanols).
2. Experimental Section
2.1. Exposure Assessment
Three hundred ninety homes the children participating in a nested case-control investigation of asthma and allergies were inspection and air and dust samples were collected [15]. Professional inspectors visually examined the homes for the water damages, the presence of mold odour, building’s structural characteristics, indoor temperature, relative humidity, absolute humidity difference between indoor and outdoor air (termed excess indoor humidity from here on), and air exchange rate [33]. The building inspectors also examined the housing demographics (i.e., type, age, quality, and ventilation system), urbaneness of the neighborhood, indoor combustion sources, and perception of air quality. Inspectors recorded wall surface materials (i.e., wall paper, plastic covered wall-paper, painted glass fiber, wooden panel, tile, wooden fabric, or other) as well as the flooring material (i.e., linoleum, PVC carpet, parquet, laminate, soft carpet, cork and plastic carpet, stone, or other). The type of home foundation, quality of home basement, crawl space, outer wall material, and building façade material were also examined. Excess indoor humidity was measured as a difference between indoor and outdoor water vapor content (g/m3). The water vapor content was higher indoors relative to the outdoors in 342 of 343 homes in which the measurement was taken. Home inspectors were unaware of the health outcome status of the child in the homes they were inspecting. Ventilation rates were measured using a passive perfluorocarbon tracer (PFT) gas method, as described in NT VVS 118 [34,35]. The PFT method measured multiple (≤ 12) sources depending on the size of the home with multiple (≤5) collectors. Separate mean ventilation rates were calculated for the entire house/week as well as the child’s bedroom/week.
Samples of dust from 390 homes were collected from moldings and shelves in the children’s bedroom with a hand-held vacuum. The dust was collected onto 90-mm membrane filters made of pure cellulose in holders made of styrene-acrylonitrile polymer mounted on a sampler made of polypropylene (VacuuMark disposable nozzle; Petersen Bach, Bjerringbro, Denmark) connected to a vacuum cleaner [36]. Of the 390 homes, 362 dust samples met the quality assurance criteria [30]. To further ensure reliability, only the dust samples with a measurable net increase in weight (≥25 mg) are included in the present study [30].
2.1.1. Air Sampling
Single VOC sample was taken by placing the air sampler 1 meter above the floor in the room. A SKC pocket pump (SKC Pocket Pump 210–1002, SKC Blandford, Dorset, UK) drew in air at 80 mL/min for 60 to 90 minutes (5 to 8 liters) through a Perkin Elmer adsorption tubes (glass, 300 mg Tenax TA). The tubes were sealed with PTFE stoppers, wrapped in alumina foil and kept at −20 °C until they were shipped to NILU, Norway for analysis within two weeks of collection. Prior to use the Tenax tubes were cleaned using thermo-desorption at 275 °C for 15 minutes for three consecutive cleaning cycles. Use of adsorbent, preparation of adsorbent tubes, sampling equipment, sampling flow and safe-sampling volumes, analytical methods and analytical equipment followed international standards on ambient air quality DIN EN 14662-1 (DIN ISO 5725-2 and 3). There is no standard procedure for VOC sampling and analysis for indoor air, but the chosen method is widely accepted for its reliability [37].
Tenax TA for indoor air analysis is well established with inter-laboratory differences reported to be <10% for benzene analysis (DIN EN 14662-1 annex H2). The temporal stability of compounds trapped on Tenax TA and the formation of artifacts from degradation of the adsorbent Tenax TA is widely discussed in literature [31,38,39]—the main degradation products are known as Benzaldehyde, Acetophenone and Benzoic acid and to a minor extent Benzene, Toluene and Xylenes. Other artifacts include aldehydes (Hexanal, Heptanal, Octanal, Nonanal, Decanal), created due to the reactions of ozone from the sample air and fatty acids. All those compounds are also common gases in indoor air. The blank values of those compounds are small compared to the amount of those gases in indoor air with a sample size of more than 5 liter. Due to the chemical structure of Tenax TA (2,6-diphenylene based polymer), formation of glycol ethers as artifact from Tenax TA is very unlikely and has never been reported.
2.1.2. Laboratory Analysis
The VOC samples were analyzed using an automated thermo desorption unit (Perkin Elmer ATD 400, Perkin Elmer Inc., Waltham, MA, USA) followed by GC-MS [31,38,39]. The samples were desorbed at 250 °C—refocused on a Tenax TA cold trap held at minus 30 °C and transferred to the GC-MS at 225 °C. A Hewlett Packard G 1800 A GC-MS was used as detector maintained at 250 °C. The separation column (DB-1701, 32 m length, 1 ≤m film, 0.32 mm in diameter) was programmed from 40 °C to 250 °C. The mass range of the detector was from mass 33 to mass 350. Further details on detection, calibration and quality assurance assessment are described in Online Supporting Documents.
2.2. Statistical Analysis
2.2.1. Descriptive analysis
In every air sample, the 50 compounds with the highest concentrations were reported as Toluene-equivalents [31,38,39]. In addition, the number of all compounds within each sample with a concentration above a baseline-threshold of 0.1 ppb (usually between 180 and 250 compounds) and the concentration-sum of all those compounds were reported [31,38,39]. This resulted in an identification of 405 compounds from 381 homes (excluding ten siblings and nine missing samples). Of 405 compounds, we restricted the compound groups of interest as four classes: summed concentrations of 16 PGEs, 8 terpene hydrocarbons (i.e., markers for water-based cleaning), Texanol A and B (i.e., markers for water-based paint), and phthalates (BBzP and DEHP). These four groups of compounds have been identified as independent risk factors of asthma and allergies [28,30]. As PGEs constitute main compounds of interest, investigating the degree of mutual correlations according to the sources and human behaviors were necessary, in order to clarify the independent risks of PGEs on asthma and allergies.
Our earlier validation analysis demonstrated that the non-reported concentrations of the compounds are likely to be lower than the 50th compound concentration range (0.33–11.24 μg/m3) of the present investigation [15]. Rather than attributing the non-reported concentrations with one-half of the median (1.11 μg/m3) of the lowest known concentrations across all samples, we restricted our analysis to the compounded with quantified concentration. This assumes that the non-reported concentrations of the compounds are below 0.33 μg/m3. Validity of this assumption is supported by the analysis of similar data in Finland [40]. All compounds were natural log(ln)—transformed, considering their right skewed distributions and varying standard deviations. Following the transformation, the distribution of the four chemical groups approximated the normal distribution (all Komogorov-Smirnov tests > 0.05) with comparable standard deviations. The geometric mean concentration patterns of the four groups of compounds were compared with the other chemical groups. Considering that the multiple comparisons of the compounds would increase the likelihood of a chance association, we defined Bonferroni corrected α = 0.00625 (shown in Table 2). Test for linear trend was conducted with linear-by-linear chi-square test. In order to ensure high reliability of the phthalates in the dust sample, the dust samples with weight > 25 mg are included for all analysis pertaining to BBzP and DEHP [30].
Table 2.
Geometric mean and geometric standard deviations of PGEs, terpenes, Texanols, BBzP and DEHP in the homes.
| PGEs | Terpenes | Texanols | BBzP a)b) | DEHP a)b) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nb) | GM ± GSD (μg/m3) | nb) | GM ± GSD (μg/m3) | nb) | GM ± GSD (μg/m3) | n | GM ± GSD (μg/m3) | n | GM ± GSD (μg/m3) | |
| Suspected Sources | ||||||||||
| Water-based cleaning frequency | ||||||||||
| P-for-linear-trend | 0.046 | 0.800 | 0.522 | 0.048 | 0.026 | |||||
| ≥ Once / week | 121 | 5.40 ± 3.42c) | 121 | 21.14 ± 2.77 | 30 | 4.16 ± 3.79 | 95 | 0.18 ± 2.77 | 109 | 0.86 ± 2.68 |
| Every other week | 99 | 3.94 ± 3.51 | 99 | 24.25 ± 2.87 | 26 | 3.91 ± 2.46 | 72 | 0.17 ± 2.57 | 85 | 0.88 ± 2.72 |
| ≤ Once / month | 165 | 3.13 ± 3.16 | 165 | 23.09 ± 2.34 | 38 | 4.71 ± 3.25 | 125 | 0.14 ± 2.42 | 151 | 0.68 ± 2.48 |
| At least one room was repainted prior to/following the child’s birth | ||||||||||
| P-for-ANOVA | 0.014 | 0.320 | 0.851 | 0.292 | 0.457 | |||||
| Yes | 247 | 4.28 ± 3.47 | 247 | 23.07 ± 2.62 | 66 | 4.64 ± 3.15 | 190 | 0.17 ± 2.67 | 224 | 0.80 ± 2.58 |
| No | 143 | 3.37 ± 3.18 | 143 | 21.49 ± 2.68 | 29 | 3.70 ± 3.18 | 106 | 0.15 ± 2.40 | 127 | 0.73 ± 2.68 |
| Flooring material, child’s bedroom | ||||||||||
| P-for-ANOVA | 0.110 | 0.382 | 0.178 | 0.000 | 0.002 | |||||
| Linoleum | 13 | 4.44 ± 3.89 | 13 | 22.77 ± 1.95 | 13 | 1.61 ± 1.03 | 12 | 0.12 ± 3.39 | 13 | 0.54 ± 2.98 |
| PVC | 211 | 4.42 ± 3.35 | 211 | 22.21 ± 2.64 | 211 | 4.25 ± 3.11 | 171 | 0.20 ± 2.46 | 188 | 0.95 ± 2.59 |
| Wood | 120 | 3.36 ± 3.43 | 120 | 24.44 ± 2.57 | 120 | 5.62 ± 3.40 | 82 | 0.11 ± 2.53 | 106 | 0.60 ± 2.42 |
| Laminate | 39 | 3.81 ± 3.11 | 39 | 21.25 ± 2.87 | 39 | 2.13 ± 1.71 | 25 | 0.16 ± 2.31 | 37 | 0.65 ± 2.81 |
| Other d) | 7 | 1.32 ± 1.89 | 7 | 10.26 ± 3.41 | 7 | 6 | 0.13 ± 1.40 | 7 | 0.79 ± 1.75 | |
| Age of surface materials in the child’s bedroom | ||||||||||
| P-for-linear-trend | 0.049 | 0.556 | 0.073 | 0.727 | 0.027 | |||||
| Very old | 19 | 3.15 ± 3.23 | 19 | 21.82 ± 2.02 | 5 | 1.22 ± 2.16 | 15 | 0.17 ± 2.90 | 18 | 0.99 ± 1.98 |
| Old | 76 | 3.51 ± 3.31 | 76 | 26.04 ± 2.39 | 16 | 3.07 ± 3.22 | 56 | 0.16 ± 2.87 | 70 | 0.88 ± 2.92 |
| Mixture of old/new | 136 | 3.77 ± 3.35 | 136 | 20.69 ± 3.02 | 33 | 6.28 ± 2.77 | 104 | 0.15 ± 2.19 | 122 | 0.74 ± 2.47 |
| Overall new | 112 | 3.65 ± 3.31 | 112 | 21.96 ± 2.52 | 31 | 3.95 ± 3.02 | 90 | 0.17 ± 2.91 | 101 | 0.76 ± 2.72 |
| Newest | 38 | 6.42 ± 3.54 | 38 | 22.65 ± 2.54 | 7 | 5.60 ± 4.31 | 27 | 0.15 ± 2.11 | 33 | 0.57 ± 2.13 |
| Building type | ||||||||||
| P-for-ANOVA | 0.805 | 0.706 | 0.096 | 0.308 | 0.509 | |||||
| Single family house | 321 | 3.85 ± 3.31 | 321 | 23.16 ± 2.64 | 83 | 4.65 ± 3.17 | 243 | 0.16 ± 2.56 | 291 | 0.75 ± 2.57 |
| Two family house | 22 | 4.65 ± 4.27 | 22 | 21.52 ± 2.08 | 3 | 7.80 ± 4.20 | 16 | 0.15 ± 2.14 | 19 | 0.81 ± 2.43 |
| Multifamily house | 44 | 3.94 ± 3.50 | 44 | 18.69 ± 2.96 | 8 | 1.83 ± 2.01 | 34 | 0.15 ± 2.94 | 39 | 0.94 ± 3.09 |
| Another | 3 | 6.35 ± 4.73 | 3 | 18.76 ± 1.53 | 1 | 2.35 ± -- | 3 | 0.43 ± 1.38 | 2 | 0.49 ± 1.34 |
| Secondhand smoke | ||||||||||
| P-for-ANOVA | 0.755 | 0.394 | 0.355 | 0.421 | 0.632 | |||||
| Yes | 67 | 4.07 ± 3.16 | 67 | 20.34 ± 3.19 | 17 | 3.16 ± 3.09 | 55 | 0.17 ± 2.54 | 63 | 0.82 ± 2.58 |
| No | 313 | 3.87 ± 3.40 | 313 | 22.73 ± 2.52 | 75 | 4.59 ± 3.20 | 235 | 0.16 ± 2.60 | 280 | 0.77 ± 2.63 |
| Suspected Modifiers | ||||||||||
| Quartiles of excess indoor water vapor content over outdoor content (g/m3) | ||||||||||
| P-for-linear-trend | <0.001 | 0.137 | 0.031 | 0.007 | 0.165 | |||||
| < 1.539 | 84 | 2.98 ± 3.18 | 84 | 21.28 ± 2.45 | 20 | 2.52 ± 2.77 | 65 | 0.12 ± 2.19 | 80 | 0.68 ± 2.61 |
| 1.539 - 2.156 | 84 | 4.04 ± 3.43 | 84 | 20.60 ± 2.75 | 18 | 5.09 ± 3.08 | 67 | 0.17 ± 2.17 | 82 | 0.78 ± 2.21 |
| 2.157 - 2.947 | 84 | 3.11 ± 3.30 | 84 | 18.92 ± 2.77 | 14 | 4.13 ± 4.06 | 59 | 0.16 ± 2.53 | 69 | 0.72 ± 2.91 |
| ≥ 2.948 | 84 | 6.51 ± 3.24c) | 84 | 27.58 ± 2.39 | 30 | 5.79 ± 3.05 | 66 | 0.20 ± 3.45 | 75 | 0.88 ± 2.82 |
| Quartiles of Temperature (°C) | ||||||||||
| P-for-linear-trend | 0.003 | 0.279 | 0.323 | 0.064 | 0.589 | |||||
| < 20.18 | 96 | 2.75 ± 2.90 | 96 | 19.95 ± 2.72 | 20 | 5.67 ± 2.53 | 71 | 0.13 ± 2.37 | 87 | 0.72 ± 2.72 |
| 20.18 - 20.96 | 98 | 4.86 ± 3.27 | 98 | 24.26 ± 2.45 | 24 | 4.21 ± 3.77 | 75 | 0.16 ± 2.36 | 87 | 0.84 ± 2.72 |
| 20.97 - 21.67 | 97 | 3.32 ± 3.36 | 97 | 21.10 ± 2.80 | 25 | 4.06 ± 3.46 | 71 | 0.17 ± 3.13 | 84 | 0.70 ± 2.69 |
| ≥ 21.68 | 98 | 5.32 ± 3.64c) | 98 | 24.50 ± 2.56 | 25 | 3.92 ± 2.95 | 78 | 0.18 ± 2.47 | 92 | 0.83 ± 2.38 |
| Quartiles of ventilation rate, child’s bedroom (air change / hr) | ||||||||||
| P-for-linear-trend | 0.198 | 0.372 | 0.111 | 0.125 | 0.006 | |||||
| < 0.220 | 94 | 4.64 ± 3.52 | 94 | 25.19 ± 2.49 | 24 | 6.28 ± 2.44 | 71 | 0.15 ± 2.25 | 82 | 0.69 ± 2.57 |
| 0.220 - 0.315 | 93 | 4.09 ± 3.48 | 93 | 22.16 ± 2.73 | 27 | 3.90 ± 3.05 | 76 | 0.14 ± 3.10 | 90 | 0.61 ± 2.78 |
| 0.316 - 0.435 | 94 | 3.65 ± 3.16 | 94 | 21.89 ± 2.42 | 22 | 4.23 ± 4.23 | 74 | 0.18 ± 2.53 | 86 | 0.85 ± 2.52 |
| ≥ 0.436 | 97 | 3.78 ± 3.40 | 97 | 22.19 ± 2.73 | 18 | 3.33 ± 3.24 | 69 | 0.17 ± 2.43 | 85 | 0.95 ± 2.44 |
| Type of ventilation system at home | ||||||||||
| P-for-ANOVA | 0.552 | 0.840 | 0.015 | 0.175 | 0.572 | |||||
| Natural, and do not use a kitchen fan | 20 | 2.81 ± 3.55 | 20 | 23.44 ± 1.83 | 8 | 3.43 ± 3.04 | 15 | 0.19 ± 3.02 | 18 | 0.75 ± 4.08 |
| Natural and use a kitchen fan | 229 | 4.29 ± 3.37 | 229 | 22.75 ± 2.85 | 62 | 5.09 ± 3.15 | 171 | 0.16 ± 2.57 | 211 | 0.76 ± 2.63 |
| Exhaust air system | 92 | 3.95 ± 3.62 | 92 | 20.80 ± 2.52 | 19 | 3.79 ± 3.03 | 73 | 0.17 ± 2.53 | 79 | 0.86 ± 2.49 |
| Exhaust & supply air system | 12 | 3.46 ± 3.02 | 12 | 15.91 ± 3.29 | 4 | 1.35 ± 1.61 | 9 | 0.16 ± 2.54 | 10 | 0.88 ± 1.93 |
| Exhaust & supply with heat recovery | 25 | 3.39 ± 2.82 | 25 | 27.41 ± 1.86 | 1 | 0.63 ± -- | 18 | 0.15 ± 2.63 | 21 | 0.74 ± 2.19 |
| Other | 7 | 1.76 ± 2.24 | 7 | 22.65 ± 1.73 | 7 | 0.08 ± 2.43 | 7 | 0.39 ± 2.52 | ||
| Water damage since spring 2000 | ||||||||||
| P-for-ANOVA | 0.915 | 0.813 | 0.110 | 0.764 | 0.625 | |||||
| Yes | 42 | 4.00 ± 3.77 | 42 | 21.94 ± 3.40 | 5 | 1.95 ± 2.65 | 33 | 0.15 ± 2.56 | 40 | 0.82 ± 3.15 |
| No | 344 | 3.91 ± 3.34 | 344 | 22.78 ± 2.55 | 89 | 4.56 ± 3.16 | 259 | 0.16 ± 2.60 | 306 | 0.76 ± 2.56 |
| Building Inspector Rating | ||||||||||
| First impression of stuffy or unventilated air | ||||||||||
| P-for-ANOVA | 0.027 | 0.029 | 0.925 | 0.207 | 0.367 | |||||
| Obvious | 33 | 6.76 ± 3.81 | 33 | 31.77 ± 2.14 | 9 | 8.22 ± 3.29 | 26 | 0.20 ± 2.09 | 29 | 1.02 ± 2.00 |
| Weak | 85 | 3.79 ± 3.41 | 85 | 19.85 ± 3.08 | 18 | 8.65 ± 3.97 | 64 | 0.15 ± 2.79 | 75 | 0.85 ± 2.74 |
| P-for-ANOVA | 0.027 | 0.029 | 0.925 | 0.207 | 0.367 | |||||
| Obvious | 33 | 6.76 ± 3.81 | 33 | 31.77 ± 2.14 | 9 | 8.22 ± 3.29 | 26 | 0.20 ± 2.09 | 29 | 1.02 ± 2.00 |
| Weak | 85 | 3.79 ± 3.41 | 85 | 19.85 ± 3.08 | 18 | 8.65 ± 3.97 | 64 | 0.15 ± 2.79 | 75 | 0.85 ± 2.74 |
| Stuffy, earthy, or microbial smell | ||||||||||
| P-for-ANOVA | 0.954 | 0.688 | 0.111 | 0.640 | 0.436 | |||||
| Obvious | 39 | 3.36 ± 3.21 | 39 | 25.38 ± 2.67 | 8 | 2.20 ± 2.56 | 30 | 0.15 ± 2.58 | 38 | 0.85 ± 2.85 |
| Weak | 47 | 3.31 ± 3.51 | 47 | 23.20 ± 2.92 | 11 | 5.80 ± 4.10 | 35 | 0.14 ± 2.48 | 42 | 0.70 ± 3.05 |
| Chemical smell | ||||||||||
| P-for-ANOVA | 0.451 | 0.950 | 0.137 | 0.865 | 0.429 | |||||
| Obvious | 13 | 2.47 ± 2.26 | 13 | 26.86 ± 3.53 | 1 | 3.84 ± -- | 7 | 0.14 ± 3.25 | 12 | 0.60 ± 4.13 |
| Weak | 29 | 3.41 ± 4.13 | 29 | 27.57 ± 3.44 | 3 | 22.33 ± 1.88 | 26 | 0.13 ± 2.57 | 27 | 0.84 ± 2.91 |
Sample size is reduced because only dust samples >25 mg are considered [30].
Reflects the number of respondents to given question.
The cut-off level of significant association for Bonferroni-corrected pair-wise comparison of specific categories was 0.00652. The cut-off value for the significant association for ANOVA was 0.05.
Includes three homes with wall-to-wall carpet and four homes with ‘other’ type.
2.2.2. Predictive model of indoor PGE concentration
In order to clarify whether the PGEs, terpenes and the Texanols are emitted from similar sources or modified by common building factors, an identical predictive model was used to examine its ability to explain the respective variability in the three groups of VOCs (Table 3). We conducted multivariate, ordinary least square regression after stratifying the data according to the median excess indoor humidity (2.158 g/m3). In order to avoid multiple collinearity in the models due to the mutual correlation among excess humidity, temperature and ventilation rate, the data were stratified according to the median indoor excess humidity. Here, water-based cleaning and repainting were considered PGE sources. Indoor excess humidity, temperature, ventilation rate in the child’s bedroom, and history of water damage were considered effect modifiers. We deemed that a significant interaction is present if the estimated effect of the given source on the PGE concentration differed by >30% between the strata. Subsequently, we formally tested the following respective interaction terms in the overall data to confirm the finding, (frequency of water-based cleaning × excess humidity), and (history of repainting × excess humidity).
Table 3.
Predictive models of indoor PGEs, Terpenes, and Texanols.
| PGEs | Terpenes | Texanols | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (95 % CI) | P | β | (95 % CI) | P | β | (95 % CI) | P | |||||
| Excess humidity | |||||||||||||
| ≤2.157 g/m3 | y-intercept | −1.567 | −4.507 | 1.373 | 0.294 | 3.977 | 1.707 | 6.247 | 0.001 | 5.667 | 0.083 | −0.776 | 12.110 |
| Wet-clean once/week | 0.477 | 0.040 | 0.915 | 0.033 | −0.247 | −0.585 | 0.091 | 0.151 | −0.417 | 0.397 | −1.408 | 0.574 | |
| Wet-clean every other week | 0.581 | 0.131 | 1.031 | 0.012 | 0.406 | 0.059 | 0.753 | 0.022 | −0.420 | 0.443 | −1.525 | 0.684 | |
| Repainted ≥ one room | 0.005 | −0.367 | 0.376 | 0.980 | 0.074 | −0.213 | 0.361 | 0.610 | −0.357 | 0.429 | −1.268 | 0.554 | |
| “Newest” surface material | 0.423 | −0.195 | 1.042 | 0.178 | 0.148 | −0.330 | 0.625 | 0.543 | 0.155 | 0.791 | −1.029 | 1.338 | |
| Temperature (quartile unit) | 0.125 | −0.017 | 0.267 | 0.084 | −0.049 | −0.159 | 0.061 | 0.380 | −0.170 | 0.255 | −0.470 | 0.129 | |
| Ventilation rate in the child‘s bedroom (quartile unit) | −0.054 | −0.222 | 0.113 | 0.523 | 0.004 | −0.126 | 0.133 | 0.955 | −0.149 | 0.432 | −0.532 | 0.234 | |
| ≥2.158 g/m3 | y-intercept | −2.824 | −6.290 | 0.642 | 0.110 | 0.398 | −2.421 | 3.217 | 0.781 | 3.496 | 0.362 | −4.189 | 11.181 |
| Wet-clean once/week | 0.519 | 0.100 | 0.938 | 0.015 | 0.104 | −0.236 | 0.444 | 0.547 | 0.246 | 0.594 | −0.682 | 1.174 | |
| Wet-clean every other week | 0.217 | −0.260 | 0.694 | 0.370 | −0.138 | −0.526 | 0.249 | 0.482 | −0.285 | 0.547 | −1.235 | 0.665 | |
| Repainted ≥ one room | 0.630 | 0.243 | 1.017 | 0.002 | 0.100 | −0.215 | 0.415 | 0.532 | 0.016 | 0.971 | −0.847 | 0.878 | |
| “Newest” surface material | 0.409 | −0.195 | 1.013 | 0.183 | −0.098 | −0.589 | 0.393 | 0.695 | 2.386 | 0.089 | −0.385 | 5.157 | |
| Temperature (quartile unit) | 0.185 | 0.020 | 0.350 | 0.028 | 0.127 | −0.008 | 0.261 | 0.065 | −0.064 | 0.725 | −0.431 | 0.302 | |
| Ventilation rate in the child‘s bedroom (quartile unit) | −0.081 | −0.255 | 0.093 | 0.358 | 0.026 | −0.116 | 0.167 | 0.718 | −0.257 | 0.201 | −0.658 | 0.143 | |
3. Results
3.1. Pattern of Mean PGE Concentration, Compared to the Patterns of Terpenes, Texanols, BBzP and DEHP
Table 2 shows the pattern of geometric means of the four VOC groups according to the suspected sources and modifiers. The geometric mean of the PGEs linearly increased with water-based cleaning frequency, history of repainting at least one of the rooms at home, “newest” age of the surface material, “obviously poor” indoor air quality, impression of “obviously” stuffy and unventilated air by the building inspector, growing excess indoor humidity, and higher indoor temperature. Mean PGE levels in the families with hard surface floor (i.e., linoleum, PVC, wood, or laminate) were about 4- fold higher (P = 0.057) than the families with the ‘other’ flooring material in the child’s bedroom.
The PGEs were weakly correlated with the total Texanol concentration (Spearman’s coefficient = 0.205, p = 0.046), and also with the total terpenes (Spearman’s coefficient = 0.205, p < 0.001). On the other hand, in a sub-analysis of the homes with a reliable increase (≥25 mg) in dust weight, non-parametric correlations between PGEs with BBzP and DEHP were weaker (Spearman’s coefficients = 0.144 and 0.070, p = 0.013 and 0.193).
3.2. Comparisons of Trends in Compound Groups
Trend in the geometric mean concentrations of PGEs differed from those for the terpene hydrocarbons, BBzP, and DEHP, respectively (Table 2).
The mean terpenes and Texanols did not increase with higher frequency of water-based cleaning. The mean terpenes and Texanols were 7% and 25% higher in the homes with a history of repainting prior to or following the child’s birth, compared to those without similar history. Among those homes with hard floor surfaces (n = 383), the mean terpene concentrations were approximately two-times higher than the homes with other flooring type (n = 7). Age of the surface material in the child’s bedroom was not associated with any apparent trend in terpenes. In addition, mean Texanol levels was not associated with the age of the surface material, building type, history of water-damage, or any of the inspector’s assessment of the air quality.
Overall disparate patterns in geometric mean BBzP and DEHP concentrations were observed with behavioral and structural predictors (Table 2). While no apparent trend emerged for the BBzP levels according to the age of the surface material, “very old” material was associated with ~74% higher mean DEHP than the homes with “newest” material (P-for-linear-trend = 0.027). “Obviously poor” indoor air quality, but no other ratings, was linearly associated BBzP (P-for-linear-trend = 0.028) and DEHP (P-for-linear-trend = 0.009) compared to the homes with “good” air quality. Other characteristics, such as the type of the ventilation system, or the history of home flooding were not associated with notable differences in any of the compound groups.
3.3. Inspector Rating of Indoor Air Quality
The internal consistency of indoor air quality (IAQ) was high (Chronbach’s alpha = 0.89) among the three ratings (Table 2). In addition, excess indoor humidity was not significantly different for the homes with “obvious” rating than the “weak” rating in all three items. Also, the ventilation rate in the child’s room was almost identical for the “obvious” rated homes compared to the “weak” rated homes in all three items.
3.4. Modifiers of Indoor PGE Concentrations
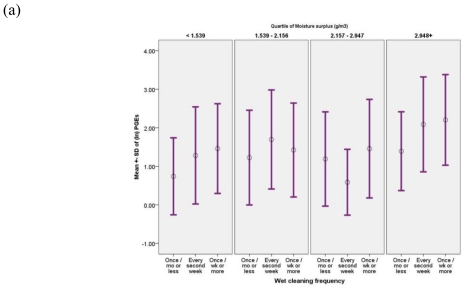
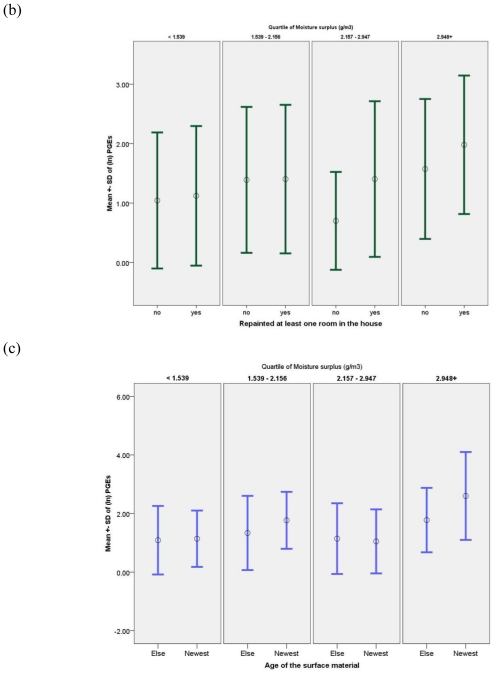
Excess indoor humidity (g/m3) was positively correlated with all groups of compounds. Figure 1 shows that the linear association between water-based cleaning and the mean PGEs in air is further enhanced by excess humidity. Geometric mean PGEs per each cleaning category was markedly higher within the highest humidity quartile (13.25, 12.23, and 5.86 ≤g/m3), compared to those within the lowest quartile (7.00, 5.82, and 3.60 ≤g/m3) (Figure 1(a)). Similarly, linear association in mean PGE concentration for those with repainting history was significantly greater within the highest quartiles of excess indoor humidity (10.85 vs. 6.68 ≤g/m3), compared to those within the lowest quartile (5.22 vs. 5.10 ≤g/m3) (Figure 1(b)). Furthermore, the mean PGEs for those with a “newest” surface material in the child’s room were augmented by excess indoor humidity (17.75 vs. 8.72 ≤g/m3 in the highest excess humidity category; 5.19 vs. 4.41 ≤g/m3 within the lowest category) (Figure 1(c)).
Figure 1.
(a) Geometric mean PGEs (≤g/m3) associated with water-based cleaning frequency, according to excess indoor humidity (g/m3). (b) Geometric mean PGEs (≤g/m3) associated with repainting history, according to excess indoor humidity (g/m3). (c) Geometric mean PGEs (≤g/m3) associated with age of the surface material, according to excess indoor humidity (g/m3).
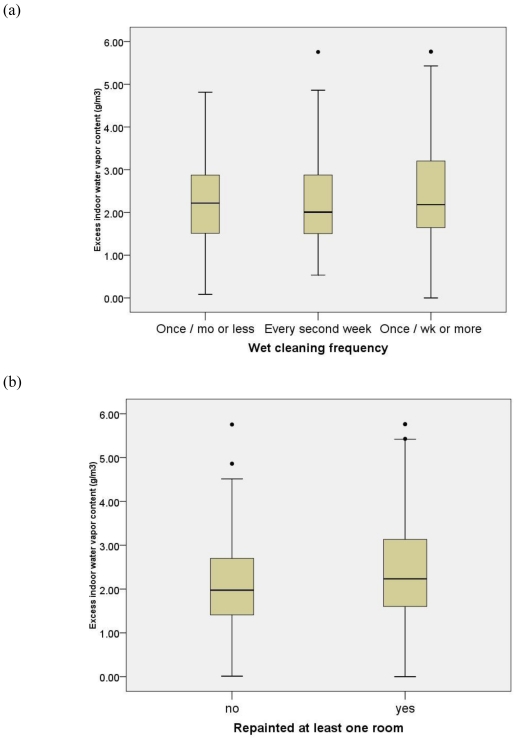
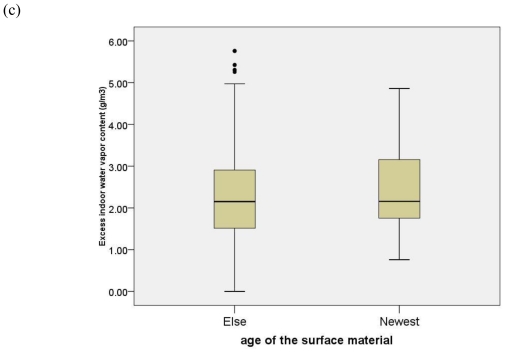
At the same time, none of the suspected sources contribute to significantly elevated excess indoor humidity, demonstrating that humidity is unlikely to confound the source-PGE relationship (Figure 2).
Figure 2.
(a) Distribution of excess indoor humidity (g/m3) according to water-based cleaning frequency. (b) Distribution of excess indoor humidity (g/m3) according to repainting history. (c) Distribution of excess indoor humidity (g/m3) according to age of the surface material in the child’s bedroom.
3.5. Final Predictive Model
In order to clarify whether the PGEs, terpenes and the Texanols are emitted and/or modified by common factors, an identical predictive models were used to examine their ability to explain the variability in each group concentration (Table 3). The water-based cleaning > once/week was associated with a larger mean increase in PGEs (52%) for the homes with a high (≥2.158 g/m3) excess humidity, compared to the homes with similar frequency of wet-cleaning within low humidity homes (48%; P-for-interaction = 0.03). Furthermore, the history of repainting was associated >10-fold larger increase in PGE level in homes with a high excess humidity (63 % vs. 0.5% increase in mean PGEs, P-for-interaction = 0.03), compared to those with lower than median excess humidity. Quartile unit increase in the indoor temperature was associated with somewhat larger mean increase in the PGEs for those with a high excess humidity, compared to the low humidity homes (19 vs. 13%). Water -based cleaning every other week, but no other factors, was associated with a higher mean terpene level (41%) among the homes with high excess indoor humidity. No sources were associated with an increase in the Texanols (Table 3).
4. Discussion
The risks of VOC compounds emitted from cleaning agents, paints, and other surface material on the asthma and allergies remain controversial [1,13]. In our earlier analysis, PGEs in indoor air significantly predicted elevated risks of multiple allergic symptoms, and the diagnoses of asthma, rhinitis and eczema, respectively [15]. In addition, a unit PGE exposure was associated with an increased likelihood of IgE-sensitization. At the same time, a review of both epidemiologic and toxicological literature concluded that VOCs from cleaning and paints are likely to be mere correlates of biological allergens, combustion products, or dampness [13]. In part, the present analysis of VOCs and phthalates in the DBH study was conducted to further examine our earlier observation on PGE compounds and childhood asthma. Specifically, the sources and the correlations of the PGEs with other risk factors of asthma and allergies (i.e., terpenes, Texanols, BBzP, and DEHP) were investigated here.
Building characteristics and occupant behaviors that contribute to elevated indoor PGE concentrations are markedly different from those of the terpenes, Texanols (Table 3) as well as BBzP and DEHP (Table 2). Such poor correlations suggest that the terpenes, BBzP, and DEHP are unlikely to confound the apparent associations of the PGEs with multiple allergic symptoms, and the diagnoses in our on-going DBH study.
In addition, significant augmentation of the PGEs in indoor air by excess humidity suggests that humidity might contribute to higher emission or retention of the PGEs [41]. The information regarding the history of repainting was collected 1.5 year prior to the present study. Also, all parents remained in the same house and have not changed most life-style practices during the 1.5 year period. To further validate this, the families (n = 18, 0.6% of cases and 1.1% controls) that renovated their house due to flooding were excluded before the onset of present investigation. A significant 63% (95% CI, 24–100%) increase in PGE concentration for those who repainted at least one of the rooms and also have a higher than median level of excess humidity suggests that PGE emission from paint might have been sustained far beyond several months period following the paint application. Such timeline supports the PGEs as risk factors, rather than mere correlates of parental allergen/adjuvant removal behavior following the clinical diagnosis of the child. Correlation structure among humidity, ventilation and temperature might additionally exert contemporary rather than long term relationship to PGE concentrations. An increase in excess humidity per unit reduction in ventilation rate was larger (2.40 g/m3) at the highest indoor temperature (21.7–25.6 °C), compared similar increase (0.97 g/m3/unit reduction in ventilation rate) at the lowest quartile (16.39–20.17 °C).
Lack of the association between the PGE sources examined here with excess indoor humidity demonstrates that humidity could not have confounded the sources—PGEs relationship (Figure 2). Rather, both water-based cleaning and repainting history significantly contribute to an increased PGE concentration in indoor air. Other experimental studies also demonstrated the water-based cleaning agents emit PGEs and terpenes [16].
PGEs, rather than limonene and terpenes are likely to be a significant risk factor of the asthma and allergy outcomes. This is supported by the persistence and the magnitude of the water-based cleaning’s association with the indoor PGE concentrations after accounting for other known predictors (Table 3). Other constituents of the cleaning agents, limonene and composite sum of the terpene hydrocarbons, were weakly correlated the PGEs (Spearman’s coefficient = 0.18 and 0.19, respectively). In the DBH study, limonene was neither an independent risk factor (adjusted-odds ratio (aOR), 1.15; 95% CI, 0.86–1.53) for the case status, nor a confounder of PGE-asthma/allergy associations. Similarly, the terpene hydrocarbons did not pose an independent risk on any of the outcomes, or confound the PGE-asthma/allergy associations. Another investigation, using only questionnaire of general domestic hygiene practice, observed somewhat elevated risks of higher home cleanliness with current wheezing symptoms (aOR, 1.16, 95% CI, 1.03–1.29) and current rhino-conjuntivitis (adjusted OR, 1.17, 95% CI, 1.04–1.31) in a large group of Australian children [42].
In the present DBH study, a large increase (41%) in PGE level for those families with “newest” surface material at home suggests that this might have additionally provided a long-term exposure. However, our present observation of the highest mean PGE concentration for the “newest” material requires a further investigation to clarify its constituent materials and to determine the exact emission patterns following the installation.
Consistent with our earlier analysis [43], PVC flooring material in the child’s bedroom (n = 188) was associated with 67% and 80% higher mean BBzP and DEHP levels, compared to those with linoleum flooring (n = 13). Multi-family houses were also more likely to use PVC flooring (n = 39) than the single-family house (n = 291) due to its strong correlation with socioeconomic position of the family [43]. The ventilation rate were also higher for the multifamily houses, compared to the single family house [43]. Linear increase in DEHP with higher ventilation rate suggests that DEHP transfer from vinyl flooring to dust occurs through the boundary layer [44]. Increased mixing of air might diminish the boundary layer and increase the DEHP transfer [44].
5. Conclusions
Use of water-based cleaning agent > once per month, repainting ≥ one room in the house, and “newest” surface material in the child’s bedroom was consistent with higher levels of PGEs in the child’s bedroom. Furthermore, the PGE levels in indoor air were significantly higher in homes with higher excess humidity in air and with higher temperatures. At the same time, these sources of PGE did not predict an elevated indoor humidity. Difference in specific sources and low correlations of the PGEs with other VOCs and the phthalates strengthen of the independence of PGE risks on the multiple allergic symptoms, and the respective diagnosis of asthma, rhinitis, and eczema.
Table 1.
Definition and distributions (μg/m3) of propylene glycol and glycol ethers (PGEs), terpene hydrocarbons, Texanols, and phthalates.
| N | Mean | S.D. | Min | 25th | 50th | 75th | Max | |
|---|---|---|---|---|---|---|---|---|
| Percentiles | ||||||||
| Propylene glycol and glycol ethers (CAS #) | ||||||||
| 1,2-propanediol (propylene glycol) (CAS # 57-55-6) | 165 | 8.20 | 8.17 | 0.51 | 2.84 | 5.54 | 10.25 | 48.62 |
| 1-methoxy-2-propanol (α-isomer of Propylene Glycol Monomethyl Ether) (CAS # 107-98-2) | 86 | 4.51 | 3.08 | 0.73 | 2.32 | 3.51 | 6.00 | 15.68 |
| 2-(2-butoxyethoxy)ethanol (CAS # 112-34-5) | 69 | 4.73 | 4.90 | 0.46 | 1.95 | 2.87 | 6.04 | 30.33 |
| 1-butoxy-2-propanol (CAS # 5131-66-8) | 65 | 7.15 | 8.87 | 0.60 | 2.21 | 4.03 | 8.08 | 53.04 |
| 2-(2-butoxyethoxy)ethanol acetate (CAS # 124-17-4) | 33 | 3.30 | 3.05 | 0.53 | 1.62 | 2.32 | 3.57 | 13.26 |
| 2-butoxy ethanol (CAS # 111-76-2) | 27 | 4.74 | 5.67 | 0.78 | 1.73 | 2.36 | 4.84 | 24.39 |
| 2-(2-(2-butoxyethoxy)ethoxy) ethanol (CAS # 143-22-6) | 20 | 2.10 | 1.80 | 0.65 | 0.96 | 1.68 | 2.60 | 8.74 |
| 2-(2-ethoxyethoxy) ethanol (cas # 111-90-0) | 16 | 9.72 | 9.39 | 2.26 | 4.38 | 5.73 | 11.59 | 36.86 |
| 1-(2-methoxypropoxy)-2-propanol (CAS # 13429-07-7) | 11 | 6.63 | 5.74 | 1.39 | 2.73 | 3.99 | 13.91 | 16.15 |
| Dipropylene glycol methyl ether (CAS # 34590-94-8) | 7 | 4.43 | 2.52 | 1.83 | 2.55 | 3.74 | 6.35 | 9.16 |
| 2-(2-methoxyethoxy) ethanol (CAS # 111-77-3) | 6 | 6.28 | 4.47 | 3.18 | 3.71 | 4.46 | 8.72 | 15.08 |
| 2-(2-hydroxypropoxy)-1-propanol (CAS # 106-62-7) | 4 | 1.42 | 0.74 | 0.62 | 0.74 | 1.37 | 2.17 | 2.34 |
| 1-(2-methoxy-1-methylethoxy)-2-propanol (CAS # 20324-32-7) | 3 | 6.66 | 2.48 | 4.13 | 4.13 | 6.76 | 9.08 | 9.08 |
| 1-propoxy-2-propanol (CAS # 1569-01-3) | 2 | 5.23 | 5.20 | 1.55 | 1.55 | 5.23 | 8.91 | 8.91 |
| 2-(2-ethoxyethoxy) ethanol acetate (CAS # 112-15-2) | 2 | 8.29 | 2.90 | 6.24 | 6.24 | 8.29 | 10.34 | 10.34 |
| 2,2-oxybis ethanol (Diethylene glycol) (CAS # 111-46-6) | 1 | 7.97 | 7.97 | 7.97 | 7.97 | 7.97 | 7.97 | |
| Ethanediol (Ethylene glycol) (CAS # 107-21-1) | 1 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | |
| Terpene hydrocarbons | ||||||||
| γ–Terpinene (CAS # 99-85-4) | 3 | 2.82 | 0.44 | 2.38 | 2.38 | 2.82 | 3.26 | 3.26 |
| iso–Terpinolene (CAS # 586-62-9) | 7 | 3.86 | 1.62 | 2.10 | 2.53 | 3.30 | 5.75 | 6.08 |
| α–Terpinene (CAS # 99-86-5) | 1 | 25.21 | 25.21 | 25.21 | 25.21 | 25.21 | 25.21 | |
| α–Pinene (CAS # 80-56-8) | 239 | 20.76 | 16.40 | 1.93 | 8.47 | 16.27 | 28.58 | 97.53 |
| Limonene (CAS # 5989-27-5) | 383 | 17.78 | 14.50 | 1.36 | 7.84 | 13.61 | 23.19 | 92.99 |
| β–Pinene (CAS # 127-91-3) | 35 | 4.41 | 2.58 | 0.62 | 2.77 | 3.85 | 5.18 | 13.21 |
| Camphene (CAS # 79-92-5) | 155 | 5.62 | 4.24 | 0.71 | 2.82 | 4.59 | 7.07 | 27.16 |
| β–Phellandrene (CAS # 555-10-2) | 14 | 5.81 | 4.34 | 1.39 | 2.37 | 4.97 | 8.08 | 16.35 |
| Texanol® | ||||||||
| Texanol A | 39 | 9.95 | 6.56 | 1.20 | 5.63 | 7.58 | 14.33 | 27.89 |
| Texanol B | 88 | 5.60 | 7.13 | 0.47 | 1.72 | 2.76 | 6.55 | 42.48 |
| Phthalate compounds in dust samplea) | ||||||||
| n-butyl benzyl phthalate (BBzP) | 296 | 0.38 | 2.64 | 0.01 | 0.09 | 0.16 | 0.30 | 45.55 |
| di(2-ethylhexyl)phthalate (DEHP) | 351 | 1.31 | 2.59 | 0.02 | 0.46 | 0.77 | 1.31 | 40.46 |
Sample size is reduced because only dust samples > 25 mg are considered [30].
Acknowledgements
The study has been supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), Swedish Asthma and Allergy Association’s Research Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research, Norwegian Research Council, the U.S. National Research Service Award (T32 ES 07069), and County Council of Värmland, Sweden. The authors declared that they have no conflict of interest.
Appendix: Online Supporting Document
Detection and Identification
An automated mass spectra library check (Wiley) gave the first preliminary identification results. Each of the library suggestions for component identification were then again cross checked against Norwegian Institute for Airway Research (NILU)’s database for indoor air pollutants which contains about 1,000 components. The database contains retention time indexes from compounds which were identified in indoor air samples at NILU on exact the same analytical system over the last 20 years. Most of the compounds within this database have been verified by direct injection of pure standard solutions or mixed standard solutions. The criteria for identification were over 80 % confidence match from the mass spectra library and a match to the retention time database within 5 seconds of relative retention together with a manual check of the retrieved mass spectrogram against the library mass spectrogram. If a peak did not meet those criteria it was named as “unidentified compound”.
Calibration
The calibration was based on toluene equivalents [31,38,39]. Ten samples were run together with two standard injections before and after each series. Internal standards consisted of a solution containing 100 ng/≤L of benzene, toluene, ethylbenzene and xylenes in methanol [31,38,39]. Standard Tenax tubes were prepared by syringe onto the adsorbent followed by 5 minutes of dry nitrogen (20mL).
Quality Assurance
Our VOC sampling approach with adsorption/thermal desorption coupled with gas chromatography-mass spectrometry (GC/MS) has been validated as sensitive, simple, and cost-effective assessment method [37,45,46]. Also, our sampling duration (60–90 min) was substantially longer than the standard protocol [28]. Compared to other methods, our present approach has an advantage of higher sensitivity [28]. In a number of indoor VOC investigations, which relied on Tenax TA as a general purpose adsorbent, overall very low inherent artifacts were observed [38]. Known artifacts of Tenax TA do not include glycol ethers. Thus, glycol ethers are unlikely to have been introduced in this investigation as sampling artifacts [28]. At the same time, no study as ever examined temporal stability of the 405 compounds in this study over time. A time period of 5 to 6 weeks between sampling and analysis could influence the artifact level and recovery rates. While this is likely to have biased the aromatic hydrocarbon level towards the null, there was no evidence that the PGEs were also influenced by the transport duration. The method used is in accordance to best laboratory practice and the recommendations given by DIN EN 14662-1 and Helmig 1996 [16].
The prevalence and concentration the VOCs detected in the present study are strikingly concordant with those detected from other Scandinavian countries. We compared the concordance of our detected VOCs with Finnish EXPOLIS study [47]. In the EXPOLIS study, air samples (2–3 L) from 183 homes were collected during the winter of 1996–1997 in Helsinki, Finland, focusing on 30 VOCs as target compounds. Extensive quality assurance and control standards were practiced. Of the 30 VOCs, 21 VOCs were also collected in our study. The prevalence (% detected in participant homes) of the VOC compounds were significantly correlated between the two studies (R2 = 0.57, p < 0.001) [15]. Also, eight VOC compounds, which were identified in ≥ 80% of the homes in both the studies (i.e., toluene, limonene, hexanal, p/m-xylene, benzaldehyde, octanal, undecane, and ethylbenzene), their concentrations were significantly correlated (R2 = 0.612, p = 0.022). This suggests that compounds with low prevalence are also expected to have low concentrations in both DBH and EXPOLIS. For example, 2-methyl-1-propanol, observed in 5% of the homes of DBH study was 1.96 μg/m3 and 3.37 μg/m3 in EXPOLIS. Striking similarities in absolute concentration and correlation of the compounds between the two studies support the validity of our sampling and analytical procedures.
Table 1.
Mean geometric concentration of individual PGE compounds according to excess indoor humidity and cleaning frequency.
| Excess Indoor Humidity (g/m3) | Cleaning frequency | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | GM | 95% | CI | N | GM | 95% | CI | ||
| 1,2-propanediol | |||||||||
| <1.539 | 29 | 5.26 | 3.5 | 7.92 | ≥once/wk | 64 | 6.12 | 4.85 | 7.73 |
| 1.539–2.156 | 35 | 4.77 | 3.56 | 6.39 | Bi-wkly | 34 | 5.94 | 4.45 | 7.92 |
| 2.157–2.947 | 30 | 5.96 | 3.93 | 9.03 | ≤once/mo | 63 | 4.98 | 3.97 | 6.24 |
| ≥2.948 | 52 | 6.32 | 5.2 | 7.67 | 161 | ||||
| 146 | |||||||||
| 1-methoxy-2-propanol | |||||||||
| <1.539 | 19 | 2.66 | 1.89 | 3.74 | ≥once/wk | 29 | 4.27 | 3.45 | 5.3 |
| 1.539–2.156 | 18 | 4.21 | 2.8 | 6.33 | Bi-wkly | 26 | 3.85 | 2.91 | 5.09 |
| 2.157–2.947 | 15 | 3.54 | 2.58 | 4.85 | ≤once/mo | 31 | 3.01 | 2.34 | 3.89 |
| ≥2.948 | 24 | 4.51 | 3.5 | 5.8 | 86 | ||||
| 76 | |||||||||
| 2-(2-butoxyethoxy)ethanol | |||||||||
| <1.539 | 11 | 1.51 | 0.8 | 2.83 | ≥once/wk | 34 | 3.2 | 2.44 | 4.2 |
| 1.539–2.156 | 10 | 4.51 | 2.28 | 8.92 | Bi-wkly | 14 | 4.42 | 2.67 | 7.33 |
| 2.157–2.947 | 13 | 3.04 | 1.95 | 4.74 | ≤once/mo | 21 | 2.74 | 1.78 | 4.2 |
| ≥2.948 | 21 | 4.30 | 3.16 | 5.83 | 69 | ||||
| 55 | |||||||||
| 1-butoxy-2-propanol | |||||||||
| <1.539 | 2 | 5.11 | 0 | ∞ | ≥once/wk | 4 | 7.44 | 1.12 | 49.34 |
| 1.539–2.156 | 5 | 3.08 | 0.97 | 9.74 | Bi-wkly | 7 | 6.24 | 2.57 | 15.11 |
| 2.157–2.947 | 2 | 2.84 | 0 | ∞ | ≤once/mo | 10 | 3.38 | 1.55 | 7.36 |
| ≥2.948 | 8 | 6.92 | 2.94 | 16.25 | 21 | ||||
| 17 | |||||||||
| 2-(2-butoxyethoxy)ethanol acetate | |||||||||
| <1.539 | 7 | 1.43 | 0.8 | 2.58 | ≥once/wk | 10 | 2.67 | 1.44 | 4.94 |
| 1.539–2.156 | 9 | 2.64 | 1.34 | 5.21 | Bi-wkly | 13 | 2.99 | 1.96 | 4.55 |
| 2.157–2.947 | 5 | 1.89 | 0.67 | 5.29 | ≤once/mo | 10 | 1.67 | 0.98 | 2.85 |
| ≥2.948 | 9 | 3.63 | 2.08 | 6.33 | 33 | ||||
| 30 | |||||||||
| 2-butoxy ethanol | |||||||||
| <1.539 | 2 | 1.7 | 0.03 | 109.77 | ≥once/wk | 14 | 3.25 | 1.95 | 5.44 |
| 1.539–2.156 | 6 | 3.38 | 0.9 | 12.73 | Bi-wkly | 5 | 4.19 | 1.21 | 14.49 |
| 2.157–2.947 | 3 | 1.83 | 0.26 | 13.07 | ≤once/mo | 8 | 2.29 | 1.21 | 4.36 |
| ≥2.948 | 13 | 3.67 | 2.31 | 5.82 | 27 | ||||
| 24 | |||||||||
| 2-(2-(2-butoxyethoxy)ethoxy) ethanol | |||||||||
| <1.539 | 4 | 1.62 | 1.12 | 2.34 | ≥once/wk | 10 | 1.98 | 1.15 | 3.4 |
| 1.539–2.156 | 3 | 0.96 | 0.34 | 2.73 | Bi-wkly | 2 | 2.2 | 0.05 | 102.27 |
| 2.157–2.947 | 3 | 1.39 | 0.33 | 5.78 | ≤once/mo | 8 | 1.28 | 0.87 | 1.89 |
| ≥2.948 | 4 | 3.64 | 1.21 | 10.98 | 20 | ||||
| 14 | |||||||||
| 2-(2-ethoxyethoxy) ethanol | |||||||||
| <1.539 | 3 | 8.07 | 2.43 | 26.79 | ≥once/wk | 7 | 6.4 | 3.63 | 11.31 |
| 1.539–2.156 | 3 | 11.03 | 1.19 | 102.15 | Bi-wkly | 4 | 4.97 | 2.89 | 8.55 |
| 2.157–2.947 | 2 | 3.53 | 0.01 | ∞ | ≤once/mo | 5 | 10.71 | 2.6 | 44.08 |
| ≥2.948 | 6 | 5.76 | 3.2 | 10.36 | 16 | ||||
| 14 | |||||||||
| 1-(2-methoxypropoxy)-2-propanol | |||||||||
| <1.539 | 2 | 5.12 | 0.21 | 122.11 | ≥once/wk | 4 | 4.43 | 0.99 | 19.81 |
| 1.539–2.156 | 3 | 4.94 | 0.31 | 79.13 | Bi-wkly | 3 | 7.76 | 1.58 | 38.18 |
| 2.157–2.947 | 1 | 3.52 | ≤once/mo | 4 | 3.48 | 0.73 | 16.48 | ||
| ≥2.948 | 3 | 3.94 | 0.17 | 91.56 | 11 | ||||
| 9 | |||||||||
| Dipropylene glycol methyl ether | |||||||||
| <1.539 | 1 | 9.16 | ≥once/wk | 2 | 3.67 | 2.95 | 4.58 | ||
| 1.539–2.156 | 2 | 4.02 | 0.01 | ∞ | Bi-wkly | 4 | 4.86 | 1.98 | 11.93 |
| 2.157–2.947 | 1 | 3.74 | ≤once/mo | 1 | 1.83 | ||||
| ≥2.948 | 2 | 2.57 | 0.03 | 193.76 | 7 | ||||
| 6 | |||||||||
| 2-(2-methoxyethoxy) ethanol | |||||||||
| <1.539 | 1 | 3.18 | ≥once/wk | 1 | 15.08 | ||||
| 1.539–2.156 | 0 | Bi-wkly | 1 | 4.84 | |||||
| 2.157–2.947 | 1 | 3.88 | ≤once/mo | 4 | 4.27 | 2.61 | 6.99 | ||
| ≥2.948 | 4 | 6.66 | 2.65 | 16.75 | 6 | ||||
| 6 | |||||||||
| 2-(2-hydroxypropoxy)-1-propanol | |||||||||
| <1.539 | 1 | 0.62 | ≥once/wk | 2 | 1.6 | 0.01 | 206.67 | ||
| 1.539–2.156 | 1 | 2.34 | Bi-wkly | 1 | 0.62 | ||||
| 2.157– 2.947 | 2 | 1.34 | 0.1 | 18.48 | ≤once/mo | 1 | 1.65 | ||
| ≥2.948 | 0 | 4 | |||||||
| 4 | |||||||||
| 1-(2-methoxy-1-methylethoxy)-2-propanol | |||||||||
| <1.539 | 1 | 9.08 | ≥once/wk | 1 | 4.13 | ||||
| 1.539–2.156 | 1 | 6.76 | Bi-wkly | 2 | 7.84 | 1.21 | 50.92 | ||
| 2.157–2.947 | 1 | 4.13 | ≤once/mo | 0 | |||||
| ≥2.948 | 0 | 3 | |||||||
| 3 | |||||||||
| 1-propoxy-2-propanol | |||||||||
| <1.539 | ≥once/wk | 0 | |||||||
| 1.539–2.156 | Bi-wkly | 1 | 1.55 | ||||||
| 2.157–2.947 | ≤once/mo | 1 | 8.91 | ||||||
| ≥2.948 | 2 | ||||||||
| 2-(2-ethoxyethoxy) ethanol acetate | |||||||||
| <1.539 | 0 | ≥once/wk | 0 | ||||||
| 1.539–2.156 | 1 | 6.24 | Bi-wkly | 1 | 10.34 | ||||
| 2.157–2.947 | 0 | ≤once/mo | 1 | 6.24 | |||||
| ≥2.948 | 1 | 10.34 | 2 | ||||||
| 2 | |||||||||
| Texanol A | |||||||||
| <1.539 | 4 | 9.06 | 3.26 | 25.15 | ≥once/wk | 12 | 9.56 | 5.9 | 15.49 |
| 1.539–2.156 | 9 | 7.27 | 4.49 | 11.77 | Bi-wkly | 9 | 6.07 | 3.16 | 11.66 |
| 2.157–2.947 | 6 | 7.12 | 3.49 | 14.52 | ≤once/mo | 16 | 8.37 | 5.9 | 11.88 |
| ≥2.948 | 13 | 10.32 | 6.52 | 16.33 | 37 | ||||
| 32 | |||||||||
| Texanol B | |||||||||
| <1.539 | 19 | 1.99 | 1.43 | 2.77 | ≥once/wk | 30 | 3.31 | 2.19 | 4.99 |
| 1.539–2.156 | 18 | 3.86 | 2.48 | 5.99 | Bi-wkly | 21 | 2.94 | 2.12 | 4.07 |
| 2.157–2.947 | 14 | 3.27 | 1.67 | 6.39 | ≤once/mo | 35 | 3.7 | 2.64 | 5.19 |
| ≥2.948 | 28 | 4.46 | 3.11 | 6.39 | 86 | ||||
| 79 | |||||||||
References
- 1.Jaakkola JJ, Jaakkola MS. Professional cleaning and asthma. Curr. Opin. Allergy Clin. Immunol. 2006;6:85–90. doi: 10.1097/01.all.0000216849.64828.55. [DOI] [PubMed] [Google Scholar]
- 2.Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int. J. Androl. 2010;33:333–345. doi: 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 3.Mendell MJ. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air. 2007;17:259–277. doi: 10.1111/j.1600-0668.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 4.Medina-Ramon M, Zock JP, Kogevinas M, Sunyer J, Torralba Y, Borrell A, Burgos F, Anto JM. Asthma, chronic bronchitis, and exposure to irritant agents in occupational domestic cleaning: a nested case-control study. Occup. Environ. Med. 2005;62:598–606. doi: 10.1136/oem.2004.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina-Ramon M, Zock JP, Kogevinas M, Sunyer J, Basagana X, Schwartz J, Burge PS, Moore V, Anto JM. Short-term respiratory effects of cleaning exposures in female domestic cleaners. Eur. Respir. J. 2006;27:1196–1203. doi: 10.1183/09031936.06.00085405. [DOI] [PubMed] [Google Scholar]
- 6.Wieslander G, Norback D, Edling C. Airway symptoms among house painters in relation to exposure to volatile organic compounds (VOCS)—a longitudinal study. Ann. Occup. Hyg. 1997;41:155–166. doi: 10.1016/S0003-4878(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 7.Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup. Environ. Med. 2001;58:649–655. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wieslander G, Norback D, Nordstrom K, Walinder R, Venge P. Nasal and ocular symptoms, tear film stability and biomarkers in nasal lavage, in relation to building-dampness and building design in hospitals. Int. Arch. Occup. Environ. Health. 1999;72:451–461. doi: 10.1007/s004200050398. [DOI] [PubMed] [Google Scholar]
- 9.Kogevinas M, Zock JP, Jarvis D, Kromhout H, Lillienberg L, Plana E, Radon K, Toren K, Alliksoo A, Benke G, Blanc PD, Dahlman-Hoglund A, D’Errico A, Hery M, Kennedy S, Kunzli N, Leynaert B, Mirabelli MC, Muniozguren N, Norback D, Olivieri M, Payo F, Villani S, Van Sprundel M, Urrutia I, Wieslander G, Sunyer J, Anto JM. Exposure to substances in the workplace and new-onset asthma: An international prospective population-based study (ECRHS-II) Lancet. 2007;370:336–341. doi: 10.1016/S0140-6736(07)61164-7. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek JM, Filios M, Willis R, Rosenman KD, Reilly MJ, McGreevy K, Schill DP, Valiante D, Pechter E, Davis L, Flattery J, Harrison R. Work-related asthma in the educational services industry: California, Massachusetts, Michigan, and New Jersey, 1993–2000. Am. J. Ind. Med. 2008;51:47–59. doi: 10.1002/ajim.20539. [DOI] [PubMed] [Google Scholar]
- 11.Obadia M, Liss GM, Lou W, Purdham J, Tarlo SM. Relationships between asthma and work exposures among non-domestic cleaners in Ontario. Am. J. Ind. Med. 2009;52:716–723. doi: 10.1002/ajim.20730. [DOI] [PubMed] [Google Scholar]
- 12.Zock J-P, Plana E, Jarvis D, Anto JM, Kromhout H, Kennedy SM, Kunzli N, Villani S, Olivieri M, Toren K, Radon K, Sunyer J, Dahlman-Hoglund A, Norback D, Kogevinas M. The use of household cleaning sprays and adult asthma: An international longitudinal study. Am. J. Respir. Crit. Care Med. 2007;176:735–741. doi: 10.1164/rccm.200612-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen GD, Larsen ST, Olsen O, Lovik M, Poulsen LK, Glue C, Wolkoff P. Do indoor chemicals promote development of airway allergy? Indoor Air. 2007;17:236–255. doi: 10.1111/j.1600-0668.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 14.Emmen HH, Muijser H, Arts JHE, Prinsen MK. Human volunteer study with PGME: Eye irritation during vapour exposure. Toxicol Lett. 2003:140–141. 249–259. doi: 10.1016/s0378-4274(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010;5:e13423. doi: 10.1371/journal.pone.0013423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer BC, Destaillats H, Hodgson AT, Nazaroff WW. Cleaning products and air fresheners: Emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air. 2006;16:179–191. doi: 10.1111/j.1600-0668.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Wieslander G, Norback D. Ocular symptoms, tear film stability, nasal patency, and biomarkers in nasal lavage in indoor painters in relation to emissions from water-based paint. Int. Arch. Occup. Environ. Health. 2010;83:733–741. doi: 10.1007/s00420-010-0552-0. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for 2-butoxyethanol and 2-butoxyethanol Acetate. Department of Health and Human Services, Public Health Service; Atlanta, GA, USA: 1998. [PubMed] [Google Scholar]
- 19.Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–751. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieslander G, Norback D, Venge P. Changes of symptoms, tear film stability and eosinophilic cationic protein in nasal lavage fluid after re-exposure to a damp office building with a history of flooding. Indoor Air. 2007;17:19–27. doi: 10.1111/j.1600-0668.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 21.Stewart RD, Baretta ED, Dodd HC, Torkelson TR. Experimental human exposure to vapor of propylene glycol monomethyl ether. Experimental human exposure. Arch. Environ. Health. 1970;20:218–223. doi: 10.1080/00039896.1970.10665577. [DOI] [PubMed] [Google Scholar]
- 22.Wieslander G, Norback D. A field study on clinical signs and symptoms in cleaners at floor polish removal and application in a Swedish hospital. Int. Arch. Occup. Environ. Health. 2010;83:585–591. doi: 10.1007/s00420-010-0531-5. [DOI] [PubMed] [Google Scholar]
- 23.Miller RR, Hermann EA, Young JT, Calhoun LL, Kastl PE. Propylene glycol monomethyl ether acetate (PGMEA) metabolism, disposition, and short-term vapor inhalation toxicity studies. Toxicol. Appl. Pharmacol. 1984;75:521–530. doi: 10.1016/0041-008x(84)90188-1. [DOI] [PubMed] [Google Scholar]
- 24.Ernstgård L, Lof A, Wieslander G, Norback D, Johanson G. Acute effects of some volatile organic compounds emitted from water-based paints. J. Occup. Environ. Med. 2007;49:880–889. doi: 10.1097/JOM.0b013e3181161ced. [DOI] [PubMed] [Google Scholar]
- 25.Wieslander G, Norback D, Bjornsson E, Janson C, Boman G. Asthma and the indoor environment: the significance of emission of formaldehyde and volatile organic compounds from newly painted indoor surfaces. Int. Arch. Occup. Environ. Health. 1997;69:115–124. doi: 10.1007/s004200050125. [DOI] [PubMed] [Google Scholar]
- 26.Jaakkola JJ, Oie L, Nafstad P, Botten G, Samuelsen SO, Magnus P. Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. Am. J. Public Health. 1999;89:188–192. doi: 10.2105/ajph.89.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaakkola JJK, Parise H, Kislitsin V, Lebedeva NI, Spengler JD. Asthma, wheezing, and allergies in Russian school children in relation to new surface materials in the Home. Am. J. Public Health. 2004;94:560–562. doi: 10.2105/ajph.94.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolkoff P, Schneider T, Kildesø J, Degerth R, Jaroszewski M, Schunk H. Risk in cleaning: chemical and physical exposure. Sci. Total Environ. 1998;215:135–156. doi: 10.1016/s0048-9697(98)00110-7. [DOI] [PubMed] [Google Scholar]
- 29.Järnström H, Saarela K, Kalliokoski P, Pasanen AL. Comparison of VOC and ammonia emissions from individual PVC materials, adhesives and from complete structures. Environ. Int. 2008;34:420–427. doi: 10.1016/j.envint.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, Hagerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ. Health Perspect. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenø JG, Wolkoff P, Clausen PA, Wilkins CK, Petersen T. Degradation of the adsorbent Tenax TA by nitrogen oxides, ozone, hydrogen peroxide, OH radical and limonene oxidation products. Environ. Sci. Technol. 2002;36:4121–4126. doi: 10.1021/es025680f. [DOI] [PubMed] [Google Scholar]
- 32.Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD. Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air. 2000;10:82–91. doi: 10.1034/j.1600-0668.2000.010002082.x. [DOI] [PubMed] [Google Scholar]
- 33.Engman L, Bornehag CG, Sundell J. How valid are parents’ questionnaire responses regarding building characteristics, mouldy odour, and signs of moisture problems in Swedish homes? Scand. J. Public Health. 2007;35:125–132. doi: 10.1080/14034940600975658. [DOI] [PubMed] [Google Scholar]
- 34.Bornehag CG, Sundell J, Sigsgaard T, Hagerhed-Engman L. Association between ventilation rates in 390 Swedish homes and allergic symptoms in children: A nested case control study. Indoor Air. 2005;15:275–280. doi: 10.1111/j.1600-0668.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 35.Nordtest method NT VVS 118. Nordtest, Finland: 1997. Nordtest, Ventilation: Local mean age of air—Homogenous emission techniques. [Google Scholar]
- 36.Bornehag CG, Sundell J, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L. Phthalates in indoor dust and their association with building characteristics. Environ. Health Perspect. 2005;113:1399–1404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CH, Feng CT, Lo YS, Lin TY, Lo JG. Determination of volatile organic compounds in workplace air by multisorbent adsorption/thermal desorption-GC/MS. Chemosphere. 2004;56:71–80. doi: 10.1016/j.chemosphere.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Helmig D. Artifact free preparation, storage and analysis of solid adsorbent sampling cartridges used in the analysis of volatile Organic compounds in air. J. Chromatogr. A. 1996;732:414–417. [Google Scholar]
- 39.Uhde E. Organic Indoor Air Pollutants: Occurrence-Measurement-Evaluation. Chapter 1 Wilhelm-Klauditz- Institut (WKl), Fraunhofer-Institut für Holzforschung; Braunschweig, Germany: 1999. Application of solid sorbents for the sampling of volatile organic compounds in indoor air. [Google Scholar]
- 40.Edwards RD, Jurvelina J, Saarela K, Jantunena M. VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLISHelsinki, Finland. Atmos. Environ. 2001;35:4531–4543. [Google Scholar]
- 41.Chou MS, Huang BJ, Chang HY. Degradation of gas-phase propylene glycol monomethyl ether acetate by ultraviolet/ozone process: A kinetic study. J. Air Waste Manage. Assoc. 2006;56:767–776. doi: 10.1080/10473289.2006.10464488. [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Spickett J, Lee AH, Rumchev K, Stick S. Household hygiene practices in relation to dampness at home and current wheezing and rhino-conjunctivitis among school age children. Pediatr. Allergy Immunol. 2005;16:587–592. doi: 10.1111/j.1399-3038.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 43.Bornehag CG, Sundell J, Hägerhed-Engman L, Sigsgaard T. Association between ventilation rates in 390 Swedish homes and allergic symptoms in children. Indoor Air. 2005;15:275–280. doi: 10.1111/j.1600-0668.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 44.Clausen PA, Lindeberg Bille RL, Nilsson T, Hansen V, Svensmark B, Bowadt S. Simultaneous extraction of di(2-ethylhexyl) phthalate and nonionic surfactants from house dust. Concentrations in floor dust from 15 Danish schools. J. Chromatogr A. 2003;986:179–190. doi: 10.1016/s0021-9673(02)02007-1. [DOI] [PubMed] [Google Scholar]
- 45.Bayer CW, Black MS, Galloway LM. Sampling and analysis techniques for trace volatile organic emissions from consumer products. J. Chromatogr. Sci. 1988;26:168–173. doi: 10.1093/chromsci/26.4.168. [DOI] [PubMed] [Google Scholar]
- 46.Kuntasal OO, Karman D, Wang D, Tuncel SG, Tuncel G. Determination of volatile organic compounds in different microenvironments by multibed adsorption and short-path thermal desorption followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. A. 2005;1099:43–54. doi: 10.1016/j.chroma.2005.08.093. [DOI] [PubMed] [Google Scholar]
- 47.Edwards RD, Jurvelin J, Saarela K, Jantunen M. VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland. Atmos. Environ. 2001;35:4531–4543. [Google Scholar]