Abstract
The photoreceptor phytochrome-A (phyA) regulates germination and seedling establishment by mediating very low fluence (VLFR) and far-red high irradiance (FR-HIR) responses in Arabidopsis thaliana. In darkness, phyA homodimers exist in the biologically inactive Pr form and are localized in the cytoplasm. Light induces formation of the biologically active Pfr form and subsequent rapid nuclear import. PhyA Pfr, in contrast to the Pr form, is labile and has a half-life of ∼30 min. We produced transgenic plants in a phyA-201 null background that express the PHYA–yellow fluorescent protein (YFP) or the PHYA686–YFP–dimerization domain (DD) and PHYA686–YFP–DD–nuclear localization signal (NLS) or PHYA686–YFP–DD–nuclear exclusion signal (NES) fusion proteins. The PHYA686–YFP fusion proteins contained the N-terminal domain of phyA (686 amino acid residues), a short DD and the YFP. Here we report that (i) PHYA686–YFP–DD fusion protein is imported into the nucleus in a light-dependent fashion; (ii) neither of the PHYA686 fusion proteins is functional in FR-HIR and nuclear VLFR; and (iii) the phyA-dependent, blue light-induced inhibition of hypocotyl growth is mediated by the PHYA686–YFP–DD–NES but not by the PHYA686–YFP–DD–NLS and PHYA686–YFP–DD fusion proteins. We demonstrate that (i) light induces degradation of all PHYA N-terminal-containing fusion proteins and (ii) these N-terminal domain-containing fusion proteins including the constitutively nuclear PHYA686–YFP–DD–NLS and predominantly cytoplasmic PHYA686–YFP–DD–NES degrade at comparable rates but markedly more slowly than PHYA–YFP, whereas (iii) light-induced degradation of the native phyA is faster compared with PHYA–YFP.
Keywords: High irradiation response, Light-induced degradation, Nuclear import, Phytochrome-A, Signaling, Very low fluence response
Introduction
Plants are sessile organisms that use the intensity, direction, duration and wavelength of light in the environment as cues to inform themselves of time and place. Light affects nearly every phase of the plant life cycle, and these light-triggered physiological processes are mediated by several groups of photoreceptors, including the phytochromes. Phytochromes are sensors of red (R) and far-red (FR) light that covalently bind an open-chain tetrapyrrole chromophore per molecule. The model plant Arabidopsis thaliana contains five genes (PHYA–PHYE) encoding the phytochrome apoprotein (Neff et al. 2000). Phytochrome-A (phyA) is a versatile photoreceptor that plays an important role in seedling establishment by mediating transition from heterotrophic to phototropic growth. PhyA mediates two types of photoresponses including very low fluence responses (VLFRs) and high irradiance responses (HIRs). It is worth noting that physiological responses frequently used to assess phyA functionality under laboratory conditions, including inhibition of hypocotyl growth, unfolding of the cotyledons, etc., exhibit both VLFR and HIR as two discrete phases of the response, where VLFR is saturated by infrequent R or FR light pulses and HIR requires very frequent or continuous FR (cFR) light at higher fluence rates (Casal et al. 2000).
Recent studies established that light-regulated translocation of phyA into the nucleus is a critical step of signaling cascades initiated by phyA. In darkness, the inactive Pr form of phyA accumulates in the cytoplasm, whereas the photo-converted Pfr form is rapidly imported into the nucleus (Kircher et al. 1999, Hisada et al. 2000, Kircher et al. 2002). It has been shown that nuclear import of phyA can be induced either by FR light pulses, leaving a pool in the cytoplasm (this is a VLFR), or by constant FR light (this is an HIR). PhyA does not contain a specific nuclear localization signal (NLS), and light-dependent accumulation of phyA in the nucleus depends on two specific plant proteins, namely FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and FHY1-LIKE (FHL) in planta (Hiltbrunner et al. 2005, Hiltbrunner et al. 2006, Genoud et al. 2008) and/or in a cell-free in vitro system (Pfeiffer et al. 2009). Characterization of the precise mechanism by which nuclear (Bae and Choi 2008) or cytosolic phyA Pfr (Rosler et al. 2007) initiates downstream signaling—underpinning physiological FR-HIR and VLFR—has attracted considerable interest in recent years.
A number of mutants have been identified that encode signaling components downstream of phyA and either specifically affect FR-HIR, including the eid1 (Dieterle et al. 2001), fhy3 (Wang and Deng 2002), pat1 (Bolle et al. 2000) and blh mutants (Staneloni et al. 2009), or influence both VLFR and FR-HIR such as the fhy1 (Desnos et al. 2001), hfr1, (Fairchild et al. 2000), spa1 (Hoecker et al. 1999), pks4 (Schepens et al. 2008), gi (Oliverio et al. 2007) and phyA-402 mutants (Muller et al. 2009). Very recently Kneissl et al. (2009) reported isolation of a new type of mutant designated owl that specifically affects nuclear VLFR.
A specific feature of phyA-controlled VLFR and HIR signaling is that the biologically active phyA Pfr is labile and is rapidly degraded. Degradation starts immediately after the onset of light (photo-conversion); thus it is assumed that the Pfr-specific proteolytic machinery is pre-existent and can become active right at the moment when the Pfr form of phyA appears (Vierstra 1994). Degradation of PHYA Pfr can occur both in the nucleus (Seo et al. 2004) and in the cytosol (Speth et al. 1987). Very recently, Debrieux et al. (2010) reported that phyA is degraded by a proteosome-dependent mechanism in both the cytoplasm and the nucleus. The same authors also showed that (i) phyA degradation is slower in the cytoplasm than in the nucleus and that (ii) phyA is degraded even in its Pr form in the nucleus. These data suggest that light-induced degradation of phyA is partially regulated by the light-induced import into the nucleus where the turnover of the photoreceptor is higher. It is evident that rapid degradation of phyA Pfr is a critical factor for phyA-mediated VLFR and FR-HIR signaling, yet so far only two PHYA-interacting proteins have been described that directly modulate the concentration of phyA Pfr. COP1 has been shown to bind to both the Pr and the Pfr forms of PHYA, and circumstantial evidence indicates that it decreases PHYA signaling by decreasing the total PHYA concentration (Seo et al. 2004). The same authors showed that COP1, acting as a multifunctional E3 ligase, mediates ubiquitination of the PHYA apoprotein in vitro (Seo et al. 2004). PAPP5 encodes a phosphatase that was shown to bind to both PHYA and PHYB and preferentially to dephosphorylate their Pfr forms (Ryu et al. 2005). The dephosphorylation of PHYA by PAPP5 increases the stability of the PHYA Pfr form, thus suggesting that PAPP5 is a versatile regulator that also enhances phyA output activity.
Cherry et al. (1993) showed that the removal of the C-terminal domain eliminates the biological activity of PhyA in transgenic tobacco, whereas Mateos et al. (2006) reported that a fusion protein of constitutive nuclear localization, containing the 595 amino acid N-terminal fragment of oat phyA fused to the β-glucuronidase (GUS) reporter, is light stable and is capable of signaling in VLFR but not in FR-HIR mode in transgenic Arabidopsis. Here we report the analysis of transgenic phyA-201 mutant plants that express the Arabidopsis phyA686 N-terminal fragment as a homodimer in different cellular compartments. We show that this Arabidopsis phyA N-terminal fragment, similarly to the native phyA, is imported into the nucleus in a light-dependent fashion, yet neither of these N-terminal fusion proteins is capable of complementing the phyA-201 mutant and restoring FR-HIR or nuclear VLFR types of signaling. In addition, we demonstrate that (i) the PHYA–yellow fluorescent protein (YFP) fusion protein degrades more slowly than the native phyA and that (ii) deletion of the C-terminal domain of phyA compromises light-induced degradation of phyA in a compartmentalization-independent fashion. Interestingly, we found that the dominantly cytoplasm localized PHYA686–YFP–dimerization domain (DD)–nuclear exclusion signal (NES) fusion protein, in contrast to the constitutively nuclear PHYA686–YFP–DD–nuclear localization signal (NLS), is capable of restoring phyA-mediated blue (B) light-induced growth inhibition, a response that is considered to be independent of FHY1/FHL.
Results
To assess the regulatory role of the phyA N-terminal region in the VLFR and HIR type of signaling, we constructed two sets of chimeric gene constructs. First we amplified a 686 amino acid fragment containing the phyA N-terminal fragment that does not contain the Quail box shown to be involved in mediating phyA and phyB signaling (Wagner et al. 1995), but carries all the domains which are necessary for the function of phyB in the nucleus (Matsushita et al. 2003). Next, we constructed a chimeric gene encoding a fusion protein consisting of the PHYA N-terminal fragment followed by the YFP and a short (46 amino acids) DD of the transcription factor CPRF as described in Pfeiffer et al. (2009). To ensure targeted subcellular localization, we produced additional chimeric genes encoding the PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES fusion proteins. Next we produced transgenic plants in the phyA-201 mutant background expressing these fusion proteins and the full-length phyA protein fused to YFP. Expression of the transgenes was under the control of the native PHYA promoter localized on a 2.5 kb fragment. Schematic illustration of the domain structure of PHYA and the fusion proteins used in this study is shown in Fig.1A. We regenerated about 10 independent transgenic lines for each construct. The selected lines were bred to homozygocity and multiplied to facilitate functional characterization of the various fusion proteins for VLFR and FR-HIR signaling.
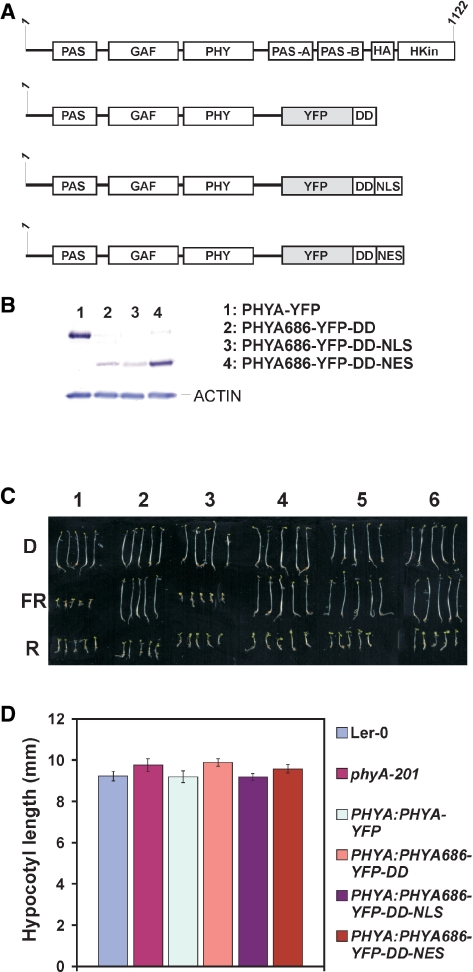
Fig. 1.
(A) Schematic illustration of the domain structure of the native Arabidopsis phyA protein and chimeric phyA N-terminal fusion proteins used in this study. The fusion proteins contain the PAS/GAF/PHY domains (amino acids 1–686) of phyA. YFP, yellow fluorescent protein; DD, 46 amino acid dimerization domain of the transcription factor CPRF; NLS, nuclear localization signal; NES, nuclear exclusion signal. (B) Expression levels of various phyA fusion proteins in homozygous seedlings used in this study. A 30 μg aliquot of total protein extracts of 4-day-old etiolated seedlings was separated by SDS–PAGE. Fusion proteins were detected by using a GFP-specific antiserum. (C) Morphology of (1) Ler wild-type, (2) phyA-201, (3) PHYA:PHYA-YFP, (4) PHYA:PHYA686-YFP-DD, (5) PHYA:PHYA686-YFP-DD-NLS, (6) PHYA:PHYA686-YFP-DD-NES 4-day-old seedlings grown in darkness (D) or irradiated with constant red light, 50 μmol m−2 s−1 (R) or far-red light, 9 μmol m−2 s−1 (FR). (D) Average hypocotyl length of 4-day-old dark-grown seedlings expressing various phyA fusion proteins.
The PHYA686–YFP fusion proteins do not initiate FR-HIR signaling
Before starting functional characterization, we determined the expression levels of the various fusion proteins in each of the homozygous transgenic lines by Western blot hybridization. After analyzing total protein extracts prepared from etiolated seedlings we chose lines that expressed these various fusion proteins (PHYA–YFP, PHYA686–YFP–DD, PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES) at the most comparable levels (Fig. 1B). We note that (i) the expression level of PHYA686–YFP–DD and PHYA686–YFP–DD–NLS fusion proteins was constantly lower (about 5- to 10-fold) in all tested lines when compared with PHYA–YFP and PHYA686–YFP–DD–NES (Supplementary Fig. S1) and that (ii) the expression level of the PHYA–YFP was sufficiently high to fully complement the phyA-201 mutant, in all aspects tested. These selected lines were then subjected to detailed physiological, microscopic and molecular analysis. First, we measured inhibition of hypocotyl elongation and cotyledon size and unfolding in transgenic seedlings grown in darkness or under different intensities of cFR and R light. Fig. 1C and D demonstrate that etiolated seedlings expressing the various fusion proteins exhibited normal skotomorphogenesis. We found that the hypocotyl elongation and cotyledon size of PHYA686–YFP–DD-, PHYA686–YFP–DD–NLS- or PHYA686–YFP–DD–NES-expressing seedlings were not affected by cFR light (Fig. 2A, B) but showed normal hypocotyl growth inhibition in continuous R light (Supplementary Fig. S2). The phenotype of these transgenic seedlings was identical to that of the phyA-201 mutant, whereas the expression of PHYA:PHYA–YFP resulted in a complete complementation of the phyA-deficient mutant in cFR light. We used the same transgenic seedlings to measure the inducibility of a selected group of genes that had been shown to respond to cFR light treatment by Teppermann et al. (2004). Supplementary Fig. S3 shows that treatment of the seedlings expressing the PHYA686 N-terminal domain with 2 h cFR light did not increase the expression of the LHY1 and PRR9 genes above threshold levels. Taken together these data suggest that neither of the PHYA686–YFP–DD-containing fusion proteins is functional in FR-HIR.
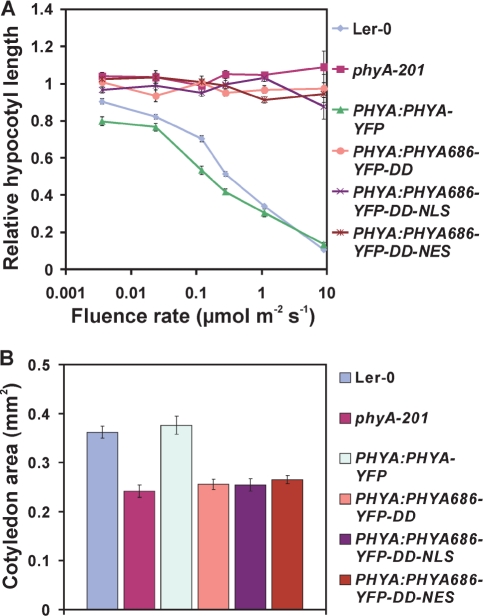
Fig. 2.
PHYA686–YFP–DD-, PHYA686–YFP–DD–NLS- and PHYA686–YFP–DD–NES-expressing transgenic seedlings do not respond to cFR irradiation. (A) Fluence rate response curves for inhibition of hypocotyl elongation. (B) Cotyledon areas of 4-day-old seedlings grown in cFR light (9 μmol m−2 s−1).
The PHYA686–YFP–DD fusion proteins do not induce nuclear VLFR signaling
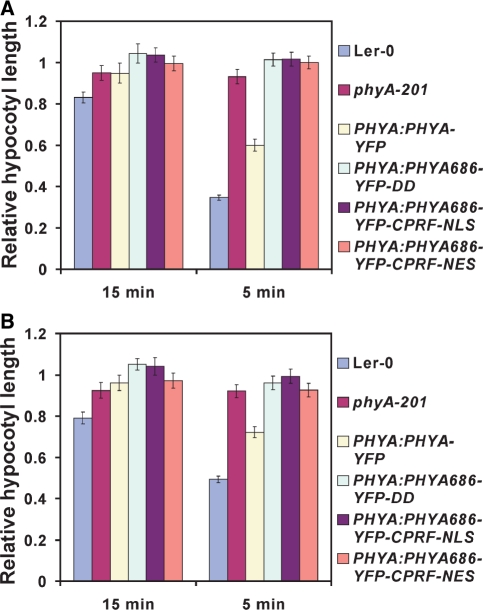
The phyA VLFR mode of signaling has been implicated in regulating several aspects of seedling development, including abrogation of gravitropism (Poppe et al. 1997) and inhibition of hypocotyl elongation by repeated R and/or FR pulses (Casal et al. 2000). First, we characterized a frequently used nuclear VLFR, namely the phyA-mediated inhibition of hypocotyl elongation in response to repeated pulses of FR light. We found that none of the transgenic seedlings expressing PHYA686–YFP–DD responded to cyclic FR pulses (2 or 10 μmol s−1 m−2) given after various time (5 or 15 min) intervals of darkness, whereas the expression of PHYA:PHYA–YFP resulted in full complementation of the phyA-201 mutant (Fig. 3A, B). Next, we measured phyA-suppressed gravitropism of transgenic seedlings in cFR light. Supplementary Fig. S4 illustrates randomization of hypocotyl growth orientation by FR light in the wild type, phyA-201 and various transgenic lines. This figure shows that phyA-201 exhibited complete insensitivity toward FR light abrogation of gravitropism, whereas transgenic lines expressing the full-length PHYA–YFP fusion protein displayed sensitivity similar to that of the wild type. All other transgenic lines expressing the PHYA686–YFP–DD, PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES fusion proteins showed a phenotype similar to that of the phyA-201 mutant, suggesting that these PHYA N-terminal fragments are also insufficient to mediate cFR light-induced gravitropism. In addition to these physiological responses, the ability of PHYA686–YFP fusion proteins to induce VLFR at the level of gene expression was also determined. It was found that irradiation of the various PHYA 686–YFP–DD-, PHYA686–YFP–DD–NLS- and PHYA686–YFP–DD–NES-expressing lines with pulses of R light that yield 0.3, 0.6 and 3.7% phyA Pfr are not sufficient to bring about detectable increases in the abundance of PRR9 (Fig. 4A) and CAB2 mRNA (Fig. 4B). In contrast, the same treatment was clearly efficient in elevating the expression of PRR9 and CAB2 above threshold levels in the wild-type or phyA-201 lines expressing the full-length PHYA–YFP fusion proteins.
Fig. 3.
PHYA686–YFP–DD-, PHYA686–YFP–DD–NLS- and PHYA686–YFP–DD–NES-expressing transgenic seedlings are insensitive to repeated pulses of 2.5 min of FR light followed by 5 or 15 min of darkness given for 3 d. Relative hypocotyl lengths of seedlings grown under repeated FR pulses of (A) (10 μmol m−2 s−1 or (B) 2 μmol m−2 s−1) are shown. Values were normalized to the hypocotyl length of the corresponding dark-grown seedlings.
Fig. 4.
Expression of PRR9 (A) and CAB2 (B) is not induced by short R light pulses in PHYA686–YFP–DD-, PHYA686–YFP–DD–NLS- and PHYA686–YFP–DD–NES-expressing transgenic seedlings. Four-day-old etiolated seedlings were treated with 1 min R light pulses to generate 0.3, 0.6 and 3.7% phyA Pfr. After the 1 min light treatment the seedlings were transferred to darkness. Samples for RNA extraction were collected 60 min after the R light treatment. Induction levels were normalized to the corresponding dark levels; the fold induction is shown.
The PHYA686–YFP–DD–NES fusion protein is functional in cytoplasmic VLFR signaling
Beside the above-described VLFRs that are thought to be mediated by phyA localized in the nucleus, we also characterized these transgenic lines for hypocotyl growth inhibition in continuous blue (cB) light. According to Rosler et al. (2007), this response is independent of FHY1/FHL, thus it is likely to be regulated by cytoplasmic phyA. Fig. 5 shows that the phenotype of wild-type and phyA-201 seedlings is indeed different in cB light and that the PHYA 686–YFP–DD–NES transgenic seedlings exhibited a phenotype similar to the wild type. In contrast, the same figure shows that the PHYA686–YFP–DD and PHYA686–YFP–DD–NLS seedlings did not respond to cB light. These data suggest that the PHYA686 N-terminal fragment constitutively localized in the cytoplasm is capable, at least partially, of mediating B light-induced phyA-regulated signaling.
Fig. 5.
Constitutively cytoplasmic PHYA686–YFP–DD–NES fusion protein complements B light-induced inhibition of hypoctyl growth of the phyA-201 mutant. Fluence rates of applied B light and relative hypocotyl lengths are shown. Values were normalized to the hypocotyl length of the corresponding dark-grown seedlings.
Nucleo/cytosolic partitioning and degradation of PHYA686–YFP–DD fusion protein is light dependent
The majority of phyA-controlled physiological responses, with a few exceptions described by Rosler et al. (2007), are mediated by phyA localized in the nucleus. The nuclear import of phyA requires FHY1/FHL and shows a characteristic dependence on the light quality and quantity, indicating that nuclear import of phyA itself can be classified as VLFR and/or FR-HIR (Kim et al. 2000). To test whether nucleo/cytoplasmic partitioning of the PHYA686 N-terminal fragments is still subject to light regulation, we monitored the cellular distribution of these fusion proteins in seedlings exposed to various light treatments. We found, as expected, that the PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES fusion proteins were constitutively localized in the nucleus and in the cytoplasm, respectively, in both etiolated and light-grown/treated cells (Fig. 6A, B). This figure, however, also illustrates that the cellular distribution of the PHYA686–YFP–DD fusion proteins, similarly to that of PHYA–YFP, is controlled by light. In etiolated seedlings the abundance of these fusion proteins in the nuclei is below the level of detection, whereas short R light pulses and/or cFR light induce rapid nuclear translocation of these chimeric photoreceptors. In addition, we found that, in contrast to PHYA–YFP seedlings (Kircher et al. 1999), irradiation of cFR light-pre-treated PHYA686–YFP–DD, PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES seedlings with R light pulses failed to induce the formation of PHYA-containing nuclear bodies (NBs) in the nucleus (Supplementary Fig. S5) and/or SAPs (sequestered areas of phytochromes) in the cytoplasm (Supplementary Fig. S6). On the other hand, Fig. 6A and B also shows that irradiation of seedlings with 24 h R light lowered the fluorescence of all types of PHYA–YFP fusion proteins below the level of detection, thereby indicating that the stability/degradation of the Pfr conformers of these chimeric PHYA photoreceptors is regulated by light.
Fig. 6.
Nucleo/cytoplasmic distribution of the PHYA686–YFP–DD fusion protein is regulated by light. (A) The cellular distribution of (1) PHYA–YFP, (2) PHYA686–YFP–DD–NLS, (3) PHYA686–YFP–DD–NES and (4) PHYA686–YFP–DD fusion proteins is shown in darkness (D), after a 3 min R light pulse (Rp) and after 24 h FR light treatment (13 μmol m−2 s−1). (B) The cellular distribution of (1) PHYA–YFP, (2) PHYA686–YFP–DD–NLS, (3) PHYA686–YFP–DD–NES and (4) PHYA686–YFP–DD fusion proteins is shown in darkness (D) and after irradiation with 1 h and 24 h R light (12 μmol m−2 s−1) respectively. Nuclear speckles (nusp), nucleus (nu), sequestered area of phytochrome (saps), scale bar 10 μm.
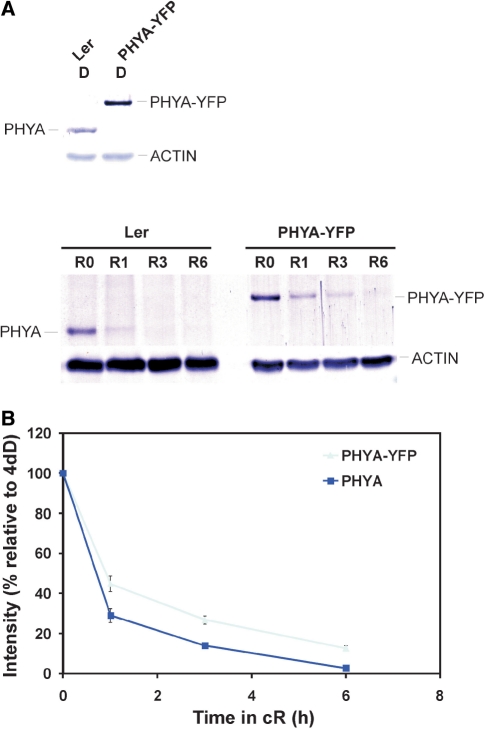
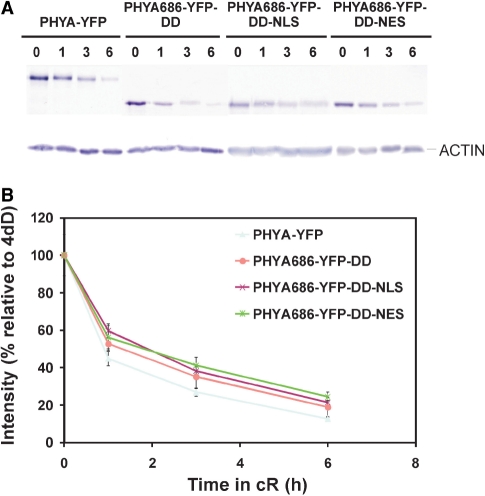
To assess degradation of these PHYA fusion proteins in more detail, we performed a series of Western blot hybridization experiments. First, we compared the kinetics of R light-induced degradation of native phyA and the PHYA–YFP fusion protein (Fig. 7). Our results demonstrate that irradiation with 6 h of R light lowered the level of the native phyA below the level of detection, whereas the PHYA–YFP fusion protein was still clearly visible (Fig. 7A). Accordingly, quantification of the Western blot experiments showed that residual levels of the PHYA–YFP fusion protein were about twice as high as that of the native phyA after 1, 3 and 6 h irradiation with R light (Fig. 7B). Next, we determined whether removal of the C-terminal domain and compartmentalization has any effect on R light-induced degradation of the PHYA686–YFP–DD, PHYA686–YFP–DD–NLS and PHYA686–YFP–DD–NES fusion proteins. Fig. 8 illustrates that R light-induced degradation of these truncated fusion proteins did not differ significantly, but was slower compared with that of PHYA–YFP. Taken together these data indicate that (i) degradation of the PHYA Pfr conformer is largely but not exclusively mediated by the N-terminal domain of PHYA and that (ii) degradation of the phyA N-terminal domain containing fusion proteins in the nucleus and cytoplasm follows a nearly identical pattern.
Fig. 7.
(A) Expression levels of PHYA and PHYA–YFP in 4-day-old etiolated seedlings or in seedlings exposed for 0, 1, 3 and 6 h to R light (12 μmol m−2 s−1). Loading was normalized to obtain identical protein levels in darkness. Total protein extracts (30 μg from Ler and 15 μg from a PHYA–YFP transgenic line) were separated by SDS–PAGE and blotted onto a PVDF membrane. PHYA proteins were detected by using a PHYA-specific polyclonal phyA antiserum. (B) Quantification of PHYA levels in R light. Results are expressed relative to the dark levels of both lines (set to 1).
Fig. 8.
R light-induced degradation of the various PHYA686–YFP N-terminal and full-length PHYA–YFP fusion proteins. (A) Expression levels of full-length PHYA–YFP and PHYA686–YFP N-terminal fusion proteins in 4-day-old seedlings exposed for 0, 1, 3 and 6 h to R light (12 μmol m−2 s−1). Total protein extracts were separetad by SDS–PAGE and blotted onto a PVDF membrane. Fusion proteins were detected using a GFP antiserum. Loading was normalized to obtain identical protein levels in darkness. Lanes contain PHYA–YFP (12 μg), PHYA686–YFP–DD (40 μg), PHYA686–YFP–DD–NLS (60 μg) and PHYA686–YFP–DD–NES (15 μg) total protein extracts. (B) Quantification of PHYA levels in R light. Results are expressed relative to the dark levels of each genotype (set to 1).
Discussion
PHYA is the prominent R/FR light-absorbing photoreceptor that mediates the establishment of young emerging seedlings. PHYA initiates VLFR- and FR-HIR-type signaling, and the characterization of molecular components and events underpinning these signaling modes has been the subject of extensive investigation. It is generally agreed that (i) rapid light-induced nuclear import of phyA is an early, critical step to launch phyA-controlled VLFR and FR-HIR modes of signaling (Kircher et al. 1999, Hisada et al. 2000) and that (ii) nuclear import of PHYA is dependent on FHY1/FHL and is mediated by the conformer- (Pfr > Pr) dependent interaction of these proteins (Hiltbrunner et al. 2005, Hiltbrunner et al. 2006, Pfeiffer et al. 2009). Based on the fact that a PHYA construct constitutively localized in the nucleus (PHYA–NLS–YFP) fully complemented the phenotype of the fhy1/fhl RNAi mutant, Genoud et al. (2008) concluded that the FHY1/FHL proteins do not play a significant role in directly regulating PHYA degradation and/or PHYA signaling. However, more recent data (Saijo et al. 2008, Shen et al. 2009, Yang et al. 2009) indicate that the function of FHY1/FHL in mediating PHYA signaling may not be fully understood. Independently of the precise molecular function of FHY1/FHL in phyA-controlled responses, here we show that a 686 amino acid N-terminal fragment of PHYA containing the GAF/PHY domains is imported into the nuclei of transgenic seedlings in a light-regulated fashion. Our data demonstrate that (i) accumulation of this truncated PHYA in the nuclei of etiolated seedlings is below the level of detection and (ii) nuclear translocation is induced by either short R light pulses or cFR light. However, unlike full-length PHYA–YFP, the truncated PHYA fusion proteins do not associate with NBs (Supplementary Fig. S5) or with SAPs (Supplementary Fig. S6) under any conditions tested. This observation indicates that formation of SAPs, first described by Speth and Schäfer (1987), may not be essential for light-induced degradation of PHYA Pfr under the conditions tested. We show that despite being imported into the nucleus in a light-regulated fashion, none of the Arabidopsis PHYA686–YFP–DD fusion proteins is functional in initiating nuclear VLFR (hypocotyl shortening after multiple FR pulses, FR-induced randomization of hypocotyl orientation, induction of gene expression after R pulses) or FR-HIR (hypocotyl growth inhibition in cFR light, cotyledon size); thus, they do not even partially complement the phyA-201 mutant. We therefore conclude that, in contrast to PHYB (Matsushita et al. 2003, Oka et al. 2004), the C-terminal domain of PHYA does not play a significant role in regulating nuclear translocation of the photoreceptor in cFR light but it is absolutely required for initiating signaling in the nucleus. These data extend and confirm earlier data reported by Cherry et al. (1993). The precise mechanism by which the C-terminal domains of phyA and B contribute to signaling is far from being understood (for a comprehensive review see Schäfer and Nagy 2006). It has been shown by Ni et al. (1999) that PIF3 binds strongly to photoactivated full-length phytochrome B but also moderately to its N-terminal domain. In contrast, this binding was only demonstrated for the phyA C-terminal region. The difference in the biological activity of the phyB and phyA N-terminal domain might be explained by the differential binding requirements of the known basic helix–loop–helix (bHLH) signaling components. (Ni et al. 1998, Ni et al. 1999, Shen et al. 2008). However, to the best of our knowledge here we provide the first evidence that nuclear translocation of and nuclear HIR and VLFR signaling launched by phyA require different domains of this photoreceptor.
Mateos et al. (2006) reported that a truncated oat PHYA fusion protein constitutively localized in the nucleus (PHYA1–595–YFP–GUS–NLS) showed physiological activity in darkness and was functional in inducing VLFR but not FR-HIR signaling in transgenic Arabidopsis phyA-201 seedlings. We found that (i) none of the analyzed transgenic PHYA686–YFP–DD–NLS lines exhibited any partially de-etiolated phenotype and that (ii) in the majority of these lines the expression level of the PHYA686–YFP–DD–NLS fusion protein was about 5-fold reduced compared with PHYA–YFP (Fig. 1B). At this abundance level the PHYA–YFP fusion protein has fully complemented the phyA-201 mutant in all VLFR assays performed. More specifically, we showed that an R light pulse converting 0.3% Pr into Pfr of PHYA was sufficient to induce a significant increase in PRR9 mRNA accumulation (Fig. 4), whereas in the case of PHYA686–YFP–DD–NLS an R light pulse resulting in >10% Pfr was still ineffective (Supplementary Fig. S7). These data clearly demonstrate that the expression levels of PHYA686–YFP–DD and PHYA686–YFP–DD–NLS fusion proteins were sufficiently high to induce PRR9 induction if they are active. However, we cannot exclude that higher expression levels of PHYA686–YFP–DD–NLS are required to cause some other type of VLFRs, such as hypocotyl growth inhibition, selectively.
Irrespective of nuclear VLFR, here we provide evidence that the PHYA686–YFP–DD–NES but not the PHYA686–YFP–DD or PHYA686–YFP–DD–NLS fusion proteins can complement B light-induced phyA-mediated hypocotyl growth inhibition of phyA-201. This response has been shown to be independent of FHY1/FHL (Rosler et al. 2007), thus it is thought to be mediated by PHYA Pfr localized in the cytoplasm. Thus our data suggest that the C-terminal domain of phyA is irrelevant for regulating PHYA signaling launched in the cytoplasm. We note that (i) the expression level of the PHYA686–YFP–DD–NES is about 5-fold higher than that of the other two PHYA N-terminal fragment-containing fusion proteins and that (ii) the PHYA686–YFP–DD–NES fusion protein is predominantly but not exclusively localized in the cytoplasm. Thus it is possible that elevated levels of the PHYA686–YFP–DD could also result in complementing B light-induced phyA-mediated hypocotyl growth inhibition of phyA-201. The molecular mechanism by which phyA and the predominantly cytoplasm localized PHYA686–YFP–DD–NES protein from the cytoplasm could integrate B and FR light signaling cascades in the nucleus is unknown. Whether this requires direct interaction of the PHYA N-terminal region with CRY1/CRY2 (Ahmad et al. 1998) or is mediated by cytoplasmic components involved in both CRY and PHYA signaling such as SUB1 (Guo et al. 2001) remains to be determined.
Rapid light-induced degradation of the PHYA Pfr form is an important aspect of attenuating and/or terminating PHYA-controlled nuclear VLFR and FR-HIR signaling. Recently, Seo et al. (2004) reported that COP1 acts as an E3 ligase to regulate phyA signaling by targeting elimination of the phyA itself. The same authors showed that the COP1 WD40 domain interacts with the PAS domain (591–850 amino acids) of phyA in yeast, COP1 ubiquitinates Pr and Pfr forms of phyA in vitro and the destruction rate of phyA is decreased in cop1 mutants. In addition, very recently, Debrieux et al. (2010) documented that phyA is degraded in both the cytoplasm and the nucleus and that degradation of phyA is slower in the cytoplasm. The same authors showed that phyA is degraded in the nucleus even in its inactive Pr form thereby preventing accumulation of nuclear phyA in darkness. Taken together, these data indicate that light-induced degradation of phyA is in part controlled by the light-regulated import into the nucleus where the turnover is faster. We found that (i) R light did induce degradation of all PHYA 686–YFP–DD fusion proteins used in this study, independent of their cellular localization, but (ii) degradation of these fusion proteins was somewhat slower than the full-length PHYA–YFP. These observations suggest that degradation of Arabidopsis PHYA either in the cytoplasm or in the nucleus is partly mediated by the N-terminal domain but accelerated degradation in the nucleus requires the PAS and HKRD domains located in the C-terminal half of the protein. Mateos et al. (2006) showed that R light-induced degradation of the oat PHYA595–YFP–GUS–NLS fusion protein is completely blocked in transgenic Arabidopsis seedlings. The apparent contradiction between our data and those reported by Mateos et al. (2006) might be explained as follows: (i) unlike the oat PHYA1–595 fragment, the Arabidopsis PHYA686 fragment still contains a small part of the PAS-A domain, and thus it is plausible to assume that it still can interact with and be ubiquitinated by COP1 and (ii) it is not known, in contrast to YFP, to what extent the addition of the GUS tag might change the stability of the fusion protein. In this respect we note that we determined the effect of the frequently used YFP reporter in altering the degradation kinetics of the phyA protein. Our data show that the PHYA–YFP fusion proteins degrades somewhat more slowly than the native phyA photoreceptor, but degradation of the fusion protein is still clearly fast and light-induced. Quantification of the degradation kinetics of the native phyA and PHYA–YFP fusion protein showed that addition of the YFP tag does not dramatically change the accumulation and degradation pattern of the biologically active fusion protein.
Taken together, our data demonstrate the PHYA686 N-terminal fragment is imported into the nucleus in a light-dependent fashion and that the presence of the C-terminal domain is absolutely required for VLFR and HIR signaling. Thus it is evident that this 686 amino acid-containing phyA fragment, or a shorter version of it, can easily be engineered to function as a light regulatory switch for any signaling pathways by fusing it to modified regulatory proteins (i.e. transcription factors in which the NLS is mutated) that are otherwise constitutively localized to the nucleus. Expression of these chimeric fusion proteins in custom-designed transgenic plants will provide a unique tool to engineer novel light-regulated pathways controlling various developmental or stress-induced pathways.
Materials and Methods
Cloning of the constructs
The native PHYA promoter and the full-length PHYA cDNA fragment were cut out from the PHYA:PHYA-GFP pPCV plasmid (Kim et al. 2000) and inserted into the YFP–NOS terminator carrying pPCV.
To create the N-terminal PHYA cDNA fusions, first we generated modified versions of the pPCV812 binary vector that contained either NLS or NES sequences. The DNA fragment containing the SV40 NLS (Kalderon et al. 1984) was obtained by annealing two oligonucleotides (5′-CAAGCTTCCTAAGAAGAAGAGAAAGGTTGGAGGATAGGAGCT-3′ and 5′-CCTATCCTCCAACCTTTCTCTTCTTCTTAGGAAGCTTGAGCT-3′) encoding the amino acid residues LPKKKRKVGG (stop). NES from PKI (Wen et al. 1995) was generated by annealing the oligonucleotides 5′-CAAGCTTAACGAGCTTGCTCTTAAGTTGGCTGGACTTGATATTAACAAGACTGGAGGATAGGAGCT-3′ and 5′-CCTATCCTCCAGTCTTGTTAATATCAAGTCCAGCCAACTTAAGAGCAAGCTCGTTAAGCTTGAGCT-3′. The encoded amino acid residues are: LALKLAGLDINKTGG (stop).
The YFP-DD-NLS/NES pPCV plasmid vectors were created as described by Pfeiffer et al. (2009). To obtain the vector without an NLS/NES signal, we PCR-amplified the DD with a STOP codon at its 3′end and replaced the DD–NLS sequences with this fragment. First the native PHYA promoter was inserted into this set of pPCV plasmids. The cDNA fragment encoding the 686 amino acid N-terminal domain of Arabidopsis PHYA was amplified by PCR and cloned into the PHYA:YFP-DD-NLS/NES or PHYA:YFP-DD vectors. The final constructs were verified by sequencing and introduced into Agrobacterium tumefaciens GV3101.
Plant transformation and regeneration of transgenic lines
Arabidopsis plants were transformed by the Agrobacterium-mediated floral dip method (Clough and Bent 1998). The chimeric constructs were transformed into the phyA-201 mutant. From each of these transformations primary transgenic plants expressing the fusion proteins were selected by their resistance to hygromycin and grown to maturation in the greenhouse. Multiple independent homozygous lines expressing one copy of the transgene were selected for further analysis.
Seedling and plant growth conditions and growth measurements
For hypocotyl length and cotyledon area measurements, seeds were sown on four layers of filter paper and imbibed in water for 48 h at 4°C. Cold-treated seeds were next irradiated with white light for 3 h at 22°C to induce seed germination and transferred to darkness for an additional 18 h at 22°C. The plates were subsequently placed under various light conditions for 4 d, as specified in the figure legends, and seedlings were laid on the surface of agar medium. Images of scanned plates were analyzed using MetaMorph Software (Universal Imaging). Hypocotyl lengths at different fluences of light were normalized to the corresponding dark-grown hypocotyl length to reflect solely the light-dependent regulation. A total of 25–30 seedlings were used for each line and each experiment, and experiments were repeated three times. Seedlings for the repeated FR pulse treatment were grown as described for microscopy, whereas the pulse treatments were performed as described in Büche et al. (2000) (light sources, SNAP-LITE LED lighting system; maximal spectral outputs, B 470 nm, R 670 nm, FR 735 nm.)
Angle of the hypocotyl
To determine gravitropism in FR light, seeds were surface sterilized and sown on Murashige and Skoog medium (MS) without sucrose along a line in square Petri dishes, then stratified at 4°C in the dark for 3 d, exposed to white light for 3 h to induce germination and returned to darkness at 22°C for 18 h. The dishes were next placed vertically while illuminated perpendicularly (from one side) by 5 μmol m−2 s−1 FR light. Images of scanned plates were analyzed using MetaMorph Software (Universal Imaging). The experiments were performed at least three times to calculate averages and standard error.
Quantitative reverse transcription–PCR (RT–PCR)
Seeds were surface sterilized and plated on half-strength MS with 1% sucrose, stratified at 4°C in the dark for 3 d, exposed to white light for 3 h to induce germination and returned to darkness at 22°C. Light induction was carried out on the fourth day and samples were harvested as described in the figure legends. RNA samples were prepared from whole seedlings using RNeasy miniprep kits (Qiagen) according to the manufacturer's instructions, and DNA was removed by DNase I treatment. cDNA was synthesized from 1 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas). RT–PCR was run for 40 cycles. Real-time RT–PCR analysis was carried out with a 7500 Real-Time PCR System with a SYBR Green JumpStart Taq ReadyMix (Sigma). The expression of PRR9 (At2g46790), CAB2 (At1g29920) and YFP was normalized to the expression of Tub2/3 (At5g62690). The primer sequences were: PRR9 RT-Fwd, CCTTCTCAAGATTTGAGGAAAGC; PRR9 RT-Rev, TTTGGCTCACCTGAAGTACTCTC; CAB2 RT-Fwd, ACAATGGCTCTCTCCTCCCC; CAB2 RT-Rev, GGAAAGGTTCACAGCCTTTCC; YFP RT-Fwd, CACAAGCTGGAGTACAACTA; YFP RT-Rev, GATCTTGAAGTTCACCTTGATG; TUB2/3 RT-Fwd, CCAGCTTTGGTGATTTGAAC; and TUB2/3 RT-Rev, CAAGCTTTCGGAGGTCAGAG. The experiments were performed at least three times to calculate averages and standard error.
Microscopy
The seeds were sown on four layers of wet filter papers and imbibed for 2 d at 4°C in the dark. Germination was induced by 6 h irradiation with R light. After the germination induction, seedlings were transferred back to darkness and incubated at 25°C either 16 h prior to FR pulse treatment or 4 d prior to light treatments for microscopy. For induction of germination, light treatment for degradation kinetics and localization studies at the microscope in R light, a standard R light field was used (12 μmol m−2 s−1 656 nm, 24 nm full width at half-maximum). For microscopic localization studies after FR light treatment, a standard FR light field (13 μmol m−2 s−1, 730 nm, 128 nm full width at half-maximum) was used. Prior to microscopy, for white light pulses the microscope lamp was used, for R light pulses red light filter KG65 (Schott, Germany) was placed onto the microscope lamp, and for FR pulse treatments, DAL filters with a transmission maximum of 715 nm were used (Schott). Microscopic analysis was performed by using an Axioskop microscope (Zeiss) with an Axiocam camera system (Zeiss) and green fluorescent protein (GFP)-, YFP- and cyan fluorescent protein (CFP)-specific filter sets (AHF Analysentechnik).
Western blot hybridization
For the immunoblot analysis, 4-day-old dark-grown seedlings were exposed to R light and harvested at different time points. Denaturing protein extraction was performed by homogenization in a potter with boiling SDS sample buffer [50 mM Tris–HCl (pH 6.8), 7% SDS, 4 M urea, 10% glycerol, 10 mM dithiothreitol (DTT), 0.05% bromphenol blue]. The protein samples were separated by SDS–PAGE and blotted onto a PVDF membrane. For detection of the PHYA or the fusion proteins, a phyA- or GFP-specific antiserum was used at a dilution of 1 : 1,000; detection of actin was performed by using a monoclonal anti-actin antibody (Sigma-Aldrich) at a dilution of 1 : 20,000. Secondary antibodies coupled to alkaline phosphatase (Biorad) were used at a dilution of 1 : 10,000. For comparison of the expression levels of transgenic lines expressing the various PHYA–YFP fusion proteins, 30 μg of total protein was loaded. For comparison of the degradation of the various fusion proteins, the loading was normalized so as to have an equal expression level in extracts prepared from dark-grown seedlings. Signal intensities were compared using the software ImageJ. The experiments were performed at least three times to calculate averages and standard error.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Howard Hughes Medical Institute International Fellowship and Hungarian Scientific Research Fund (OTKA) [grants to F.N. (OTKA-81399) and L.K.-B. (OTKA-73362) for work in Szeged, Hungary]; Deutsche Forschungsgemeinschaft [(NA650/2-1) grant to F.N. for work in Freiburg, Germany]; Sonderforschungsbereich 592 [grant to E.S.]; the Hungarian Academy of Sciences [János Bólyai Research Scholarship to L.K.-B.].
Acknowledgments
We thank Katalin Joszai for excellent technical assistance.
Glossary
Abbreviations
- B
blue
- cB
continuous blue
- CFP
cyan fluorescent protein
- cFR
continuous far-red
- DD
dimerization domain
- FR
far-red
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- HIR
high irradiance response
- NB
nuclear body
- NES
nuclear exclusion signal
- NLS
nuclear localization signal
- phyA
phytochrome-A
- R
red
- RT–PCR
reverse transcription–PCR
- SAPs
sequestered areas of phytochromes
- VLFR
very low fluence response
- YFP
yellow fluorescent protein.
References
- Ahmad M., Jarillo J.A., Smirnova O., Cashmore A.R. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell. 1998;1:939–48. doi: 10.1016/s1097-2765(00)80094-5. [DOI] [PubMed] [Google Scholar]
- Bae G., Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- Bolle C., Koncz C., Chua N.H. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 2000;14:1269–1278. [PMC free article] [PubMed] [Google Scholar]
- Büche C., Poppe C., Schäfer E., Kretsch T. eid1: a new Arabidopsis mutant hypersensitive in phytochrome A-dependent high-irradiance responses. Plant Cell. 2000;12:547–558. [PMC free article] [PubMed] [Google Scholar]
- Casal J.J., Yanovsky M.J., Luppi J.P. Two photobiological pathways of phytochrome A activity, only one of which shows dominant negative suppression by phytochrome B. Photochem. Photobiol. 2000;71:481–486. doi: 10.1562/0031-8655(2000)071<0481:tppopa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cherry J., Hondred D., Walker J., Keller J., Hershey H.P., Vierstra R.D. Carboxy-terminal deletion analysis of oat phytochrome A reveals the presence of separate domains required for structure and biological activity. Plant Cell. 1993;5:565–575. doi: 10.1105/tpc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Debrieux D., Fankhauser C. Light-induced degradation of phyA is promoted by transfer of the photoreceptor into the nucleus. Plant Mol. Biol. 2010;73:687–695. doi: 10.1007/s11103-010-9649-9. [DOI] [PubMed] [Google Scholar]
- Desnos T., Puente P., Whitelam G., Harberd N.P. FHY1: a phytochromeA-specific signal transducer. Genes Dev. 2001;15:2980–2990. doi: 10.1101/gad.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle M., Zhou Y.C., Schäfer E., Funk M., Kretsch T. EID1, an F-box protein involved in phytochrome A-specific light signaling. Genes Dev. 2001;15:939–944. doi: 10.1101/gad.197201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild C.D., Schumaker M.A., Quail P.H. HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 2000;14:2377–2391. [PMC free article] [PubMed] [Google Scholar]
- Genoud T., Schweizer F., Tscheuschler A., Debrieux D., Casal J., Schäfer E., et al. FHY1 mediates nuclear import of the light activated phytochrome A photoreceptor. PloS Genet. 2008;4:e1000143. doi: 10.1371/journal.pgen.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Mockler T., Duong H., Lin C. SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science. 2001;291:487–490. doi: 10.1126/science.291.5503.487. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Viczian A., Bury E., Tscheuschler A., Kircher S., Toth R., et al. Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 2005;15:2125–2133. doi: 10.1016/j.cub.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Tcsheuschler A., Viczian A., Kunkel T., Kircher S., Schäfer E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006;47:1023–1034. doi: 10.1093/pcp/pcj087. [DOI] [PubMed] [Google Scholar]
- Hisada A., Hanzawa H., Weller J.L., Nagatani A., Reid J., Furuya M. Light-induced nuclear translocation of the endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell. 2000;12:1063–1078. doi: 10.1105/tpc.12.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker U., Tepperman J.M., Quail P. SPA1, a WD-40 repeat protein specific to phytochrome A signal transduction. Science. 1999;284:496–499. doi: 10.1126/science.284.5413.496. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B.L., Richardson W.D., Smith A.E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kim L., Kircher S., Tóth R., Ádám É., Schäfer E., Nagy F. Light induced nuclear import of phytochrome-A:GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 2000;22:125–133. doi: 10.1046/j.1365-313x.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- Kircher S., Gil P., Kozma-Bognar L., Fejes E., Speth V., Husselstein-Muller T., et al. Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell. 2002;14:1541–1555. doi: 10.1105/tpc.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S., Kozma-Bognar L., Kim L., Adam E., Harter K., Schäfer E. Light quality-dependent import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneissl J., Wachtler V., Chua N.H., Bolle C. OWL1: an Arabidopsis J-domain protein involved in perception of very low light fluences. Plant Cell. 2009;21:3212–3225. doi: 10.1105/tpc.109.066472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos J., Luppi J., Ogorodnikova O., Sineshchekov V., Yanovsky M., Braslavsky S., et al. Functional and biochemical analysis of the N-terminal domain of phytochrome-A. J. Biol. Chem. 2006;281:34421–34429. doi: 10.1074/jbc.M603538200. [DOI] [PubMed] [Google Scholar]
- Matsushita T., Mochizuki N., Nagatani A. Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature. 2003;424:571–574. doi: 10.1038/nature01837. [DOI] [PubMed] [Google Scholar]
- Muller R., Fernandez A.P., Hiltbrunner A., Schäfer E., Kretsch T. The histidine kinase related domain of Arabidopsis phytochrome A controls the spectral sensitivity and the subcellular distribution of the photoreceptor. Plant Physiol. 2009;150:1297–1309. doi: 10.1104/pp.109.135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.M., Fankhauser C., Chory J. Light: an indicator of time and space. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- Ni M., Tepperman J.M., Quail H.P. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix–loop–helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- Ni M., Tepperman J.M., Quail H.P. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- Oka Y., Matsushita T., Mochizuki N., Suzuki T., Tokutomi S., Nagatani A. Functional analysis of a 450-amino acid N-terminal fragment of phytochrome B in Arabidopsis. Plant Cell. 2004;8:2104–2116. doi: 10.1105/tpc.104.022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio K.A., Crepy M., Martin-Tryon E.L., Milich R., Harmer S.L., Putterill J., et al. GIGANTEA regulates phytochrome A-mediated photomorphogenesis independently of its role in the circadian clock. Plant Physiol. 2007;144:495–502. doi: 10.1104/pp.107.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A., Kunkel T., Hiltbrunner A., Neuhaus G., Wolf I., Speth V., et al. A cell-free system for light dependent nuclear import of phytochrome. Plant J. 2009;57:680–689. doi: 10.1111/j.1365-313X.2008.03721.x. [DOI] [PubMed] [Google Scholar]
- Poppe C., Schäfer E. Seed germination of Arabidopsis thaliana phyA/phyB double mutants is under phytochrome control. Plant Physiol. 1997;14:1487–1492. doi: 10.1104/pp.114.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler J., Klein I., Zeidler M. Arabidopsis fhy1/fhl double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl Acad. Sci. USA. 2007;25:10737–10742. doi: 10.1073/pnas.0703855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J.S., Kim J.I., Kunkel T., Kim B.C., Cho D.S., Hong S.H., et al. Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer. Cell. 2005;120:395–406. doi: 10.1016/j.cell.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Saijo Y., Zhu D., Li J., Rubio V., Zhou Z., Shen Y., et al. Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell. 2008;31:607–613. doi: 10.1016/j.molcel.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer E., Nagy F. Photomorphogenesis in Plants and Bacteria: Function and Signal Transduction Mechanisms. Dordrecht, The Netherlands: Springer; 2006. [Google Scholar]
- Schepens I., Boccalandro H.E., Kami C., Casal J.J., Fankhauser C. PHYTOCROME KINASE SUBSTRATE4 modulates phytochrome mediated control of hypocotyl growth orientation. Plant Physiol. 2008;147:661–671. doi: 10.1104/pp.108.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Watanabe E., Tokutomi S., Nagatani A., Chua N.H. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 2004;18:617–622. doi: 10.1101/gad.1187804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Zhu L., Castillon A., Majee M., Downie B., Huq E. Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell. 2008;6:1586–1602. doi: 10.1105/tpc.108.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhou Z., Feng S., Li J., Tan-Wilson A., Qu L.J., et al. Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell. 2009;21:494–506. doi: 10.1105/tpc.108.061259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth V.O., Schäfer E. Intracellular localization of phytochrome and ubiquitin in red-light-irradiated oat coleoptiles by electron microscopy. Planta. 1987;171:332–338. doi: 10.1007/BF00398678. [DOI] [PubMed] [Google Scholar]
- Staneloni R.J., Rodriguez-Batiller M.J., Legisa D., Scarpin M., Agalou A., Cerdan P.D., et al. Bell-like homeodomain selectively regulates the high irradiance response of phytochrome A. Proc. Natl Acad. Sci. USA. 2009;106:13624–13629. doi: 10.1073/pnas.0906598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman J.M., Hudson M.E., Khanna R., Zhu T., Chang S.H., Wang X., et al. Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling deetiolation. Plant J. 2004;38:725–739. doi: 10.1111/j.1365-313X.2004.02084.x. [DOI] [PubMed] [Google Scholar]
- Vierstra R.D. Phytochrome degradation. In: Kendrick R.E., Kronenberg G.H.M., editors. Photomorphogenesis in Plants. 2nd edn. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 141–162. [Google Scholar]
- Wagner D., Quail P.H. Mutational analysis of phytochrome B identifies a small COOH-terminal-domain region critical for regulatory activity. Proc. Natl Acad. Sci. USA. 1995;92:8596–8600. doi: 10.1073/pnas.92.19.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Deng X.W. Arabidopsis FHY3 defines a key phytochrome A signalling component directly interacting with its homologous partner FAR1. EMBO J. 2002;21:1339–1349. doi: 10.1093/emboj/21.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Meinkoth J.L., Tsien R.Y., Taylor S.S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Yang S., Jang I.C., Henriques R., Chua N.H. FAR-RED ELONGATED HYPOCOTYL 1 and FHY1-like associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell. 2009;21:1341–1359. doi: 10.1105/tpc.109.067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.