Abstract
Objective
To examine the current medical management of arteriopathic patients attending a vascular surgical service at a university teaching hospital over a 6-month period. The prescribing of antiplatelets, statins, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers and beta-blockers was specifically examined. Vascular patients are often under the care of multiple specialties, and therefore the influence of different medical specialties on the patients’ medical management was also examined.
Design
Between January and June 2009, data were recorded on sequential patients with arterial disease attending the vascular surgical service. Patients’ demographics, type of arterial disease, medical consultations within the previous 12 months, and current medications were recorded.
Results
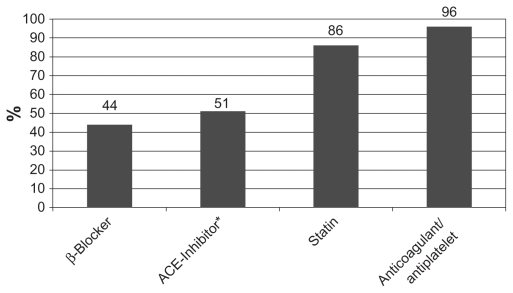
The study included 180 patients with a mean age of 69 years (39–88 years). All but 4% were taking an antiplatelet or anticoagulant, predominantly aspirin. There were 86% taking a statin, 44% taking a beta-blocker, and 51% taking an ACE inhibitor. Suboptimal prescription of ACE inhibitors and beta-blockers was evident regardless of the type of medical consultations in the previous year. No specialty group differed significantly from vascular surgeons in their prescribing pattern.
Conclusions
While almost all arteriopaths receive some form of antiplatelet and statin in line with clinical evidence, ACE inhibitors and beta-blockers appear to be under-prescribed in this arteriopathic population. We conclude that opportunity exists for vascular surgeons to embrace recent guidelines and lead the way in both surgical and medical optimization of arteriopathic patients through improving links with primary care physicians or taking greater responsibility themselves for the medical as well as the surgical care of their arteriopathic patients.
Keywords: ACE inhibitors, arteriopathic disease, vascular disease, statins, beta-blockers
Introduction
Atherosclerosis is the leading cause of death in Western society. It is a systemic disease leading to arterial lesions which typically develop unnoticed. Peripheral arterial disease (PAD) affects 12% of the population of the Western world.1 Intermittent claudication is the most common symptom of mild to moderate PAD, occurring at an annual incidence of 2% in patients aged over 65 years.2 These patients are at significantly higher risk of cardiovascular death compared to healthy controls of similar age.3
Vascular surgeons are uniquely placed to identify and initiate medical treatment in atherosclerotic patients, as well as to choose from the ever-expanding arsenal of endovascular and open procedures linked with advances in technology, techniques, and materials.
Two large multinational randomized controlled clinical trials (RCCTs) have shown that the use of angiotensin-converting enzyme (ACE) inhibitors and statins in arteriopathic patients significantly improves both morbidity and mortality (HOPE and Prospective Study of Pravastatin in the Elderly at Risk [PROSPER]).4,5 The benefits of ACE inhibitors appear to be independent of their antihypertensive effects. It is similarly proven that an antiplatelet medication independently reduces morbidity and overall mortality in arteriopathic patients.6 There is also strong evidence for beta-blockade of patients with a history of ischemic heart disease or an equivalent risk factor.
While smoking cessation and weight loss and exercise programs are commonly ‘prescribed’ interventions for arteriopaths, it is argued that all PAD patients (ankle–brachial index <0.9) should also be medically optimized with prescription of all four drug subclasses mentioned above.7 In an observational study by Feringa et al,8 of 2420 consecutive patients (64 ± 11 years) with PAD, followed over a median of 8 years, of which 1067 patients (44%) died, after adjustment for risk factors, statins (hazard ratio [HR] = 0.46), beta-blockers (HR = 0.68), aspirin (HR = 0.72), and ACE inhibitors (HR = 0.80) were all significantly associated with a reduced risk of long-term mortality. These HRs demonstrate a significant benefit for each of these four medications in a study population demographically very similar to our own study group.
We hypothesized that vascular patients presenting with arterial disease to a vascular surgical service are receiving suboptimal medical management relative to those with arterial disease presenting to other specialties. The perceived enhanced influence by other medical specialties on this medical management was also examined.
Methods
Between January and June 2009, data were prospectively recorded on patients attending the vascular service at Cork University Hospital. Data were recorded by a member of the vascular surgical team on questionnaires completed at the time of inpatient admission or during an outpatient consultation. Inclusion criteria were patients with documented arterial disease or those with significant clinical symptoms being investigated for arterial disease. Patients’ demographics, location of arterial disease, prior medical consultations within the last 12 months, and current medications were recorded. A record of the patients’ prescribed medications was available at the time of consultation, and patient compliance was confirmed. The patients were specifically asked if they had been seen by their general practitioner (GP), a medical consultant, a cardiologist, or a member of the vascular surgical team in the preceding 12 months.
Results
Data were collected on a total of 180 patients (143 males, 37 females) with a mean age of 69 (39–88). Patients’ demographics, comorbidities, and medication use are presented in Table 1. The frequency of medication use is graphically represented in Figure 1.
Table 1.
Patient details (n = 180)
| Patient demographics | ||
| Age (years, mean, range) | 69 | 39–88 |
| Sex (male:female) | 143:37 | |
| Patient comorbidities | Number of patients | % |
| Claudication | 111 | 61.7 |
| Coronary angio/stenting | 11 | 6.1 |
| Peripheral angioplasty | 17 | 9.4 |
| CABG | 31 | 17.2 |
| Carotid disease | 56 | 31.1 |
| Peripheral bypass | 31 | 17.2 |
| AAA | 23 | 12.7 |
| MI/CVA | 31 | 17.2 |
| Diabetes | 38 | 21.1 |
| Patient medication use | ||
| Beta-blocker | 79 | 43.8 |
| ACE inhibitor | 79 | 43.8 |
| Angiotensin receptor blocker | 13 | 7.2 |
| Statin | 155 | 86.1 |
| Aspirin | 107 | 59.4 |
| Clopidogrel | 12 | 6.7 |
| Aspirin and clopidogrel | 36 | 20.0 |
| Warfarin | 14 | 7.8 |
| Warfarin and aspirin | 3 | 1.6 |
Abbreviations: AAA, abdominal aortic aneurysm; ACE, angiotensin-converting enzyme; CABG, coronary artery bypass graft; CVA, cardiovascular accident; MI, myocardial infarction.
Figure 1.
Percentage of study population using individual medications.
Note: *Including ARBs.
Abbreviations: ARB, angiotensin-receptor blockers; ACE, angiotensin-converting enzyme.
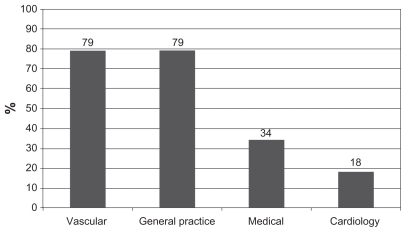
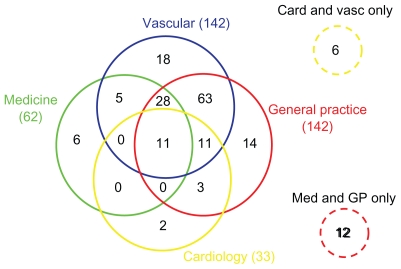
Within the previous 12 months, 142/180 (79%) patients had seen a vascular surgeon (return patient), 142/180 (79%) had seen their GP, 62/180 (34%) a medical consultant, and 33/180 (18%) a cardiologist (Figure 2). The majority (78%) of patients had seen more than one specialty in the previous 12 months. Of note, 95/180 (53%) patients had seen only a GP, a vascular surgeon, or both in the previous 12 months. Only 40/180 (22%) had seen one specialty in the previous 12 months (vascular 18/180, GP 14/180, medical 6/180, and cardiology 2/180). All 180 patients had been seen by at least one specialty in the previous 12 months. This is represented in a modified Venn diagram in Figure 3.
Figure 2.
Percentage of patients reviewed by each specialty in previous 12 months.
Figure 3.
Modified Venn diagram representing the subgroups per specialty seen in the previous 12 months.
Abbreviation: GP, general practice.
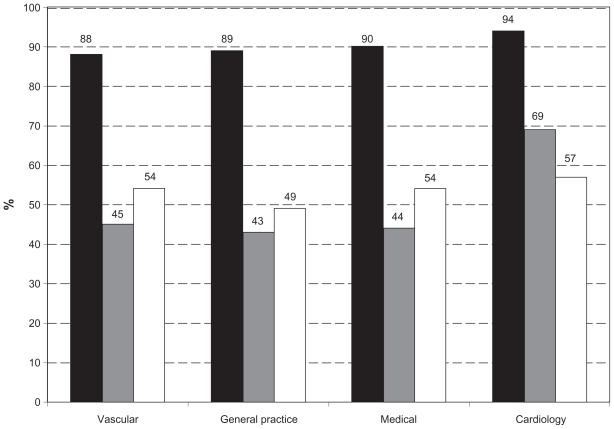
The study population was analyzed according to which of the four specialty groups had seen them in the previous 12 months. It emerged that regardless of whether patients had seen a GP, vascular, medical, or cardiology doctor or any combination in the previous 12 months, prescribing patterns were similar. Suboptimal prescription of ACE inhibitors and beta-blockers was evident across all subgroups. The types of specialties seen in the previous 12 months did not appear to influence the prescribing patterns (with the exception of a minor increase in beta-blocker usage in those patients seen by a cardiologist). These results are charted in Figure 4.
Figure 4.
Percentage of patients on different medications per specialty groups.
Notes: Black, statins; gray, beta-blockers; white, ACE inhibitors (or ARBs).
Abbreviations: ARB, angiotensin-receptor blockers; ACE, angiotensin-converting enzyme.
Discussion
Almost all arteriopaths receive some form of antiplatelet and a statin in line with clinical evidence. However, ACE inhibitors and beta-blockers appear to be under-prescribed in this arteriopathic population despite significant evidence that they reduce morbidity and mortality in such patients. Several landmark randomized clinical control trials in recent years have identified clear guidelines for the best medical management of arteriopathic patients.
The use of antiplatelets in arteriopathic patients is well established. The benefits of antiplatelets are best described in a meta-analysis of 129 RCCTs published by the Antiplatelet Trialists’ Collaboration in the British Medical Journal in 1994.6 The meta-analysis included >100,000 patients and demonstrated a 25% decrease in myocardial infarction (MI), stroke, and death in arteriopathic patients on low dose–prolonged antiplatelet treatment. Since this publication, the prescription of antiplatelet therapy has increased significantly, as is clearly demonstrated in our study with >96% of patients on some form of antiplatelet or anticoagulant therapy. The use of the anticoagulant warfarin in our study population was almost exclusively for risk reduction of embolic events secondary to the presence of atrial fibrillation and was not due to the presence of their arterial disease. However, the use of warfarin did deter the coprescribing of an antiplatelet due to the increased risk of bleeding complications, with only 3 of 17 patients on warfarin also receiving aspirin.
All arteriopathic patients should be prescribed HMG CoA reductase inhibitors (statins). Arteriopathic patients should be aggressively treated with a lipid-lowering therapy even if their baseline cholesterol levels are normal.9 Low-density lipoprotein (LDL) cholesterol should be the primary target of cholesterol-lowering therapy as a 1% reduction in LDL levels reduces the relative risk of a major cardiovascular event by 1% over a 5-year period, independent of age, gender, and baseline levels.10 Statin therapy typically dropped LDL levels by 30%–40% in all of the treatment arms of the major clinical trials.5,9,11–13 The doses used are comparable to current clinical doses, representing a significant risk reduction benefit when used in arteripathic patients. PROSPER was a multicenter RCCT of pravastatin use in 5800 patients with vascular disease.5 Mortality from coronary artery disease fell by 24% in the pravastatin group. While the risk for stroke was unaffected, the HR for transient ischemic attacks was 0.75 in the treatment group compared to placebo. As well as improving overall survival, statins improve symptoms of PAD through pleiotropic effects, thought to be mediated through a reduction in endothelial dysfunction, plaque stabilization, and anti-inflammatory effects.14,15 The Scandinavian Simvastatin Survival Study found a 38% decrease in ‘new or worsening claudication’ over a 5.4-year period in 4444 patients treated with simvastatin.13 This further supports the use of statins in vascular patients.
The use of beta-blockers is well established in coronary artery disease. A meta-analysis of 82 RCCTs incorporating >54,000 patients demonstrated the effect of beta-blockade in long-term secondary prevention after MI with a proven reduction in mortality.16 Carotid artery disease, peripheral vascular disease, and abdominal aortic aneurysms are termed coronary risk equivalents as they represent a comparable increased risk of developing new coronary events equivalent to patients with established coronary artery disease (>20% over 10 years). Patients with coronary risk equivalents should have the same target blood pressure as patients with coronary artery disease.17 The achievement of optimal blood pressure control appears more important than the antihypertensive agent used in overall risk reduction in patients without established coronary artery disease. The prospective observational study by Feringa et al8 demonstrated a HR of 0.68 for patients with PAD receiving beta-blockers. In this study of 2420 patients, beta-blockers were the second most beneficial drug after statins in reducing long-term mortality.
Unfounded fears have existed with regard to the use of beta-blockers in patients with intermittent claudication. A recent Cochrane review of six RCCTs of beta-blocker versus placebo in PAD showed no statistically significant worsening effect of beta-blockers on maximum walking distance, claudication distance, calf blood flow, or skin temperature.18 An earlier meta-analysis of 11 RCCTs again showed no evidence of adverse effects on walking capacity or symptoms of intermittent claudication in patients with mild to moderate PAD.19 Both of these publications support the use of beta-blockers in patients with coronary artery disease and PAD.
An observational study performed on 575 men and women, mean age 80 years, with symptomatic PAD and prior MI, demonstrated a 53% significant independent decrease in the incidence of new coronary events in this elderly population over a 32-month follow-up period when prescribed a beta-blocker. 20 This study recommended beta-blockers for these patients in the absence of contraindications to these drugs. In the same study, 15% of patients were reported to have contraindications to beta-blockers, and of those patients that commenced beta-blockade, 12% discontinued treatment due to adverse effects. Although this elderly cohort of patients is likely to have more contraindications and adverse side effects to beta-blockers than our younger study population, 73% of its patients were eligible for long-term beta-blockade. This compares to only 44% of our patient group on long-term beta-blockade. Consequentially, contraindications and adverse effects to beta-blockers are unlikely to explain this low prescribing rate in our own study population.
The increased use of beta-blockers in patients in the cardiology group of our study relative to the other groups can be explained by the increased recognition of established coronary artery disease within this group. An under-recognition of ‘coronary risk equivalents’ in the other groups may be leading to the under-prescription of beta-blockers in all of these high-risk patients. The cardioprotective effects of beta-blockers make them an important treatment option for risk reduction in vascular patients without specific contraindications to beta-blockade.
ACE inhibitors act on the renin–angiotensin–aldosterone system by inhibiting the ACE-mediated conversion of angiotensin I to angiotensin II. Angiotensin II is a potent vasoconstrictor. Within the kidneys, angiotensin II preferentially constricts the efferent arterioles leading to increased perfusion pressure in the glomeruli. It is a drop in this glomerular filtration pressure that initially stimulates renin release. Angiotensin II also stimulates the adrenal cortex to release aldosterone, which causes retention of sodium and excretion of potassium in the kidneys which leads to increased water retention, blood volume, and consequentially blood pressure. It also stimulates the release of antidiuretic hormone from the posterior pituitary which again increases water retention and increases blood pressure. By blocking the conversion of angiotensin I to angiotensin II with ACE inhibitors, antihypertensive effects are achieved.
However, ACE inhibitors have been shown to reduce the cardiovascular morbidity and mortality rates in patients with peripheral vascular disease by 25% regardless of the presence or absence of hypertension. This was demonstrated eloquently in the HOPE trial, a multicenter international RCCT with >9000 high-risk vascular patients assigned to either a placebo group or a ramipril (10 mg) group.4 In fact, the beneficial effects of ramipril were so evident that the trial was concluded after only 2 years instead of the initially planned 4.5 years. The 2006 AHA/ACC guidelines state that it is reasonable to treat patients with peripheral vascular disease with ACE inhibitors to reduce the risk of adverse cardiovascular events. As well as reducing mortality, a small double-blind placebo-controlled trial published by Ahimastos in 2006 demonstrated that ACE inhibitors improve the symptoms of peripheral vascular disease, increasing walking time by >200%, although the patient numbers were small and patients with hypertension and diabetes were excluded. 21 Data from the same cohort of patients suggested that this improvement was due to reduced arterial wall stiffness caused by ACE inhibitors in the treatment group.22 Like statins, ACE inhibitors have pleiotropic vascular protective effects including plaque stabilization, improved vasomotor dysfunction, and many biochemical mechanisms including inhibition of platelet adhesion and aggregation, inhibition of platelet-derived growth factor, endothelin, and stimulation of endothelial relaxation via stimulation of nitric oxide and prostacyclin.15
Despite the evidence for the use of ACE inhibitors being as compelling as those that support the use of antiplatelets and statins, there are significant differences in the prescribing rates seen in our study (51% vs 96%). Why do the majority of vascular patients who should be treated with an ACE inhibitor remain untreated?
We feel that there is a perception among vascular surgeons that the prescription of aspirin and a statin is ‘safe,’ but that the prescription of ACE inhibitors and beta-blockers has traditionally been left as the responsibility of ‘medical’ doctors. This may be due to a lag time in embracing new guidelines or a fear of the contraindications and potential side effects of ACE inhibitors.
The contraindications to ACE inhibitors include bilateral renal artery stenosis (or unilateral with a solitary functioning kidney) and angioedema associated with ACE inhibitor therapy in the past, both of which are rare. These contraindications may be causing an overcautious reluctance among vascular surgeons to prescribe ACE inhibitors. In the HOPE trial, 0.5% of the ramipril group and 0.6% of the placebo group stopped treatment due to increase in serum creatinine.4 This represents a very small incidence of renal impairment secondary to ACE inhibitors in a population that was at high risk for renal artery stenosis. It should be noted also that 10 mg of ramipril was used in the HOPE trial, as opposed to 2.5 mg, which would be the current initial recommended starting dose.6
Side effects from ACE inhibitors are rare but include hypotension, renal impairment, angioedema, cough, and anaphylactoid reactions. In the ONTARGET trial, the largest clinical trial involving the use of ACE inhibitors, 1.7% of 8576 patients discontinued ramipril due to hypotension, 0.7% due to sufficient renal impairment, 3.3% due to hyperkalemia, 0.3% due to angioedema, and 4.4% due to cough. A total of 10.4% of patients discontinued treatment due to side effects from ramipril.23 The rare amount of contraindications and the side effect prevalence of 10.4% (as seen in the ONTARGET trial) can be dismissed as a possible explanation for the low prescribing level of ACE inhibitors within our study.
Some vascular patients may have preexisting renal impairment especially with the high incidence of diabetes (21% in our study population), leading to diabetic nephropathy. ACE inhibitors have a proven renal protective effect in diabetic patients, independent of the antihypertensive effects.24,25 The same is true in nondiabetic patients. An RCCT of benazepril use in nondiabetic patients with advanced renal insufficiency found that the treatment group had a 43% reduction in the risk of doubling of serum creatinine, end stage renal disease, or death.26 ACE inhibition also reduced the rate of decline in renal function by 23%. Strong evidence exists proving the benefits of ACE inhibitors in vascular patients grossly outweigh the potential risks.
While we argue that the low prevalence of contraindications and adverse side effects to ACE inhibitors and beta-blockers in previous studies make these an unlikely explanation for the low prescribing rate of these drugs in our own study, we accept that not specifically recording the contraindications and side effects from these drugs in our own study population is a weakness of our study. However, any medications that were temporarily stopped to facilitate an intravenous contrast study or another procedure at the time of data collection were regarded as being prescribed and taken.
The influence of gender on prescription was briefly analyzed. There were four times as many men to women in the study population as would be typical of a vascular surgical service population. Prescription rates were similar, with 45% of men and 38% of women being prescribed beta-blockers. The same percentages were evident for ACE inhibitors, although the groups were made up of different individual patients.
Regarding the influence of the different medical specialties on prescribing rates, most patients attend multiple specialties in parallel. Of note, in Ireland, all patients attending a specialty consultant must be initially referred by their family doctor (GP) or a consultant colleague and will not be seen directly without this referral. This partially explains the high rate of attendance to multiple specialties. This makes it difficult to attribute changes in prescribing patterns to the attendance of a patient to a specific specialty. As seen in Figure 2, a significantly lower percentage of patients were seen by a cardiologist or a medical consultant. Although those patients who did see a cardiologist or medical consultant appeared to have similar medication lists (Figure 4), the overall low percentage of patients reviewed by these specialists may partially explain the under-prescription of ACE inhibitors and beta-blockers. The low prescribing rate of appropriate medications is more of a reflection of the entire health care system rather than that of the individual specialties. However, it emphasizes the importance of good quality communication among consultants looking after patients with multiple comorbidites, and it particularly emphasizes the essential link required between hospital consultants and primary care physicians working in general practice. It is important to recognize that our study is a single-center study from Ireland with a limited number of patients, and therefore, our findings may not be applicable to other health care systems and practices.
Conclusion
Vascular surgeons have unique access to arteriopathic patients. In our study population, 53% of patients had seen only a GP or a vascular surgeon in the preceding year. Of those patients who had seen only one doctor in the preceding year, the vascular surgeon had been the most commonly seen doctor. These facts highlight the opportunity vascular surgeons must take in leading the medical optimization of arteriopathic patients. This is particularly the case in Ireland where the medical equivalent of the vascular surgeon, the ‘angiologist,’ does not exist, as seen in some European countries and North America. A comparison of the medical management of Irish and European arteriopathic patients may identify a need to develop this specialty role in countries where it is absent.
Overwhelming evidence exists in support of prescribing an antiplatelet agent, a statin, and an ACE inhibitor for all arteriopathic patients without specific contraindications. Beta-blockers should also be used in patients with coronary artery disease or coronary risk equivalents. We have highlighted that while most vascular patients receive aspirin and a statin, approximately only half receive an ACE inhibitor or a beta-blocker. This inconsistency in prescribing habits is evident across medical specialties. Vascular surgeons are uniquely placed to lead in the medical optimization of the arteriopathic patient population they serve through the increased use of ACE inhibitors as well as the continued use of antiplatelets and statins. Beta-blockers should remain the antihypertensive of choice in all patients with coronary artery disease or a coronary risk equivalent in the absence of contraindications.
Acknowledgments
We are grateful to all the patients who cooperated with this study and to Dr Edel Quinn, Dr Patrica Cronin, Dr G Gosi, and the administration staff at the Department of Vascular Surgery for their assistance in data collection.
Footnotes
Disclosure
The authors report no financial support or conflicts of interest in this work.
References
- 1.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33(1):13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 6.Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81–106. [PMC free article] [PubMed] [Google Scholar]
- 7.Rice TW, Lumsden AB. Optimal medical management of peripheral arterial disease. Vasc Endovascular Surg. 2006;40(4):312–327. doi: 10.1177/1538574406291835. [DOI] [PubMed] [Google Scholar]
- 8.Feringa HH, van Waning VH, Bax JJ, et al. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. J Am Coll Cardiol. 2006;47(6):1182–1187. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 11.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 12.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288(23):2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 13.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 14.Kinlay S. Potential vascular benefits of statins. Am J Med. 2005;118(Suppl 12A):62–67. doi: 10.1016/j.amjmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Faggiotto A, Paoletti R. State-of-the-Art lecture. Statins and blockers of the renin-angiotensin system: vascular protection beyond their primary mode of action. Hypertension. 1999;34(4 Pt 2):987–996. doi: 10.1161/01.hyp.34.4.987. [DOI] [PubMed] [Google Scholar]
- 16.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318(7200):1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 18.Paravastu SC, Mendonca D, da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev. 2008;4:CD005508. doi: 10.1002/14651858.CD005508.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Intern Med. 1991;151(9):1769–1776. [PubMed] [Google Scholar]
- 20.Aronow WS, Ahn C. Effect of beta blockers on incidence of new coronary events in older persons with prior myocardial infarction and symptomatic peripheral arterial disease. Am J Cardiol. 2001;87(11):1284–1286. doi: 10.1016/s0002-9149(01)01521-1. [DOI] [PubMed] [Google Scholar]
- 21.Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Brief communication: ramipril markedly improves walking ability in patients with peripheral arterial disease: a randomized trial. Ann Intern Med. 2006;144(5):660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- 22.Ahimastos AA, Dart AM, Lawler A, Blombery PA, Kingwell BA. Reduced arterial stiffness may contribute to angiotensin-converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26(5):1037–1042. doi: 10.1097/HJH.0b013e3282f8e3b6. [DOI] [PubMed] [Google Scholar]
- 23.ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 24.ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med. 2001;134(5):370–379. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- 25.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin- converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 26.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]