Abstract
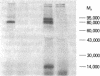
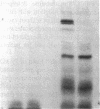
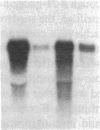
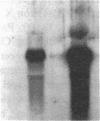
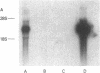
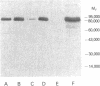
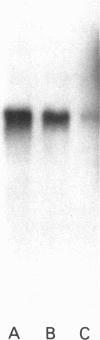
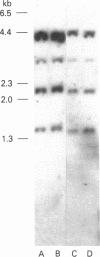
Neutrophil specific granule deficiency (SGD) is a congenital disorder associated with an impaired inflammatory response and a deficiency of several granule proteins. The underlying abnormality causing the deficiencies is unknown. We examined mRNA transcription and protein synthesis of two neutrophil granule proteins, lactoferrin and myeloperoxidase in SGD. Metabolically labeled SGD nucleated marrow cells produced normal amounts of myeloperoxidase, but there was no detectable synthesis of lactoferrin. Transcripts of the expected size for lactoferrin were detectable in the nucleated marrow cells of two SGD patients, but were markedly diminished in abundance when compared with normal nucleated marrow cell RNA. Because lactoferrin is secreted by the glandular epithelia of several tissues, we also assessed lactoferrin in the nasal secretions of one SGD patient by ELISA and immunoblotting. Nasal secretory lactoferrin was the same molecular weight as neutrophil lactoferrin and was secreted in normal amounts. From these data, we conclude that lactoferrin deficiency in SGD neutrophils is tissue specific and is secondary to an abnormality of RNA production. We speculate that the deficiency of several granule proteins is due to a common defect in regulation of transcription that is responsible for the abnormal myeloid differentiation seen in SGD patients.
Full text
PDF

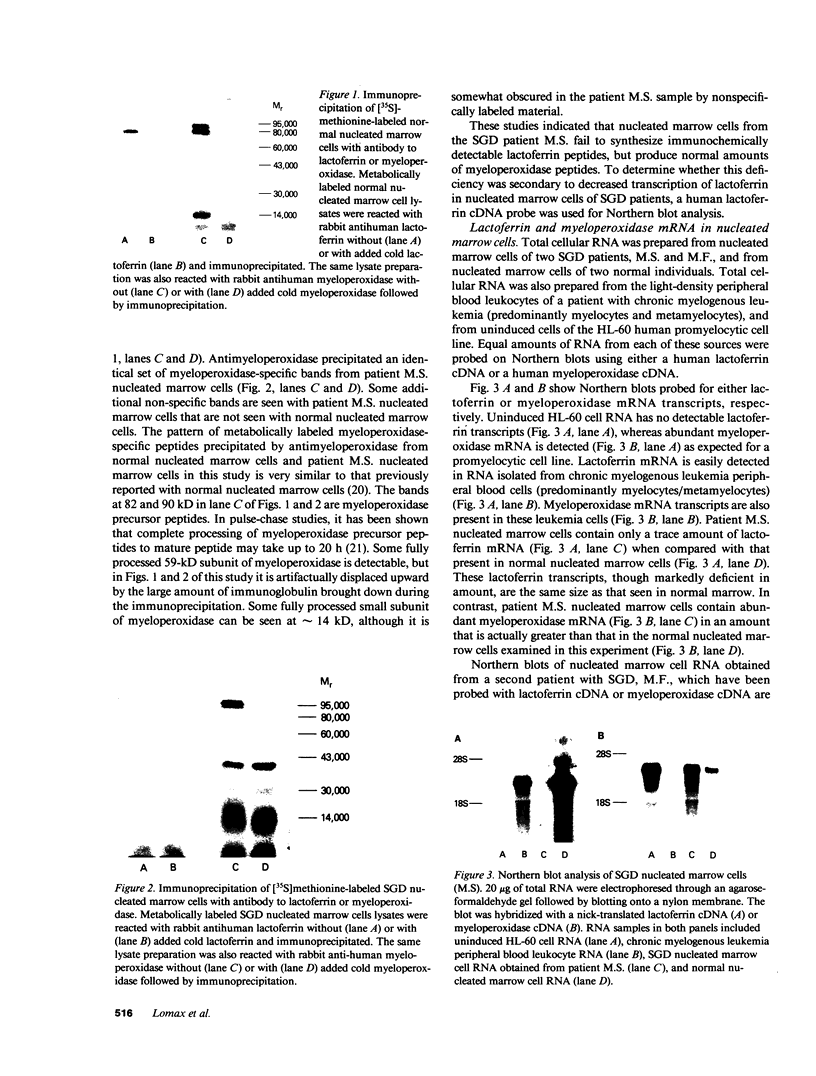

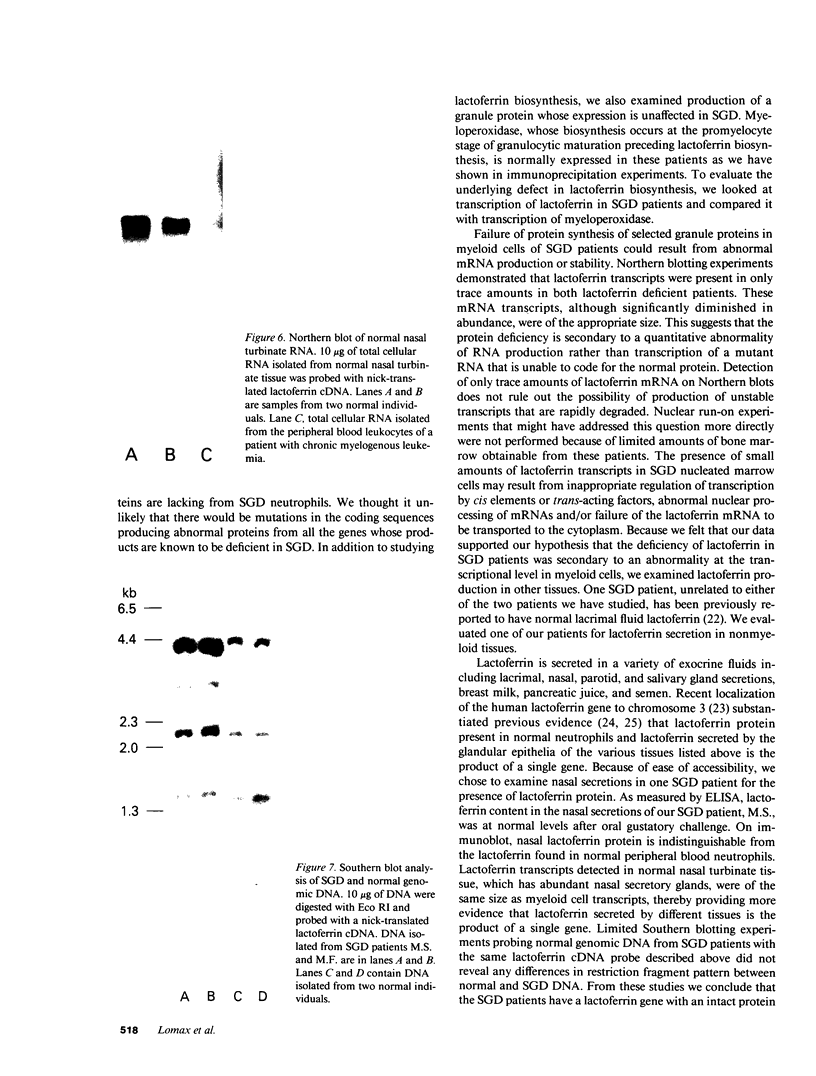

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Sasada M., Nishiyama H., Kubo A., Komiyama A., Allen R. H. Defective bactericidal activity and absence of specific granules in neutrophils from a patient with recurrent bacterial infections. J Clin Immunol. 1984 Jan;4(1):23–30. doi: 10.1007/BF00915283. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Boxer L. A., Smolen J. E., Tauber A. I. Anomalous neutrophil granule distribution in a patient with lactoferrin deficiency: pertinence to the respiratory burst. Am J Hematol. 1985 Mar;18(3):255–260. doi: 10.1002/ajh.2830180306. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Coates T. D., Haak R. A., Wolach J. B., Hoffstein S., Baehner R. L. Lactoferrin deficiency associated with altered granulocyte function. N Engl J Med. 1982 Aug 12;307(7):404–410. doi: 10.1056/NEJM198208123070704. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Mason D. Y., Buriot D., Vilde J. L., Griscelli C. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am J Pathol. 1980 May;99(2):413–428. [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bicknell D. C., Gillis S., Harris E. L., Pelus L. M., Sledge G. W., Jr Lactoferrin: affinity purification from human milk and polymorphonuclear neutrophils using monoclonal antibody (II 2C) to human lactoferrin, development of an immunoradiometric assay using II 2C, and myelopoietic regulation and receptor-binding characteristics. Blood Cells. 1986;11(3):429–446. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Falloon J., Gallin J. I. Neutrophil granules in health and disease. J Allergy Clin Immunol. 1986 May;77(5):653–662. doi: 10.1016/0091-6749(86)90404-5. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Gallin J. I., Fletcher M. P., Seligmann B. E., Hoffstein S., Cehrs K., Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982 Jun;59(6):1317–1329. [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granule deficiency. Annu Rev Med. 1985;36:263–274. doi: 10.1146/annurev.me.36.020185.001403. [DOI] [PubMed] [Google Scholar]
- Ganz T., Metcalf J. A., Gallin J. I., Boxer L. A., Lehrer R. I. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and "specific" granule deficiency. J Clin Invest. 1988 Aug;82(2):552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. P., Melnick D. A., Malech H. L. Characterization of the formyl peptide chemotactic receptor appearing at the phagocytic cell surface after exposure to phorbol myristate acetate. J Immunol. 1986 Feb 15;136(4):1400–1405. [PubMed] [Google Scholar]
- Gierschik P., Falloon J., Milligan G., Pines M., Gallin J. I., Spiegel A. Immunochemical evidence for a novel pertussis toxin substrate in human neutrophils. J Biol Chem. 1986 Jun 15;261(17):8058–8062. [PubMed] [Google Scholar]
- Johnson K. R., Nauseef W. M., Care A., Wheelock M. J., Shane S., Hudson S., Koeffler H. P., Selsted M., Miller C., Rovera G. Characterization of cDNA clones for human myeloperoxidase: predicted amino acid sequence and evidence for multiple mRNA species. Nucleic Acids Res. 1987 Mar 11;15(5):2013–2028. doi: 10.1093/nar/15.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moguilevsky N., Retegui L. A., Masson P. L. Comparison of human lactoferrins from milk and neutrophilic leucocytes. Relative molecular mass, isoelectric point, iron-binding properties and uptake by the liver. Biochem J. 1985 Jul 15;229(2):353–359. doi: 10.1042/bj2290353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M., Root R. K., Malech H. L. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983 May;71(5):1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Seligmann B. E., Gallin J. I. Cytochrome b translocation to human neutrophil plasma membranes and superoxide release. Differential effects of N-formylmethionylleucylphenylalanine, phorbol myristate acetate, and A23187. J Biol Chem. 1985 Feb 25;260(4):2409–2414. [PubMed] [Google Scholar]
- Olsson I., Lantz M., Persson A. M., Arnljots K. Biosynthesis and processing of lactoferrin in bone marrow cells, a comparison with processing of myeloperoxidase. Blood. 1988 Feb;71(2):441–447. [PubMed] [Google Scholar]
- Petty H. R., Francis J. W., Todd R. F., 3rd, Petrequin P., Boxer L. A. Neutrophil C3bi receptors: formation of membrane clusters during cell triggering requires intracellular granules. J Cell Physiol. 1987 Nov;133(2):235-42, 256. doi: 10.1002/jcp.1041330206. [DOI] [PubMed] [Google Scholar]
- Rado T. A., Bollekens J., St Laurent G., Parker L., Benz E. J., Jr Lactoferrin biosynthesis during granulocytopoiesis. Blood. 1984 Nov;64(5):1103–1109. [PubMed] [Google Scholar]
- Rado T. A., Wei X. P., Benz E. J., Jr Isolation of lactoferrin cDNA from a human myeloid library and expression of mRNA during normal and leukemic myelopoiesis. Blood. 1987 Oct;70(4):989–993. [PubMed] [Google Scholar]
- Teng C. T., Pentecost B. T., Marshall A., Solomon A., Bowman B. H., Lalley P. A., Naylor S. L. Assignment of the lactotransferrin gene to human chromosome 3 and to mouse chromosome 9. Somat Cell Mol Genet. 1987 Nov;13(6):689–693. doi: 10.1007/BF01534490. [DOI] [PubMed] [Google Scholar]
- White M. V., Kaliner M. A. Neutrophils and mast cells. I. Human neutrophil-derived histamine-releasing activity. J Immunol. 1987 Sep 1;139(5):1624–1630. [PubMed] [Google Scholar]