Abstract
Epidemiological studies indicate that maternal influenza viral infection increases the risk for schizophrenia in the adult offspring. The serotonin and glutamate systems are suspected in the etiology of schizophrenia, as well as in the mechanism of action of antipsychotic drugs. The effects of hallucinogens, such as psilocybin and mescaline, require the serotonin 5-HT2A receptor, and induce schizophrenia-like psychosis in humans. In addition, metabotropic glutamate receptor mGlu2/3 agonists show promise as a new treatment for schizophrenia. Here, we investigated the level of expression and behavioral function of 5-HT2A and mGlu2 receptors in a mouse model of maternal influenza viral infection. We show that spontaneous locomotor activity is diminished by maternal infection with the mouse-adapted influenza A/WSN/33 (H1N1) virus. The behavioral responses to hallucinogens and glutamate antipsychotics are both affected by maternal exposure to influenza virus, with increased head-twitch response to hallucinogens and diminished antipsychotic-like effect of the glutamate agonist. In frontal cortex of mice born to influenza virus-infected mothers, the 5-HT2A receptor is upregulated and the mGlu2 receptor is downregulated, an alteration that may be involved in the behavioral changes observed. Additionally, we find that the cortical 5-HT2A receptor-dependent signaling pathways are significantly altered in the offspring of infected mothers, showing higher c-fos, egr-1, and egr-2 expression in response to the hallucinogenic drug DOI. Identifying a biochemical alteration that parallels the behavioral changes observed in a mouse model of prenatal viral infection may facilitate targeting therapies for treatment and prevention of schizophrenia.
Introduction
Maternal infection with a variety of agents, such as influenza virus, rubella virus, herpes simplex virus, and poliovirus, has been associated with development of schizophrenia in the adult offspring (Pearce, 2001; Patterson, 2009; Yudofsky, 2009; Brown and Derkits, 2010). In preclinical research, the influenza virus is among the most widely used models to study the effects of prenatal viral infection on brain development (Patterson, 2002; Meyer et al., 2009a). The offspring of mothers infected with mouse-adapted influenza virus display a series of behavioral abnormalities that are relevant to schizophrenia (Shi et al., 2003). As yet, the biochemical changes underlying the behavioral abnormalities induced by maternal viral infection are not definitely established.
The human psychoactive effects of drugs such as lysergic acid diethylamide (LSD) and phencyclidine (PCP) share several features with schizophrenia, including changes in time and sensory perception and in thought, speech, mood, and affect (Geyer and Vollenweider, 2008; González-Maeso and Sealfon, 2009). It has been demonstrated that the serotonin 5-HT2A receptor is necessary for the effects of hallucinogenic drugs, such as LSD, mescaline, and psilocybin, in both murine and human model systems (Vollenweider et al., 1998; González-Maeso et al., 2003, 2007). Indeed, LSD effects were one of the earliest models of schizophrenia (Keeler, 1965), and are currently believed to model the symptoms that occur at the onset of this disorder (Geyer and Vollenweider, 2008).
Preclinical findings in rodents have demonstrated that metabotropic glutamate receptor mGlu2/3 agonists block both the locomotor hyperactivity induced by PCP-like drugs (Moghaddam and Adams, 1998) and the head-twitch behavioral response induced by LSD-like drugs (Gewirtz and Marek, 2000). In addition, in a recent clinical trial, an mGlu2/3 receptor agonist has shown promise as a new treatment for schizophrenia (Patil et al., 2007). It has been recently demonstrated that 5-HT2A and mGlu2 form a receptor heterocomplex that may be responsible for some of the effects induced by hallucinogenic 5-HT2A agonists and antipsychotic mGlu2/3 agonists (González-Maeso et al., 2008). The effect of maternal viral infection on the level of expression and behavioral function of 5-HT2A and mGlu2 receptors remains unknown.
The natural hosts for influenza viruses are avian species, and a limited number of influenza virus subtypes infect mammalian species, including humans (Palese, 2004; Bouvier and Palese, 2008). Although mice are not natural hosts for influenza virus, following an intranasal inoculation they develop an illness that closely resembles the disease in humans. In this study, using mouse-adapted influenza A/WSN/33 (H1N1) virus, we investigated the effects of maternal viral infection on the level of expression of 5-HT2A and mGlu2 receptors in the adult offspring. We show that the cellular and behavioral responses induced by hallucinogenic 5-HT2A and antipsychotic mGlu2/3 receptor agonists are affected in mice born to influenza virus-infected mothers. In humans, influenza virus replication is typically restricted to the respiratory track. Being aware of the differences in the primary target tissue for influenza virus infection in mouse and human (Li et al., 1993; Takahashi et al., 1995), we also considered and evaluated whether mouse-adapted influenza virus directly infects and replicates in the embryonic brain.
Materials and Methods
Cells and viruses.
Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC) and were maintained in minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS) and penicillin-streptomycin. Mouse-adapted A/WSN/33 (H1N1) influenza virus was routinely propagated in MDCK cells as previously reported (Martínez-Sobrido et al., 2010). Virus titers were measured by plaque assay on MDCK cells. The titer of viral stocks was calculated in plaque-forming units (pfu), and stored at −80°C until viral infections were performed. All experiments with live virus were performed under biosafety level 2 (BSL-2) containment.
Mouse viral infection.
Timed pregnant CD1 mice were obtained from Charles River Laboratories. On day 9.5 of pregnancy, mice were anesthetized with ketamine/xylazine before intranasal (i.n.) infection with 5 × 103 pfu of influenza A/WSN/33 (H1N1) virus in 50 μl of PBS. Human epidemiological studies highlight a direct association between infections during the first trimester of pregnancy and schizophrenia. Infectious agents during early pregnancy that have been shown to increase the risk of schizophrenia in the adult offspring include influenza virus (Brown et al., 2004a), rubella virus (Brown et al., 2001), genital and reproductive infections (Babulas et al., 2006), and bacterial infection (Sørensen et al., 2009). We chose day 9.5 of pregnancy for infection because it is approximately midpregnancy in mouse, and this equates to the end of the first trimester of human pregnancy (Clancy et al., 2007). Mock-infected mothers were treated identically but were infused with PBS. Offspring were separated from their mothers after 3 weeks, and males and females were caged separately in groups of three to five. Subsequent experiments were performed in adult (10–12 weeks) mice unless otherwise indicated.
The sublethal dose of infection was determined by inoculating groups of three mice with serial 10-fold dilutions of virus (5 × 102 to 5 × 105 pfu). Infection of pregnant mice with 5 × 103 pfu caused sickness behavior (lethargy, sleepiness, ruffled fur, and lack of grooming) for several days, but loss of pregnancy was not common as long as mice were not disturbed (data not shown). No decrease in litter size or in the appearance or capacity of the mice to thrive was noted in mice born to influenza virus-infected mothers (5 × 103 pfu) relative to controls; infected mice gave birth to 7.2 ± 0.8 (n = 5), whereas control mice gave birth to 7.8 ± 0.6 pups (n = 5). This observation is consistent with previous results (Asp et al., 2005). The litter size was reduced in mice born to mothers infected with higher doses of influenza virus (data not shown; see also Shi et al., 2003). Animals from at least three separate litters were sex-matched and subjected to the different protocols (Meyer et al., 2009b).
Animals were fed with food and water ad libitum, and maintained in laminar flow racks under pathogen-free conditions for 12 h light and 12 h darkness. The Institutional Animal Use and Care Committee approved all experimental procedures at Mount Sinai School of Medicine.
Virus titration.
Timed pregnant mice were infected with influenza virus (see above), and lungs were collected at 3 and 6 d after infection. Embryos from the same mother were pooled and processed as a single sample. Mock-infected mothers and embryos were used as negative controls. Tissue samples were stored at −80°C until they were analyzed. Lung samples from virus-infected mothers and embryo samples were homogenized and resuspended in sterile PBS. Virus titers were determined by plaque assay using 10-fold serial dilutions of tissue samples on MDCK cells as previously described (Martínez-Sobrido et al., 2010). Briefly, MDCK cells were infected with 100 μl of tissue homogenate for 1 h at 37°C. Cells were washed with PBS, and overlaid with MEM medium (see above) containing 0.9% agar. Plates were incubated for 3 d at 37°C, and plaques were counted by crystal violet staining (Martínez-Sobrido et al., 2010).
Detection of influenza A/WSN/33 (H1N1) virus-specific antibody from mice sera by ELISA.
Blood samples were obtained from the tail vein and allowed to clot for 30 min at room temperature. Serum samples, obtained after centrifugation at 16,000 × g for 10 min, were stored at −80°C until further analysis.
The presence of influenza virus-specific antibodies in mice sera was determined by an ELISA using mock- and influenza virus-infected MDCK cell extracts as antigens (Tumpey et al., 2005). Briefly, 50 μl of mock- and influenza virus-infected cell extracts (dilution 1:1000) were added to 96-well plates and incubated overnight at 4°C. Cells extracts were previously titrated with an anti-WSN polyclonal antibody (data not shown; for specificity of the antibody, see Martínez-Sobrido et al., 2010). Plates were blocked with blocking solution (1% bovine serum albumin, BSA, in PBS) for 1 h and, after, incubated with 50 μl of the indicated dilutions of mice sera in blocking solution for 1 h at 37°C. After three washes, plates were incubated with 50 μl of the goat anti-mouse (IgG, IgM, and IgA) horseradish peroxidase conjugate antibody (1:1000; Thermo Scientific) in blocking solution for 30 min at room temperature. After three washes, assay was developed with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Liquid Substrate System for ELISA following the manufacturer's instructions (Sigma-Aldrich). The absorbance was measured at 405 nm with an ELISA microplate reader. Representative results from two separate experiments are indicated. Results obtained with a 1:100 dilution of sera from mice born to infected mothers and controls are shown in Figure 8 d.
Figure 8.
Influenza virus is not detected in the fetus. a, b, Determination of maternal lung and embryo virus titers over a 6 d period for pregnant females inoculated (i.n.) with influenza A/WSN/33 (H1N1) virus. Three pregnant females were killed at each time point. a, Viral titers in tissue homogenates were determined by plaque assay in MDCK cells. Forty-eight hours postinfection, cells were stained with crystal violet. b, Virus titers following influenza virus inoculation (i.p.). Pregnant females were killed 3 and 6 d after inoculation. Animals were also mock-infected (day after infection = 0). Viral titers of tissue homogenates were determined by plaque assay in MDCK cells. No virus could be detected in the fetus of influenza virus-infected pregnant females (N.D., not detected). c, d, Influenza A/WSN/33 (H1N1) virus-specific antibody response. c, Pregnant females were infected with influenza A/WSN/33 (H1N1) virus (white) or PBS as a negative control (black), and serum samples were collected 8–10 weeks after the birth. Antibody response in serial dilutions of the sera from four representative mice is shown. d, Antibody response of mice born to influenza virus-infected mothers (white) and that of mice born to mock-infected mothers (black). Sera of the individual mice were tested for the presence of virus-specific antibodies at the age of 10–14 weeks. Elevated antibodies against influenza virus were detected in 2 of 31 mice born to influenza virus-infected mothers. Influenza A/WSN/33 (H1N1) virus-specific antibodies were detected by ELISA as described in Materials and Methods.
Drug administration.
1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI; Sigma-Aldrich); (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate [dizocilpine, (+)-MK-801; Sigma-Aldrich]; and (1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268; Tocris Bioscience) were dissolved in saline and injected intraperitoneally (i.p.). The injected doses were DOI, 0.5 mg/kg; LY379268, 5 mg/kg; and MK-801, 0.5 mg/kg; unless otherwise indicated. The administered doses have been shown previously to induce different behavioral responses in mice (González-Maeso et al., 2007, 2008).
Behavioral studies.
Behavioral testing took place between 9:00 A.M. and 6:00 P.M. Head-twitch behavioral response was performed as follows. Animals were injected (i.p.) with appropriate treatments, and 15 min later, they were placed into the center of a Plexiglas cage (28 × 18 × 15 cm) for 30 min, during which they were videotaped at close range by a video camcorder positioned directly above the cage. Videotapes were scored for head twitches by an experienced observer blind to prenatal conditions and treatment. Testing cages were thoroughly cleaned after each animal was tested to eliminate odor cues. The differences between the head-twitch response elicited by vehicle and the hallucinogenic drug DOI (0.5 mg/kg) in mice born to influenza virus-infected mothers and controls were assessed by Bonferroni's post hoc test of two-way ANOVA.
Motor function was assessed using a computerized three-dimensional activity monitoring system (AccuScan Instruments). The activity monitor has 32 infrared sensor pairs with 16 along each side spaced 2.5 cm apart. The system determines motor activity based on frequency of interruptions to infrared beams traversing the x, y, and z planes. Horizontal activity and vertical activity were automatically determined from the interruptions of beams in the horizontal and vertical planes, respectively. The differences between the locomotor response in mice born to influenza virus-infected mothers and controls were assessed by Bonferroni's post hoc test of two-way repeated-measures ANOVA.
Radioligand binding.
Tissue samples of mouse frontal cortex were homogenized using a Teflon-glass grinder (10 up-and-down strokes at 1500 rpm) in 1 ml of homogenization buffer (50 mm Tris-HCl, pH 7.4), supplemented with 0.25 m sucrose. The crude homogenate was centrifuged at 1000 × g for 5 min at 4°C, and the supernatant was recentrifuged at 40,000 × g for 15 min at 4°C. The resultant pellet (P2 fraction) was washed twice in homogenization buffer and recentrifuged in similar conditions. Aliquots were stored at −80°C until assay. Protein concentration was determined using the Bio-Rad protein assay.
[3H]Ketanserin (DuPont-NEN) binding (0.0625–10 nm; 10 concentrations) to 5-HT2A receptor was measured at equilibrium in 500 μl aliquots (50 mm Tris-HCl; pH 7.4) of membrane preparations (20–40 μg of protein per tube) that were incubated at 37°C for 60 min. Nonspecific binding was determined in the presence of 10 μm methysergide (Tocris Bioscience), and ranged from 12 ± 1% to 65 ± 1% of total binding in all experimental groups.
[3H]LY341495 (American Radiolabeled Chemicals) binding (0.0625–30 nm; 11 concentrations) to mGlu2/3 receptors was measured at equilibrium in 500 μl aliquots (potassium phosphate buffer supplemented with 100 mm potassium bromide, pH 7.6) of membrane preparations (20–40 μg of protein per tube) that were incubated at 4°C for 60 min. Nonspecific binding was determined in the presence of 1 mm l-glutamic acid (Tocris Bioscience), and ranged from 5 ± 0% to 15 ± 1% of total binding in all experimental groups.
Incubations were terminated by dilution with 3 ml of ice-cold incubation buffer and free ligand was separated from bound ligand by rapid filtration under vacuum through GF/C glass fiber filters. The filters were then rinsed twice with 3 ml of incubation buffer, air dried, and counted for radioactivity by liquid scintillation spectrometry using a Betaplate counter (Wallac-PerkinElmer).
Quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) assays were performed in quadruplicate as previously described with minor modifications (González-Maeso et al., 2003). See González-Maeso et al. (2008) for primer sequences. Briefly, during the 7 d preceding the qRT-PCR experiments, mice were habituated to injections by daily saline administration (i.p.). The day of the experiment, mice were killed by cervical dislocation 60 min after drug or vehicle injection (i.p.). Frontal cortex was dissected and immediately frozen at −80°C until processing for total RNA isolation following the manufacturer's instructions (Stratagene).
Results
Spontaneous locomotor activity
The activity in the open-field represents a complex behavior that mimics the natural conflict in mice between their tendency to explore a novel environment and safety. Spontaneous locomotor activity in the open field is generally considered as one of the variables reflecting exploratory behavior. To investigate the effect of maternal viral infection on spontaneous locomotor activity in the offspring, we first examined horizontal activity, vertical activity, and time spent in the center of the arena in the open field exploration test. Two-way ANOVA repeated measures indicated that there was a significant effect of prenatal influenza infection on horizontal activity (Fig. 1 a) (F (1,464) = 41.31; p < 0.001). Analysis of the total locomotor activity for the entire 80 min indicated that locomotion was significantly decreased in mice born to virus-infected mothers (Fig. 1 b) (p < 0.05). Two-way ANOVA repeated measures also indicated that there was a significant effect of prenatal influenza on vertical activity (Fig. 1 c) (F (1,464) = 100.89; p < 0.001). Analysis of the total vertical activity for the entire 80 min indicated that rearing was significantly decreased in mice born to virus-infected mothers (Fig. 1 d) (p < 0.01). Under brightly lit conditions, the center of the open field is aversive and potentially risk-laden, whereas exploration of the periphery provides a safer choice. We found that exploration of the center portion of the environment was not affected by prenatal viral infection (Fig. 1 e,f).
Figure 1.
Mice born to influenza virus-infected mothers show decreased exploratory activity in the open field test, and unaffected locomotor activity occurring in the center of the arena. Mice born to influenza virus-infected mothers (white) and controls (black) were placed in the locomotor chamber and allowed to habituate for 5 min. After habituation, the locomotor activity was measured for 80 min. a–f, Horizontal activity (a, b), vertical activity (c, d), and number of entries into the center field (e, f). Both time course (a, c, e) and total counts (b, d, f) of the effect of maternal viral infection are shown for each measurement (n = 30). Data are means ± SEM (*p < 0.05; **p < 0.01; n.s., not significant; Student's t test).
Behavioral responses induced by hallucinogens
It has been demonstrated that hallucinogenic 5-HT2A agonists, such as mescaline, psilocybin, and LSD, produce symptoms in normal subjects comparable to those seen in acutely ill unmedicated or first episode schizophrenics (Young, 1974; Vollenweider et al., 1998), and that hallucinogens aggravate hallucinations in schizophrenic patients (Hoch et al., 1952). We have previously demonstrated that head-twitch behavior is reliably and robustly elicited by hallucinogenic 5-HT2A agonists, and is absent in 5-HT2A knock-out mice (González-Maeso et al., 2007). Consistent with previous studies, two-way ANOVA indicated that there was a significant effect of the hallucinogenic drug DOI on head-twitch behavior (Fig. 2 a) (F (1,12) = 577.03; p < 0.001). Post hoc analysis indicated that DOI significantly increased head-twitch response in adult mice born to mock-infected mothers (Fig. 2 a) (p < 0.001). Post hoc analysis also indicated that DOI significantly increased head-twitch response in adult mice born to influenza virus-infected mothers (Fig. 2 a) (p < 0.001). Two-way ANOVA indicated that there was a significant effect of maternal viral infection on the head-twitch response to DOI (Fig. 2 a) (F (1,12) = 51.39; p < 0.001). Notably, post hoc analysis revealed that the hallucinogen DOI elicited significantly more head-twitch behavior in adult mice born to virus-infected mothers than in mice born to mock-infected mothers (Fig. 2 a) (p < 0.001).
Figure 2.
Head-twitch behavioral response is increased in adult mice born to influenza virus-infected mothers. Head-twitch response was determined in adult (10–12 weeks old; a) and prepubertal (21–28 d old; b) mice injected with vehicle or DOI (0.5 mg/kg). Data are means ± SEM (n = 4–6 per group). ***p < 0.001, Bonferroni's post hoc test of two-factor ANOVA.
In humans, the onset of schizophrenia typically occurs during late adolescence or early adulthood. Notably, our results show that alterations in the head-twitch response induced by DOI are not observed in prepubertal mice (21–28 d old). Thus, two-way ANOVA indicated that, similar to adult mice (10–12 weeks old) (Fig. 2 a), there was a significant effect of DOI on head-twitch behavior in prepubertal mice (Fig. 2 b) (F (1,12) = 140.85; p < 0.001). Post hoc analysis indicated that DOI significantly increased head-twitch response in both prepubertal mice born to mock-infected mothers (Fig. 2 b) (p < 0.001) and prepubertal mice born to influenza-infected mothers (Fig. 2 b) (p < 0.001). However, two-way ANOVA indicated that there was not a significant effect of prenatal viral infection on the head-twitch response to DOI in prepubertal mice (Fig. 2 b) (F (1,12) = 0.49; p = 0.49).
We next examined the effect of treatment with DOI on locomotor and investigatory behavior in the adult offspring of influenza virus-infected mothers and control mice. As in the previous study (see Fig. 1), two-way ANOVA indicated that there was a significant effect of prenatal viral infection on horizontal (Fig. 3 a) (F (1,60) = 12.07; p < 0.001) and vertical (Fig. 3 b) (F (1,60) = 11.59; p < 0.01) spontaneous activity. Two-way ANOVA also indicated that there was a significant effect of DOI on horizontal (Fig. 3 a) (F (1,60) = 18.12; p < 0.001) and vertical (Fig. 3 b) (F (1,60) = 11.59; p < 0.01) activity in mice born to mock- and influenza virus-infected mothers. Post hoc analysis revealed that the hallucinogen DOI significantly decreased horizontal (p < 0.05) and vertical (p < 0.01) activity in mice born to mock-infected mothers. Notably, and opposite to what was found in mice born to mock-infected mothers, post hoc analysis revealed that the hallucinogen DOI significantly increased horizontal (p < 0.01) and vertical (p < 0.01) activity in mice born to influenza virus-infected mothers.
Figure 3.
Effect of the hallucinogen DOI on locomotor activity in adult mice born to influenza virus-infected mothers and controls. Mice born to influenza virus-infected mothers (white) and controls (black) were placed in the locomotor chamber and allowed to habituate for 60 min. Mice were then injected with vehicle or DOI (0.5 mg/kg), and locomotor activity was measured 15 min after injection for another 60 min. Total horizontal activity (a) and total vertical activity (b) are shown for each measurement (n = 13–22 per group). Data are means ± SEM (*p < 0.05; **p < 0.01; ***p < 0.001; Bonferroni's post hoc test of two-factor ANOVA). Note that locomotor activity is decreased by DOI in mice born to mock-infected mothers, and increased by DOI in mice born to influenza virus-infected mothers.
Antipsychotic-like response induced by LY379268
Noncompetitive NMDA receptor antagonists, such as PCP, ketamine, and MK-801, are used as pharmacological models for schizophrenia in animals because of their capacity to evoke positive and negative symptoms as well as sensorimotor gating deficits resembling those seen in this disease (Morris et al., 2005; Kristiansen et al., 2007). Consistent with previous studies (Geyer and Ellenbroek, 2003), MK-801 (0.5 mg/kg) induced a robust increase in locomotor activity (Fig. 4). Two-way ANOVA revealed that prenatal infection did not affect the locomotor response induced by MK-801 (Fig. 4 a–c) (F (1,36) = 0.45; p = 0.51). Two-way ANOVA also indicated that there was a significant effect of LY379268 (5 mg/kg) on the locomotor response induced by MK-801 (Fig. 4 a,c) (F (1,36) = 8.06; p < 0.01). Interestingly, post hoc analysis revealed that LY379268 reduced the locomotor response induced by MK-801 in mice born to mock-infected mothers (Fig. 4 a,c) (p < 0.01), but not in mice born to influenza virus-infected mothers (Fig. 4 b,c) (p > 0.05).
Figure 4.
The MK-801-stimulated locomotor activity is attenuated by LY379268 (5 mg/kg) in control mice (black; a, c), but it is not affected in the offspring of infected mothers (white; b, c). The panels depict the time course of MK-801-induced locomotion in 5 min blocks (a, b). Time of injections is indicated by arrows. c, Data summary of the total MK-801-induced locomotion as a summation of horizontal activity from t = 15 to t = 90. Mice were administered LY379268 (circles), or vehicle (squares) followed by MK-801 (n = 8–12 per group). Data are mean ± SEM (**p < 0.01; n.s., not significant; Bonferroni's post hoc test of two-factor ANOVA).
Level of expression of 5-HT2A receptor and mGlu2 receptor in frontal cortex
The alterations in the behavioral responses induced by hallucinogens and LY379268 prompted us to study the level of expression of 5-HT2A receptor and mGlu2/3 receptor in the offspring of influenza virus-infected mothers. The specific binding of the antagonist [3H]ketanserin, a selective 5-HT2A receptor ligand, to mouse frontal cortex membrane preparations was a saturable process to a single population of high-affinity sites with dissociation constant (K D) values in the narrow range of 0.6–5.1 nm (Fig. 5 a) (F (2,102) = 0.76; p = 0.47) in control mice. The simultaneous analysis of multiple saturation curves indicated the existence of a significantly different [3H]ketanserin binding saturation curve in the offspring of mice born to influenza virus-infected mothers (Fig. 5 a) (F (2,200) = 20.03; p < 0.001). Analysis of individual maximum number of binding sites (B max) values demonstrated a higher density of 5-HT2A receptors in mice born to influenza virus-infected mothers (Fig. 5 b) (p < 0.05). The affinity (K D values) for [3H]ketanserin did not differ from those in the control group (p > 0.05).
Figure 5.
Prenatal viral infection affects the expression of 5-HT2A and mGlu2/3 receptors in mouse frontal cortex. a, c, [3H]Ketanserin (a) and [3H]LY341495 (c) binding saturation curves in frontal cortex of mice born to influenza virus-infected mothers (white) and controls (black; n = 12–16 per group). b, d, Maximum number of binding sites (B max) for [3H]ketanserin (b) and [3H]LY341495 (d) obtained from individual saturation curves. Data are mean ± SEM (*p < 0.05, Student's t test).
The specific binding of the antagonist [3H]LY341495, a selective mGlu2/3 receptor ligand (Wright et al., 2001), to mouse frontal cortex membrane preparations was a saturable process to a single population of high-affinity sites with K D values in the narrow range of 2.1–10.7 nm (Fig. 5 c) (F (2,84) = 0.74; p = 0.29) in control mice. The simultaneous analysis of multiple saturation curves indicated the existence of a significantly different [3H]LY341495 binding saturation curve in the offspring of mice born to influenza virus-infected mothers (Fig. 5 c) (F (2,171) = 9.39; p < 0.001). Analysis of individual B max values demonstrated a lower density of mGlu2/3 receptors in mice born to influenza-infected mothers (Fig. 5 d) (p < 0.05). The K D values for [3H]LY341495 did not differ from those in the control group (p > 0.05).
We next tested the level of expression of 5-HT2A, 5-HT2C, mGlu2, and mGlu3 mRNA in mouse frontal cortex by qRT-PCR assays (Fig. 6). Expression level was significantly higher for 5-HT2A (p < 0.05; Student's t test) and significantly lower for mGlu2 (p < 0.001; Student's t test) in mice born to influenza virus-infected mothers. Neither 5-HT2C nor mGlu3 mRNA expression was significantly changed by prenatal infection (Fig. 6).
Figure 6.
Expression of 5-HT2A, 5-HT2C, mGlu2, and mGlu3 mRNA in frontal cortex of adult mice born to influenza virus-infected mothers (white) band controls (black) determined by qRT-PCR assays (n = 11–22 per group). Data are mean ± SEM (*p < 0.05; ***p < 0.001). Student's t test.
Cellular response to hallucinogens
We have previously demonstrated that hallucinogenic 5-HT2A agonists cause induction of c-fos, egr-1, and egr-2 in mouse cortical neurons (González-Maeso et al., 2003, 2007). We have also found that the induction of c-fos by DOI is not affected by LY379268. In contrast, the hallucinogen-specific induction of egr-1 and egr-2 is selectively blocked by LY379268 in both mouse cortex and primary cortical neurons (González-Maeso et al., 2008). Here we tested the effect of maternal viral infection on the induction of c-fos, egr-1, and egr-2 by the hallucinogen DOI in the adult offspring (Fig. 7). Consistent with previous studies, DOI elicited a dose-dependent gene response in mouse frontal cortex (c-fos, F (3,40) = 34.99, p < 0.001; egr-1, F (3,48) = 96.66, p < 0.001; egr-2, F (3,56) = 102.47, p < 0.001). Notably, the cellular response induced by DOI was significantly different in the adult offspring of influenza virus-infected mothers (c-fos, F (1,40) = 4.41, p < 0.05; egr-1, F (1,48) = 10.24, p < 0.01; egr-2, F (1,56) = 788, p < 0.01). Post hoc analysis revealed that the cellular response to DOI was significantly higher in mice born to infected mothers (Fig. 7).
Figure 7.
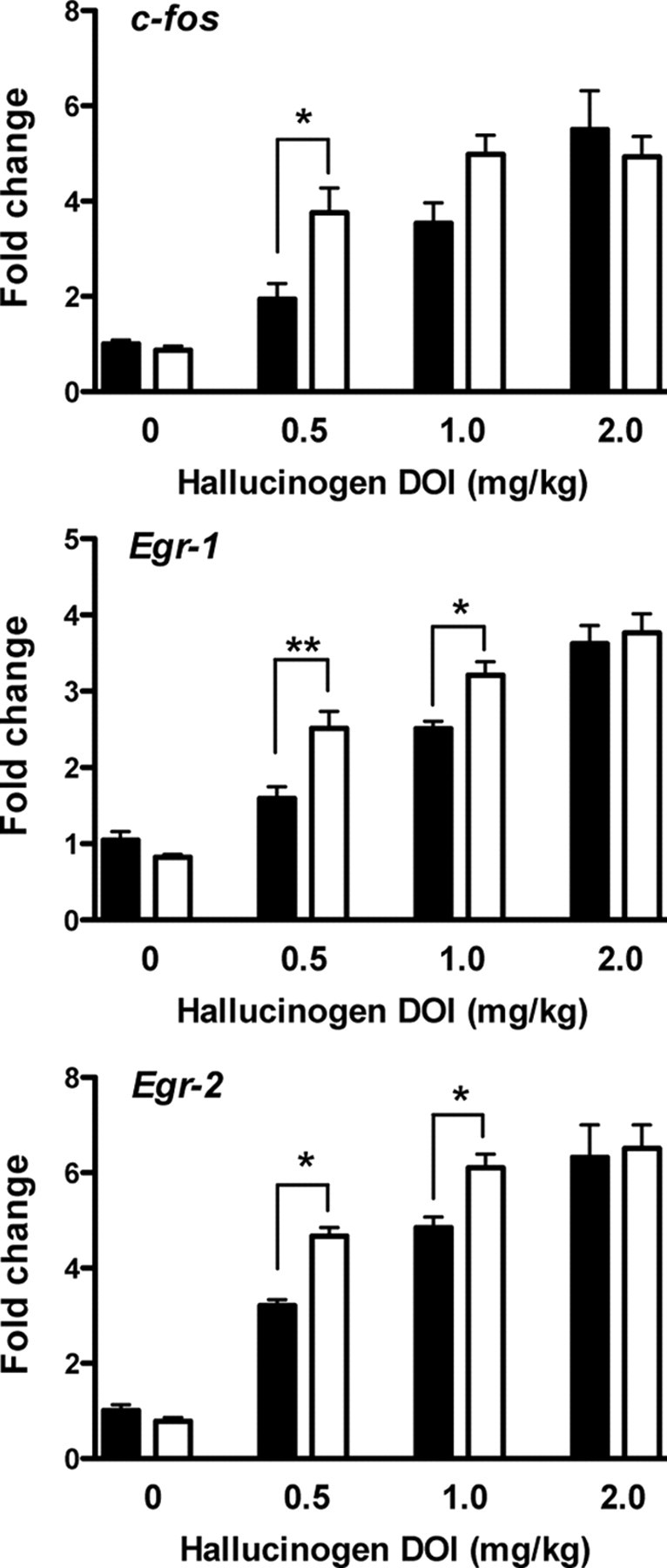
Cellular response induced by the hallucinogen DOI in frontal cortex of adult mice born to influenza virus-infected mothers (white) and controls (black) determined by qRT-PCR assays. Mice were injected (i.p.) with the doses indicated, and killed after 60 min. Relative fold changes of mRNA expression of c-fos, egr-1, and egr-2 are shown (n = 6–8). Data are mean ± SEM (*p < 0.05; **p < 0.01; Bonferroni's post hoc test of two-factor ANOVA).
Absence of replication of influenza A/WSN/33 (H1N1) virus in mouse embryos
To determine whether influenza A/WSN/33 (H1N1) virus can spread to fetuses during maternal infection, mice were infected on day 9.5 of pregnancy under identical conditions to the ones described above. On days 3 and 6 after infection, embryo samples from influenza virus- and mock-infected mothers were collected and titrated in MDCK cells. Lung samples from influenza virus- and mock-infected mothers were used as positive and negative controls, respectively. As shown in Figure 8, a and b, virus replication was detected in the lungs of infected mothers on days 3 and 6 after infection. However, virus replication was undetectable in embryo samples from infected mothers.
Detection of influenza A/WSN/33 (H1N1) virus-specific antibody in maternal and offspring serum
To further investigate the virulence of mouse-adapted influenza A/WSN/33 (H1N1) virus in mouse embryos, we assayed the detection of virus-specific antibodies in serum samples. All the mothers were bled 8–10 weeks after the birth, and the serum samples were analyzed for the presence of influenza A/WSN/33 (H1N1) virus-specific antibodies. Serial dilutions of the sera demonstrated elevated antibodies against influenza virus in all the serum samples from influenza virus-infected mothers compared to those of control mice (see Fig. 8 c for representative examples). We next tested the presence of anti-influenza virus antibodies in adult mice born to infected mothers and controls to determine whether mouse-adapted influenza A/WSN/33 (H1N1) virus can spread to the fetuses during maternal viral infection. Blood samples were collected at the age of 10–14 weeks. Elevated antibodies against influenza A/WSN/33 (H1N1) virus were detected in 2 of 31 mice born to influenza virus-infected mothers, using mice born to mock-infected mothers as controls (Fig. 8 d). Positive and negative controls in ELISA tests included anti-influenza virus antibody, mock- and influenza virus-infected MDCK cell extracts, and blood samples from mice exposed neither to mock nor to viral infection (data not shown).
Discussion
Our study investigates the effects of maternal influenza viral infection on the level of expression and function of 5-HT2A and mGlu2 receptors as a mouse model of schizophrenia. We found that head-twitch response, a mouse behavior induced only by hallucinogenic 5-HT2A agonists, was significantly increased in adult mice born to influenza virus-infected mothers. It has been described that mGlu2, and not mGlu3, is the metabotropic glutamate receptor responsible for the effects of the mGlu2/3 agonist LY379268 on the locomotor response induced by the noncompetitive NMDA receptor antagonist MK-801 (Woolley et al., 2008); see also (Fell et al., 2008). Our data demonstrate that the antipsychotic-like behavioral effects of LY379268 are absent in the offspring of influenza virus-infected mothers. The behavioral responses were associated with biochemical alterations in frontal cortex, showing a higher and a lower level of expression of 5-HT2A and mGlu2, respectively, in mice with prenatal exposure to maternal infection. We also found that maternal viral infection leads to an increased cellular response induced by the hallucinogenic 5-HT2A agonist DOI. Thus our results demonstrate that maternal viral infection dysregulates the cellular and behavioral responses that require the 5-HT2A-mGlu2 complex in the adult offspring.
Frontal cortex presents a wide array of cognitive functions, such as learning–reward processing, decision making, attention, and social cognition. From the viewpoint of the model of psychosis paradigm, there is an association between acute psychosis episodes and hyperactivity in frontal cortical regions (Wiesel et al., 1987; Szechtman et al., 1988; Parellada et al., 1994). Several laboratories have reported alterations in frontal cortex of mouse models of maternal viral infection and/or immune challenge, including changes in gene expression (Fatemi et al., 1999, 2002a, 2005), abnormal cortical neurogenesis (De Miranda et al., 2010), pyramidal cell atrophy (Fatemi et al., 2002b; Baharnoori et al., 2009), and loss of GABA-ergic interneurons (Meyer et al., 2008a). The behavioral effects of hallucinogenic drugs result from their activity at 5-HT2A receptors expressed by cortical pyramidal neurons (González-Maeso et al., 2007). It has been shown that activation of mGlu2 receptors specifically blocks the cellular and behavioral responses induced by hallucinogenic 5-HT2A agonists (Marek, 2004; González-Maeso et al., 2008). We have recently reported that, in postmortem prefrontal cortex from young untreated schizophrenic subjects, the 5-HT2A receptor is upregulated, and the mGlu2 receptor is downregulated, a pattern that could predispose to psychosis (González-Maeso et al., 2008). Since the same pattern of dysregulated expression was found in frontal cortex of mice born to influenza virus-infected mothers, these results are of considerable importance for future studies to unravel the etiology of schizophrenia and the neurochemical mechanisms responsible for the alterations observed in cortical regions in schizophrenia patients.
It is important to note that many influenza viruses do not optimally replicate or cause disease in mice (Matsuoka et al., 2009). However, some influenza viruses, including influenza A/WSN/33 (H1N1) virus (Ward et al., 1993), have been adapted to mice through serial passage, and some highly pathogenic influenza viruses can cause disease and replicate in the respiratory tract and extrapulmonary sites in mice without adaptation (Li et al., 1993; Takahashi et al., 1995; Ward, 1995). Since human influenza viruses replicate mainly in the respiratory tract, a potential factor that may question the validity of the mouse model of maternal viral infection is the presence of influenza virus in fetal brain. Discrepant results have been reported in the literature regarding detection of A/WSN/33 and A/NWS/33 influenza virus strains in the fetus (Aronsson et al., 2002; Shi et al., 2005). Our data found that mouse-adapted influenza virus is not detectable in embryo samples from infected mothers. Interestingly, our findings show that influenza A/WSN/33 (H1N1)-specific antibody is detected in ∼5% of all the adult mice (10–14 weeks) whose mothers were infected with influenza virus. Although immature, the fetal immune system is still capable of responding to certain antigenic stimuli, which might account for the antibodies against influenza detected in some of the mice born to influenza virus-infected mothers. Fetuses and neonates are also deficient in several components of inflammatory, innate, and specific immune responses. In humans, maternal antibody transfer to the offspring is mostly transplacental (Leach et al., 1996). In mice, however, the offspring receive almost all the maternally derived antibody in the colostrum and milk, and this passive antibody typically declines over the first period of life. Although further investigation is necessary, the presence of anti-influenza virus antibodies that we found in some of the adult mice born to infected mothers may be then antibodies passively acquired during lactation. Together with the absence of detectable virus in embryo samples, these data also suggest that the changes observed in the offspring are a consequence of maternal response to viral infection, and not of direct fetal infection by mouse-adapted influenza virus.
Schizophrenia patients show “positive” symptoms (e.g., hallucinations, delusions, and other thought disorders) and negative symptoms (e.g., social withdrawal, apathy, and abnormal emotional responses). In addition to the positive and negative symptoms of schizophrenia, the importance of anxiety, depression, and cognitive deficits has gained increasing attention. Significant correlations between anxiety symptoms and positive symptoms in schizophrenia have been replicated in several studies (Muller et al., 2004). Behavior in the open field is used as an anxiety test (Nestler et al., 2002). In the open field, mice face a conflict between avoidance and exploration of the center, which is more aversive than the peripheral area proximal to the walls. It has been shown that measures of behavior in the open field fall into two main categories: (1) time and distance traveled in the center, which may be related to anxiety; and (2) total distance traveled and rearing, which reflect general behavioral activation or exploration. We found that mice born to influenza virus-infected mothers displayed strong deficits in locomotor and exploratory activity, whereas anxiety-like behavior was unchanged. This reduction of locomotor activity suggests abnormal habituation to a novel environment in mice born to influenza virus-infected mothers. A subset of schizophrenic patients exhibits psychomotor agitation, which includes hyperactivity or increased stereotypic movements (Powell and Miyakawa, 2006). Locomotor response to novelty has been shown to be higher (Rojas et al., 2007) and lower (Mukai et al., 2004) in different knock-out mouse models of schizophrenia. However, previous studies have repeatedly demonstrated lower exploratory behavior score in mouse schizophrenia models of perinatal insults (Shi et al., 2003; Smith et al., 2007; Meyer et al., 2008b; Lodge et al., 2009). Speculatively, the decreased activity that we observed in the adult offspring of influenza virus-infected mothers could be related to negative symptoms of schizophrenia. Earlier reports have shown that mice born to influenza virus-infected mothers spend significantly less time in the center of the squares (Shi et al., 2003). Here we found that the amount of time spent in the center of the open field was not affected by maternal influenza viral infection. These divergent results may be explicable by strain differences in exploratory behavior between CD1 and BALB/c mice.
Antenatal and postnatal environmental factors have profound implications on the behavior of the adult offspring (Meyer et al., 2009b). Maternal behavior also alters the offspring epigenome (Weaver et al., 2004). Thus, litter effects are substantial, and their outcome might not always be independent of the effects of other factors, including maternal viral infection. To avoid litter effects, our experiments included animals originating from at least three different litters (see Materials and Methods). Although the possibility of litter effects cannot be firmly excluded (Meyer et al., 2009b), the use of offspring from different mothers, together with the association between biochemical and behavioral results obtained in mice from different litters, suggests that the results described here are consistent with the hypothesis that maternal viral infection affects the level of expression and function of 5-HT2A and mGlu2 receptors.
It has been suggested that prenatal exposure to maternal stress increases the risk of schizophrenia in the offspring (Khashan et al., 2008; Malaspina et al., 2008). In rodents, prenatal stress has been shown to induce learning deficits (Lemaire et al., 2000), as well as alterations in the immune system (Vanbesien-Mailliot et al., 2007) and feeding behavior (Pankevich et al., 2009). During shipping, animals are likely to be exposed to noise and vibrations, as well as temperature, humidity, and light–dark changes. Shipping might be then a stressful experience because many of these factors are actually used as independent variables in stress research. In our experiments, timed pregnant mice were shipped on the fifth day of gestation. Although confounding effects from other sources cannot be excluded, mice to be mock- and influenza virus-infected were shipped under the same conditions to minimize a possible bias due to maternal stress.
Some researchers have suggested that maternal infection during pregnancy produces behavioral changes in the offspring that include increased MK-801-induced locomotion (Meyer and Feldon, 2009). We found that the locomotor activity elicited by MK-801 in mice born to influenza virus-infected mothers and control mice was indistinguishable. These differences may be due to the dose of MK-801 used. The MK-801-stimulated locomotor activity was significantly attenuated by the glutamate antipsychotic LY379268 in control mice, but it was not affected in the offspring of influenza virus-infected mothers. In concordance with these effects of maternal viral infection in our mouse behavior models, the density of mGlu2 was reduced in frontal cortex of mice born to influenza virus-infected mothers.
It has been shown that a low dose of the hallucinogen DOI increases locomotor activity, an effect that is absent in 5-HT2A knock-out mice (Halberstadt et al., 2009). In contrast, a high dose of DOI decreases the locomotor activity, an effect that is blocked by a 5-HT2C selective antagonist (Halberstadt et al., 2009). These findings suggest that 5-HT2A and 5-HT2C receptors exert opposite effects on locomotor activity in mice, and they propose the involvement of 5-HT2A receptor in the locomotor-stimulating effects of DOI. We examined the locomotor activity induced by DOI in mice born to influenza virus-infected mothers and controls. We found that the locomotor activity was decreased by DOI in mice born to mock-infected mothers, whereas it was increased by DOI in mice with prenatal exposure to maternal influenza viral infection. Our data also demonstrate an increased head-twitch response to DOI in mice born to influenza virus-infected mothers (see above). Regulation of c-fos, egr-1, and egr-2 robustly predicts the head-twitch behavioral response induced by hallucinogenic drugs (González-Maeso et al., 2007). In the present study, we found that the dose-dependent cellular response induced by the hallucinogenic 5-HT2A receptor agonist DOI was significantly different in the adult offspring, with a higher cellular response to DOI in mice born to influenza virus-infected mothers. Concurrently, these cellular and behavioral responses correlate with the higher density of 5-HT2A receptors in mice prenatally exposed to maternal viral infection.
Several laboratories suggest that schizophrenia is a result of neurodevelopmental defects triggered by cytokine-related inflammatory events (Kronfol and Remick, 2000; Brown et al., 2004b; Meyer et al., 2009a; Patterson, 2009). Thus, cytokines and chemokines such as IL-6, CXCL8 (IL-8), IL-10, and TNF-α (Brown et al., 2004b; Meyer et al., 2006; Smith et al., 2007; Meyer et al., 2008b) have been proposed as responsible for pathways whereby maternal immune activation induces schizophrenia-like behaviors in the adult offspring. Influenza virus infections trigger a profound cytokine and chemokine response at both local and systemic levels, more pronounced with virus strains that appear to be more pathogenic (Kobasa et al., 2007). Further investigation will be necessary to unravel the role, if any, of cytokines and chemokines on the abnormal behavioral function of the 5-HT2A-mGlu2 receptor complex in schizophrenia murine models of maternal viral infection.
In humans, the onset of schizophrenia typically occurs during late adolescence or early adulthood. Notably, our results demonstrate that alterations in the head-twitch behavioral response are only observed after puberty in prenatally infected mice—a finding that parallels the adult onset of the disease in humans and supports our mouse schizophrenia model of perinatal insult. The antipsychotics currently available are designed only to treat the symptoms of schizophrenia, and not the underlying causes of the disease. Because of this, schizophrenia remains an incurable illness, and treatment of the symptoms with the available antipsychotics typically continues for life. The prevention of psychotic disorders is therefore a worthwhile goal. Remarkably, a prodromal state commonly precedes the acute phase of a first psychotic episode. Recent findings suggest that it is possible to identify young patients at imminent risk of psychosis. Unfortunately, results of several clinical trials that have been published recently demonstrate that the available anti-schizophrenia drugs do not delay or prevent conversion to psychosis in subjects with prodromal symptoms of schizophrenia (McGlashan et al., 2006; Cannon et al., 2008; Phillips et al., 2009). On the other hand, experiments in rodents suggest that prepubertal treatment with atypical antipsychotics such as clozapine and risperidone prevents postpubertal schizophrenia-like alterations induced by prenatal exposure to maternal immune challenge (Piontkewitz et al., 2009; Meyer et al., 2010; Piontkewitz et al., 2010). Our findings may provide a better understanding of the biochemical alterations responsible for the behavioral abnormalities observed in mouse models of prenatal insults, and may lead to the identification of new therapeutic approaches for not only treatment, but also prevention of schizophrenia and other neurodevelopmental psychiatric conditions.
Footnotes
This work was supported by National Institute of Mental Health Grant 5R01MH084894 and Dainippon Sumitomo Pharma, as well as a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and Maltz Family Foundation Award to J.G.-M. This study was also partly funded by the Center for Research on Influenza Pathogenesis to A.G.-S. through National Institute of Allergy and Infectious Diseases Contract HHSN266200700010C. J.L.M. was the recipient of a postdoctoral fellowship from Ministerio de Ciencia e Innovación, Spain. We thank C. Alberini and S. Salton for critical reading of the manuscript and E. Nistal-Villan for providing viral stocks.
The authors declare that J.G.-M. has received support for research from Dainippon Sumitomo Pharma in the past two years. The present study is not related to this professional relationship.
References
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- Asp L, Beraki S, Aronsson F, Rosvall L, Ogren SO, Kristensson K, Karlsson H. Gene expression changes in brains of mice exposed to a maternal virus infection. Neuroreport. 2005;16:1111–1115. doi: 10.1097/00001756-200507130-00016. [DOI] [PubMed] [Google Scholar]
- Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, Gorman JM, Susser ES. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004a;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004b;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI. Induction of toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio. 2010;1:e00176-10. doi: 10.1128/mBio.00176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol Psychiatry. 2002a;7:633–640. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002b;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch PH, Cattell JP, Pennes HH. Effects of mescaline and lysergic acid (d-LSD-25) Am J Psychiatry. 1952;108:579–584. doi: 10.1176/ajp.108.8.579. [DOI] [PubMed] [Google Scholar]
- Keeler MH. Similarity of schizophrenia and the psilocybin syndrome as determined by objective methods. Int J Neuropsychiatry. 1965;1:630–634. [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Schulman J, Itamura S, Palese P. Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J Virol. 1993;67:6667–6673. doi: 10.1128/jvi.67.11.6667-6673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ. Metabotropic glutamate 2/3 receptors as drug targets. Curr Opin Pharmacol. 2004;4:18–22. doi: 10.1016/j.coph.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Martínez-Sobrido L, Cadagan R, Steel J, Basler CF, Palese P, Moran TM, García-Sastre A. Hemagglutinin-pseudotyped green fluorescent protein-expressing influenza viruses for the detection of influenza virus neutralizing antibodies. J Virol. 2010;84:2157–2163. doi: 10.1128/JVI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Lamirande EW, Subbarao K. The mouse model for influenza. Curr Protoc Microbiol Chapter. 2009;15:15G.13. doi: 10.1002/9780471729259.mc15g03s13. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009;206:587–602. doi: 10.1007/s00213-009-1504-9. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008a;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008b;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009a;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009b;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology (Berl) 2010;208:531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- Muller JE, Koen L, Soraya S, Emsley RA, Stein DJ. Anxiety disorders and schizophrenia. Curr Psychiatry Rep. 2004;6:255–261. doi: 10.1007/s11920-004-0074-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Mueller BR, Brockel B, Bale TL. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol Behav. 2009;98:94–102. doi: 10.1016/j.physbeh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parellada E, Catafau AM, Bernardo M, Lomeña F, González-Monclús E, Setoain J. Prefrontal dysfunction in young acute neuroleptic-naive schizophrenic patients: a resting and activation SPECT study. Psychiatry Res. 1994;55:131–139. doi: 10.1016/0925-4927(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Nelson B, Yuen HP, Francey SM, Simmons M, Stanford C, Ross M, Kelly D, Baker K, Conus P, Amminger P, Trumpler F, Yun Y, Lim M, McNab C, Yung AR, McGorry PD. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: study design and baseline characteristics. Aust N Z J Psychiatry. 2009;43:818–829. doi: 10.1080/00048670903107625. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas P, Joodmardi E, Hong Y, Perlmann T, Ogren SO. Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry. 2007;12:756–766. doi: 10.1038/sj.mp.4001993. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–637. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Nahmias C, Garnett ES, Firnau G, Brown GM, Kaplan RD, Cleghorn JM. Effect of neuroleptics on altered cerebral glucose metabolism in schizophrenia. Arch Gen Psychiatry. 1988;45:523–532. doi: 10.1001/archpsyc.1988.01800300019002. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamada T, Nakajima S, Nakajima K, Yamamoto T, Okada H. The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med. 1995;181:2161–2169. doi: 10.1084/jem.181.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solórzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, García-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- Vanbesien-Mailliot CC, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J, Chartier-Harlin MC, Maccari S. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinology. 2007;32:114–124. doi: 10.1016/j.psyneuen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Ward AC. Changes in the neuraminidase of neurovirulent influenza virus strains. Virus Genes. 1995;10:253–260. doi: 10.1007/BF01701815. [DOI] [PubMed] [Google Scholar]
- Ward AC, Azad AA, Macreadie IG, McKimm-Breschkin JL. Complete nucleotide sequence of the non-structural gene of the human influenza virus strain A/WS/33. Nucleic Acids Res. 1993;21:2257. doi: 10.1093/nar/21.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wiesel FA, Wik G, Sjögren I, Blomqvist G, Greitz T, Stone-Elander S. Regional brain glucose metabolism in drug free schizophrenic patients and clinical correlates. Acta Psychiatr Scand. 1987;76:628–641. doi: 10.1111/j.1600-0447.1987.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther. 2001;298:453–460. [PubMed] [Google Scholar]
- Young BG. A phenomenological comparison of LSD and schizophrenic states. Br J Psychiatry. 1974;124:64–74. doi: 10.1192/bjp.124.1.64. [DOI] [PubMed] [Google Scholar]
- Yudofsky SC. Contracting schizophrenia: lessons from the influenza epidemic of 1918–1919. JAMA. 2009;301:324–326. doi: 10.1001/jama.2008.980. [DOI] [PubMed] [Google Scholar]









