ABSTRACT
Anterior access to the craniocervical junction has traditionally been through a transoral approach. With the advent of newer techniques, recent literature suggests a possible role for a transnasal endoscopic approach to the craniocervical junction. A review of the literature primarily consists of case reports and three anatomic cadaveric studies demonstrating the feasibility of an endonasal approach. In this retrospective review, we report our experience with four patients who underwent an endoscopic transnasal approach to the C1–C2 region. The surgical technique using a binasal endoscopic approach is described. The results indicate that the procedure is well tolerated with no significant deleterious sequelae. Although the use of this technique is in its early stages, the transnasal approach may offer a safe and effective alternative with minimal morbidity. Larger clinical studies are necessary to further explore the risks and benefits of this procedure.
Keywords: Transnasal approach, craniocervical junction, endoscope, surgical technique
The traditional1,2,3,4,5,6,7,8,9,10,11 transoral approach to the craniocervical junction has been used successfully to approach various pathologies of this region. However, this approach has several disadvantages due to disruption of palate and tongue anatomy, including the use of tracheotomy,7,8,9,10,11 which is routinely performed by many surgeons. It is also associated with the risk of bacterial contamination5,7,8 and subsequent wound dehiscence.5,8,11Furthermore, many patients require a feeding tube3,4,9,10 due to postoperative tongue edema.3,10 There is also a significant risk of velopharyngeal incompetence,3,8,10 especially if the palatal musculature or mucosa has been disrupted. Palate splitting is frequently necessary to access certain pathologies via the transoral technique.3,5,8,10,11
A transcervical approach has also been used to reach the craniocervical junction. It is associated with other potential complications, such as damage to the esophagus, trachea, carotid sheath, recurrent laryngeal nerve, and surrounding cranial nerves. This approach also necessitates a skin incision.
Using an endoscopic transnasal approach, most of the previously mentioned complications can be avoided or minimized. Furthermore, due to the proximity of the endoscope to the target location, the optical field tends to be wider than that of the microscope, therefore allowing superior visualization to that of the microscope used in transoral surgery. To date, the literature consists primarily of case reports12,13,14,15,16,17 and three anatomic cadaveric studies18,19,20 demonstrating the feasibility of this approach. We describe this technique and our experience in four patients.
SURGICAL TECHNIQUE
A preoperative computed tomography (CT) scan is performed for intraoperative image guidance. A 0-degree endoscope is used for visualization throughout the procedure. The patient is placed supine with the head fixed in a three-pin Mayfield head holder. A limited ethmoidectomy is performed. To allow for mobility of the instruments and visualization, the posterior aspect of the nasal septum is removed. Monopolar cautery is used to create a septal flap based on the sphenopalatine artery, with the boundaries including the choanae, base of the septum, anterior middle turbinate, and 1 cm below the nasal roof. The septal flap is mobilized and placed in the nasal cavity during the procedure and preserved for closure. A sphenoidotomy is performed and the pituitary fossa and internal carotid arteries are identified. The dissection then proceeds inferiorly, where the eustachian tubes, torus tubarius, and the fossa of Rosenmüller are identified on both sides. The posterior nasopharyngeal and pharyngobasilar fasciae are incised and an inferiorly based U-shaped flap is created. The longus colli and paravertebral muscles are stripped from the inferior clivus and anterior arches of C1 and C2. The rostrum and anterior portion at the clivus are drilled down, followed by the arch of C1, and finally the odontoid process. For closure, the U-shaped flap is replaced, the septal flap is laid superiorly, and Gelfoam (Pharmacia & Upjohn Company, Kalamazoo, MI) and Tisseel (Baxter Healthcare Corp., Deerfield, IL) are applied. Silastic nasal splints and Merocel packing (Medtronic XOMED, Jacksonville, FL) are inserted. Refer to Figs. 1–4 for images.
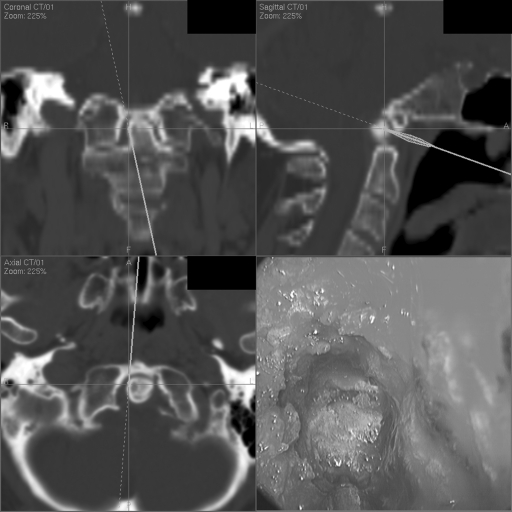
Figure 1.
Endonasal view with corresponding image guidance localization during the start of odontoid drilling.
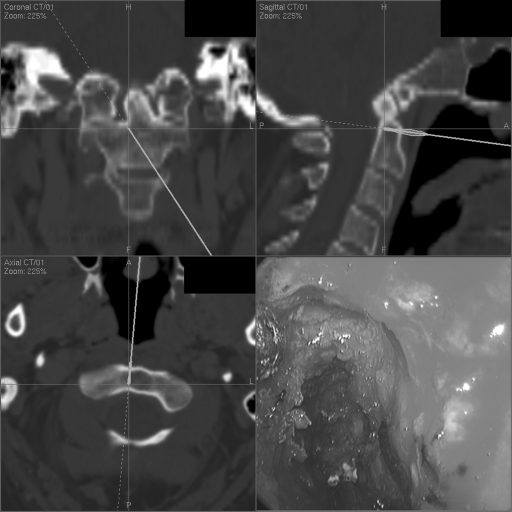
Figure 2.
Endonasal view with corresponding image guidance localization at the finale of odontoidectomy.
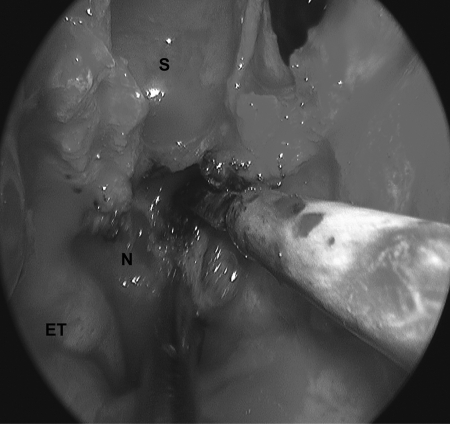
Figure 3.
Endonasal view illustrating the creation of the nasopharyngeal flap. S, sphenoid sinus; ET, eustachian tube; N, nasopharyngeal mucosa.
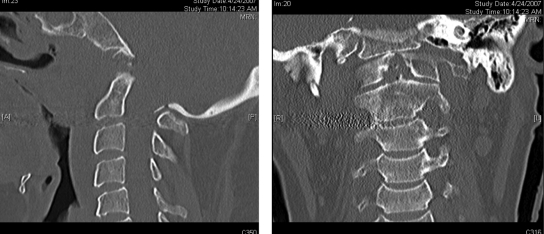
Figure 4.
Sagittal and coronal postoperative computed tomography images revealing absence of the odontoid process.
CASE REPORTS
Case 1
An 85-year-old man presented with a 2-year history of progressively worsening cervical myelopathy with quadriparesis ultimately causing the patient to be bedridden. Examination revealed severe weakness of his arms, with a grade 3/5 for power in the left arm, a grade 4/5 in the right arm, and a power of 4/5 in the lower extremities. He had hyperreflexia with sustained clonus. Imaging revealed a large rheumatoid pannus causing severe spinal stenosis with spinal cord compression at the C1–C2 region. The patient underwent surgical resection of the pannus through C1 and C2. Intraoperative cerebrospinal fluid (CSF) leak occurred, which was controlled at the time of the procedure using Surgicel (Johnson & Johnson Company, Somerville, NJ), Gelfoam, Tisseel, and an infraumbilical fat graft to close the CSF leak defect. The vascularized mucosal flap was then laid to cover the defect. There were no other complications associated with this procedure. On follow-up 2 weeks postoperatively, the patient had an improvement in his strength in all extremities; however, it had not yet completely returned. Radiological evidence of cervical spine instability was present; therefore, the patient underwent a posterior occipital-cervical instrumented fusion with C1 and C2 decompression 1 month following transnasal surgery. He showed gradual clinical improvement and by 6 months postoperatively he was ambulating independently and had grade 5/5 strength in all extremities except hand grip, which was 4/5.
Case 2
A 52-year-old man endured a fall while playing hockey and subsequently complained of neck stiffness and persistent headaches. Neurological examination was unremarkable, but a CT scan revealed a lytic lesion of the C2 vertebra with evidence of instability. Magnetic resonance imaging study revealed an abnormal marrow signal with mild enhancement involving the odontoid process, body and lateral masses of C2, with extension into the anterior pedicles. No evidence of cord compression was detected. A neoplasm was suspected, and the patient underwent partial resection of the odontoid lesion and subsequent occiput to C2 instrumented fusion. There were no periprocedural complications. The pathology report revealed metastatic carcinoma and a full workup yielded no known primary sites. The patient underwent postoperative radiation therapy.
Case 3
A 52-year-old woman presented with complaints of balance problems causing her to veer toward the left with a propensity to fall. She noted worsening of imbalance when looking up and down. Neurological examination revealed horizontal and vertical nystagmus, absence of gag reflex and palate movement, hyperreflexia of all limbs, positive Romberg, and unsteady gait. A CT scan revealed a Chiari malformation with platybasia with basilar invagination causing significant distortion of the cervicomedullary junction and compression up on the mid pons and medulla by the odontoid process. Surgical resection of the clivus and odontoid were performed to decompress the midbrain. There were no surgical complications. However, the patient had a longer stay in the intensive care unit due to airway distress necessitating reintubation. This is likely a result of the residual compression of the brain stem from the Chiari malformation. After extubation, there were no neurological deficits and the patient had significant improvement in her gait and stability.
Approximately 2 weeks postoperatively, the patient returned to the hospital with complaints of neck pain and dysphagia. A cervical X-ray did not reveal an instability or subluxation. It was suspected that the pain was secondary to the Chiari malformation. Therefore, a correction of the Chiari malformation, as well as posterior fusion of the cervical spine, was performed.
Case 4
A 6-year-old boy had previously undergone surgery and chemotherapy for rhabdomyosarcoma of the left cheek. A routine surveillance CT scan 4 years after the initial presentation revealed a lytic lesion involving the C2 vertebral body. The patient was asymptomatic. Neurological examination was found to be unremarkable. He underwent an endoscopic transnasal biopsy of the lytic lesion and posterior instrumented fusion of C1 to C3 for stabilization. There were no complications. Pathologic diagnosis was challenging and multiple opinions were obtained, with the final diagnosis of a telangiectatic osteosarcoma. A repeat CT scan revealed a progression of the lytic C2 lesion. The patient was taken to the operating room 4 months later for partial excision and instrumented fusion of the lesion via a transcervical approach with reconstruction of the odontoid and with an allograft bone. Again, the patient had no sequelae from the surgery apart from mild hoarseness. On 7-month follow-up, there has been no progression of the lesion on imaging and no evidence of cervical instability. More recently, the patient presented with a lesion of his right femur and a biopsy was positive for a high-grade chondroblastic osteosarcoma. He is currently undergoing chemotherapy.
DISCUSSION
Similar to the experience of Kassam and colleagues17 and Alfieri et al,20 we have found that transnasal endoscopy yields an effective means of accessing the craniocervical junction with less morbidity than with a transoral approach. Table 1 provides an overview of the patients. In this series, one patient had an intraoperative CSF leak that was controlled and closed during the operation. All patients had closure of the nasopharyngeal flap using Tisseel with no complications. Early in this experience, the main challenge was the inadequate length of the instruments that made it difficult to access the odontoid. This was overcome by obtaining a drill with a longer and thinner shaft to reach the bottom of C2. We have also attempted to extend the spine and use a 0-degree scope whenever possible. Control of bleeding was a concern early in our experience of over 300 purely endoscopic skull base cases. We have found that the two-surgeon technique using suction to enhance visualization, the use of endoscopic bipolar cautery, and selected packing to be adequate for dealing with bleeding. On average, the blood loss was 200 mL. One patient lost approximately 1 L of blood primarily due to venous bleeding arising directly from a vascular tumor. None of the patients suffered velopharyngeal insufficiency and no patient required a tracheostomy. All four patients were able to tolerate oral fluids on the same day postoperatively, obviating the need for a feeding tube.
Table 1.
Outcome of Patients Undergoing Endoscopic Transnasal Approach to the Craniocervical Junction
| Patient Age (y)/Sex | Indication for Surgery | Duration of Surgery (min) | Blood Loss | Complication | Cervical Spine Fusion | Surgical Outcome |
|---|---|---|---|---|---|---|
| 85/male | Rheumatoid pannus with C1–C2 stenosis | 220 | <250 mL | Intraoperative cerebrospinal fluid leak | One month postoperative | Improved symptoms |
| 52/male | Biopsy lytic lesion of C2, c-spine fusion | 470 | 1 L | Venous bleed from tumor | Intraoperative | Postoperative radiation |
| 52/female | Platybasia, Chiari 1 malformation, basilar invagination | 320 | <200 mL | None | Three weeks postoperative | Correction of Chiari malformation three weeks postoperatively |
| 6/male | Biopsy C2 lesion, c-spine fusion | 295 | <200 mL | None | Intraoperative | Excision of tumor via external approach |
Two patients underwent cervical spine fusion intraoperatively, and the remaining two patients underwent delayed cervical spine fusion approximately 1 month postoperatively. It is expected that the majority of the patients will likely require posterior instrumentation because of the very high likelihood of instability resulting from the resection of the arch of C1 and disruption of ligaments. A transcervical approach may provide some ability to instrument anteriorly, but a robust instrumentation technique for instrumentation at this level has not been described thus far, thereby necessitating posterior instrumentation. In addition, a transcervical approach is more invasive and has higher morbidity than the procedure described here.
Table 2 outlines the advantages and proposed disadvantages of each approach to the craniocervical junction. Several concerns have been raised with respect to the endoscopic transnasal approach. The fossa of Rosenmüller is an important landmark that the surgeon encounters upon utilizing this approach. There is a potential risk of injuring surrounding structures, such as the eustachian tube, vidian nerve, and carotid artery.21 However, we feel that this risk is minimized with the use of image guidance in addition to remaining within the boundary of the eustachian tube and using the sphenoid as a landmark. Another criticism is the loss of the three-dimensional view associated with an endoscope.1 We feel that this is offset by tactile feedback and with experience does not hinder our ability to adequately visualize and identify structures. Certainly, there is a learning curve associated with using the endoscope, which may translate into a longer operative time at least initially. However, we hope that further experience will reduce surgical time and be accompanied by lower morbidity. Crusting did not appear to be a major concern in our patients, despite the fact that they all underwent removal of the posterior aspect of the nasal septum.
Table 2.
A Comparison of Advantages and Disadvantages Associated with Various Approaches to the Craniocervical Junction
| Approach | Advantages | Disadvantages |
|---|---|---|
| Transoral | • Direct route to the craniocervical junction | • Tracheotomy common |
| • Wider surgical field | • Tongue edema common | |
| • Wound infection and dehiscence | ||
| • Feeding tube often required | ||
| • Velopharyngeal insufficiency risk | ||
| • Soft and hard palate splitting for exposure | ||
| Transnasal endoscopic | • No tracheotomy | • Longer operative duration |
| • No tongue edema | • Learning curve | |
| • No velopharyngeal insufficiency | • Nasal crusting | |
| • No feeding tube required | • Hemostasis | |
| • Theoretical decreased risk of infection and dehiscence | • Length of instruments | |
| • Superior visualization | • Risk to surrounding structures | |
| • ?Visualization | ||
| • Closure (theoretical) | ||
| Transcervical | • Enhanced exposure | • Risk of injury to surrounding structures (esophagus, carotid sheath, trachea, recurrent laryngeal nerve) |
| • Skin incision |
Finally, the fourth patient underwent a biopsy of the lytic C2 lesion via the transnasal endoscopic approach. Definitive resection several months later required an external approach due to growth of the lesion beyond that of C2. This highlights the limitation of the endonasal technique, whereby the caudal limit of accessing the craniocervical junction via this approach is C2.17
CONCLUSION
We have illustrated the successful surgical access to the C1–C2 region via a transnasal endoscopic approach in four patients. This approach is an effective means to access the craniocervical junction and avoids many of the disadvantages associated with using the traditional transoral approach. Preliminary studies have shown the potential in using the transnasal endoscopic approach in select patients. Further studies are needed to fully define its utility.
REFERENCES
- Frempong-Boadu A K, Faunce W A, Fessler R G. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51(5 Suppl):S60–S66. [PubMed] [Google Scholar]
- Mummaneni P V, Haid R W. Transoral odontoidectomy. Neurosurgery. 2005;56:1045–1050. discussion 1045–1050. [PubMed] [Google Scholar]
- Crockard H A. Transoral surgery: some lessons learned. Br J Neurosurg. 1995;9:283–293. doi: 10.1080/02688699550041304. [DOI] [PubMed] [Google Scholar]
- Shaha A R, Johnson R, Miller J, Milhorat T. Transoral-transpharyngeal approach to the upper cervical vertebrae. Am J Surg. 1993;166:336–340. doi: 10.1016/s0002-9610(05)80327-7. [DOI] [PubMed] [Google Scholar]
- Hadley M N, Spetzler R F, Sonntag V KH. The transoral approach to the superior cervical spine. A review of 53 cases of extradural cervicomedullary compression. J Neurosurg. 1989;71:16–23. doi: 10.3171/jns.1989.71.1.0016. [DOI] [PubMed] [Google Scholar]
- Dickman C A, Locantro J, Fessler R G. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525–530. doi: 10.3171/jns.1992.77.4.0525. [DOI] [PubMed] [Google Scholar]
- Menezes A H, VanGilder J C. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg. 1988;69:895–903. doi: 10.3171/jns.1988.69.6.0895. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo N. Transoral approach to extradural lesions of the lower clivus and upper cervical spine: an experience of 19 cases. Neurosurgery. 1989;24:37–42. doi: 10.1227/00006123-198901000-00006. [DOI] [PubMed] [Google Scholar]
- Merwin G E, Post J C, Sypert G W. Transoral approach to the upper cervical spine. Laryngoscope. 1991;101(7 Pt 1):780–784. doi: 10.1288/00005537-199107000-00016. [DOI] [PubMed] [Google Scholar]
- Kingdom T T, Nockels R P, Kaplan M J. Transoral-transpharyngeal approach to the craniocervical junction. Otolaryngol Head Neck Surg. 1995;113:393–400. doi: 10.1016/S0194-59989570074-9. [DOI] [PubMed] [Google Scholar]
- Yang S Y, Gao Y Z. Clinical results of the transoral operation for lesions of the craniovertebral junction and its abnormalities. Surg Neurol. 1999;51:16–20. doi: 10.1016/s0090-3019(97)00501-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Huang W, Liang M, et al. Endoscopic transnasal transclival odontoidectomy: a new approach to decompression: technical case report. Neurosurgery. 2008;63(1 Suppl 1):ONSE92–ONSE94. discussion ONSE94. doi: 10.1227/01.neu.0000335020.06488.c8. [DOI] [PubMed] [Google Scholar]
- Magrini S, Pasquini E, Mazzatenta D, Mascari C, Galassi E, Frank G. Endoscopic endonasal odontoidectomy in a patient affected by Down syndrome: technical case report. Neurosurgery. 2008;63:E373–E374. doi: 10.1227/01.NEU.0000315285.84524.74. [DOI] [PubMed] [Google Scholar]
- Nayak J V, Gardner P A, Vescan A D, Carrau R L, Kassam A B, Snyderman C H. Experience with the expanded endonasal approach for resection of the odontoid process in rheumatoid disease. Am J Rhinol. 2007;21:601–606. doi: 10.2500/ajr.2007.21.3089. [DOI] [PubMed] [Google Scholar]
- Laufer I, Greenfield J P, Anand V K, Härtl R, Schwartz T H. Endonasal endoscopic resection of the odontoid process in a nonachondroplastic dwarf with juvenile rheumatoid arthritis: feasibility of the approach and utility of the intraoperative Iso-C three-dimensional navigation. Case report. J Neurosurg Spine. 2008;8:376–380. doi: 10.3171/SPI/2008/8/4/376. [DOI] [PubMed] [Google Scholar]
- Hansen M A, da Cruz M J, Owler B K. Endoscopic transnasal decompression for management of basilar invagination in osteogenesis imperfecta. J Neurosurg Spine. 2008;9:354–357. doi: 10.3171/SPI.2008.9.10.354. [DOI] [PubMed] [Google Scholar]
- Kassam A B, Snyderman C, Gardner P, Carrau R, Spiro R. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57(1 Suppl):E213. doi: 10.1227/01.neu.0000163687.64774.e4. [DOI] [PubMed] [Google Scholar]
- Cavallo L M, Cappabianca P, Messina A, et al. The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst. 2007;23:665–671. doi: 10.1007/s00381-007-0332-7. [DOI] [PubMed] [Google Scholar]
- Messina A, Bruno M C, Decq P, et al. Pure endoscopic endonasal odontoidectomy: anatomical study. Neurosurg Rev. 2007;30:189–194. discussion 194. doi: 10.1007/s10143-007-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Jho H D, Tschabitscher M. Endoscopic endonasal approach to the ventral cranio-cervical junction: anatomical study. Acta Neurochir (Wien) 2002;144:219–225. discussion 225. doi: 10.1007/s007010200029. [DOI] [PubMed] [Google Scholar]
- Wolinsky J P, Sciubba D M, Suk I, Gokaslan Z L. Endoscopic image-guided odontoidectomy for decompression of basilar invagination via a standard anterior cervical approach. Technical note. J Neurosurg Spine. 2007;6:184–191. doi: 10.3171/spi.2007.6.2.184. [DOI] [PubMed] [Google Scholar]