ABSTRACT
We sought to quantify the mean surface area of the exposed diaphragma sellae and the mean sellar volume in the subfrontal and anterolateral approaches to pituitary adenomas and to detail our expansion of the superficial and deep window in the anterolateral approach. We performed a retrospective data analysis and cadaveric study in a clinical and skull base laboratory. We studied eight patients who had anterolateral approach for transcranial resection of pituitary macroadenoma and seven cadaveric specimens. Main outcome measures were degree of tumor resection, cerebrospinal fluid (CSF) leak, cranial nerve outcome, and quantification of the exposed sella via the anterior (subfrontal) and anterolateral approach. We observed complete resection in one; visual outcome: stable in three, improved in four, worsened in one; CSF leakage in two; transient CN III palsy in three; mean surface area (mm2) of exposed diaphragma sellae,115.3 (subfrontal approach) versus 94.7 (anterolateral approach; p = 0.1); mean sellar volume (mm3) exposed, 224.8 (subfrontal approach) versus 569.3 (anterolateral approach; p < 0.0001). Our technical note supports the increased exposure of sellar volume via the anterolateral approach. Despite the relatively high complication rate, complex cranial surgeons should maintain the skills and knowledge of transcranial approaches. Indeed, the rapid expansion of transsphenoidal techniques will continue to decrease the number of cases but will also continue to increase the complexity of those adenomas that are referred for transcranial resection.
Keywords: Pituitary, transcranial, FTOZ, tumor, sella turcica
In 2004, we were invited to write a paper1 on the transcranial removal of pituitary adenomas because, despite the advancements of transsphenoidal and expanded transsphenoidal techniques, transcranial approaches were still needed to resect a small subset of these tumors. The anterior (subfrontal) and anterolateral (pterional) approaches have been traditionally used to remove these tumors.2,3,4,5,6 In this technical note, we compare the difference in surface area of the exposed diaphragma sella and the sellar volume in seven cadaveric specimens between these two approaches. We also report our experience over 6 years using the skull base modification of the anterolateral corridor.
SURGICAL TECHNIQUE
The anterolateral approach to pituitary adenomas is a skull base variation of the standard pterional craniotomy that expands both the superficial and deep working windows.
Expansion of the Superficial Window
Expansion of the superficial working window is achieved by either removal of the orbital rim and/or removal of the zygomatic arch. Both of these expansions can be achieved in a single or two-piece bone flap. In our most recent experience, the orbital rim is routinely removed as a one-piece orbitopterional flap whereas the zygomatic arch is down-fractured as a separate piece to preserve the insertion of the masseter muscle. The orbital osteotomy is routinely included in the approach, but the zygoma is only removed in selected cases if the tumor has significant middle fossa extension. Expansion of the superficial working window decreases the depth of the surgical field and allows the viewing trajectory to be different from the working trajectory. This expansion also allows the sellar space to be approached from different trajectories with minimal manipulation of the optic nerves, chiasm, and tracts.
Expansion of the Deep Window
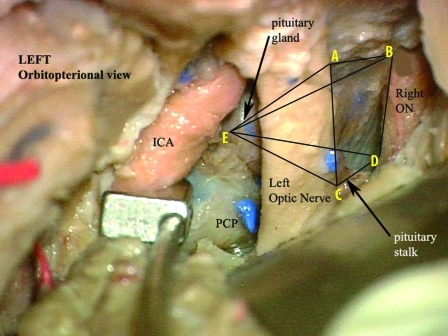
The deep working window is expanded by unroofing the optic canal, anterior clinoidectomy, and sectioning the distal dural ring of the carotid. The anterior clinoidectomy expands the opticocarotid window and allows the surgeon to approach the lateral aspect of the tumor and sellar wall with no retraction of the optic nerve. Finally, sectioning of the distal dural ring allows lateral retraction of the carotid and further expansion of the opticocarotid space (Fig. 1).
Figure 1.
Cadaveric dissection photograph of a left-sided approach. The anterior clinoid process and the distal dural ring of the carotid has been removed and cut. The internal carotid artery (ICA) is retracted laterally to increase the size of the opticocarotid window. Measurements of the area and volume were based on five different points (A, B, C, D, and E). These were the points reachable with a straight probe without retraction of the optic nerve or chiasm. These points were determined using the anterolateral approach and the midline subfrontal approach and used to calculate the surface area and volume exposed using a contact digitizer. PCP, posterior clinoid process; ON, optic nerve. (With permission from the University of South Florida, Department of Neurosurgery.)
Sylvian Fissure and Lesser Sphenoid Wing
Opening of the sylvian fissure and complete flattening of the lesser wing of the sphenoid are basic steps of the standard pterional approach. Similarly, in our technique, the complete removal of the roof and lateral wall of the orbit allows an unobstructed subfrontal view to the sella and optic chiasm. Opening of the sylvian fissure allows the frontal and temporal lobe to fall away from each other and further enhances the wide working space with minimal retraction.
Tumor Removal
Once the superficial and deep windows have been appropriately expanded, the removal of the tumor can be tailored to each part of the tumor mass and its relationship to the optic nerve, chiasm, and tracts. The working trajectory can easily interchange between the subfrontal, trans-sylvian, prechiasmatic, and opticocarotid trajectories. To access the intrasellar portion of the tumor, a cut is made in the lateral wall of the sella starting at the posterior clinoid process and extending forward toward the decompressed optic nerve. This cut opens the intrasellar compartment and allows direct access to the tumor via the opticocarotid window (Fig. 2). Minor bleeding from the circular sinus is occasionally encountered and controlled with packing of hemostatic material. Major bleeding from the cavernous sinus is hardly ever encountered as the sinus itself is either compressed or invaded by tumor. The anterolateral approach allows the surgeon to maintain a direct vision of the pituitary stalk on the posterior aspect of the adenoma during the resection of the tumor.
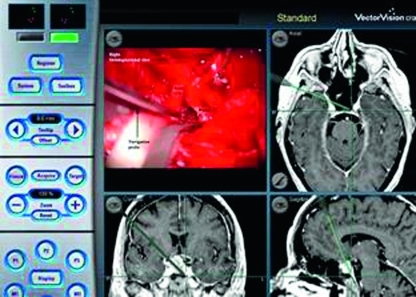
Figure 2.
Intraoperative photograph of a right orbitopterional approach; microscopic (upper left corner) and neuronavigation (Brainlab AG, Feldkirchen, Germany). The neuronavigation probe is in the sellar compartment through the opticocarotid window. (With permission from the University of South Florida, Department of Neurosurgery.)
Tumor Capsule
We believe that the same principles of tumor resection in the transsphenoidal approach should be maintained when the tumor is approached transcranially. The intimate relationship of large pituitary adenomas with the optic nerve and chiasm involves sharing of microvascularization between the tumor capsule and these structures. We have stayed clear from attempting a complete resection of the tumor capsule, in particular, the parts that are in contact with the optic pathways. Although part of the capsule has to be resected to access the tumor, our resection technique remains an intracapsular debulking similar to the technique applied when these tumors are resected transsphenoidally.
CADAVERIC STUDY
Seven formalin-fixed, latex-injected cadaveric specimens were analyzed to determine the extent of exposure of the sellar compartment obtained after either the anterolateral (fronto temporal orbito zygomatic [FTOZ]) approach (14 sides) or anterior (subfrontal) approach (seven midline exposures). After placement in a Mayfield three-point head holder, a low bifrontal craniotomy was performed, the frontal lobes were retracted, and the interhemispheric fissure was partially opened to release arachnoidal adhesions. Under the operating microscope (Carl Zeiss AG, Oberkochen, Germany), we identified the furthest corners of the diaphragma sellae that was reachable with a straight probe without retraction of the optic nerve and chiasm (points A, B, C, D; Fig. 1). This surface area was measured using a 3-D contact digitizer (Microscribe G2, Immersion Corporation, San Jose, CA). After incision of the diaphragma sellae, the contact digitizer probe was inserted as far and as deeply as possible without compressing or retracting the optic nerves to determine the volume of sellar space that was readily accessible using a straight instrument under direct vision (point E). The volume delimited by the points A, B, C, D, and E was again calculated using the contact digitizer (Fig. 1).
On each side, a frontotemporo-orbital craniotomy and extradural anterior clinoidectomy were performed with unroofing of the optic canal. The dura was opened along the sylvian fissure ending in a T-shaped manner under the frontal and temporal lobes. The distal dural ring was then cut and the anterior loop of the internal carotid artery was retracted laterally. At this point, the ipsilateral tuberculum sellae and posterior clinoid process were identified. The pituitary stalk was visualized and preserved. The same points A, B, C, D, and E were then measured from the anterolateral exposure to determine the surface of diaphragma sellae and the sellar volume reachable without retraction on the optic nerves and chiasm.
Measurements of sellar surface and volume obtained with the right and left FTOZ were averaged for each head. Two single-factor analyses of variance were used to compare mean differences in volume and surface between the subfrontal and FTOZ approaches. Alpha was set to the 0.05 level of probability to control for type I error.
Anatomic Morphometric Results
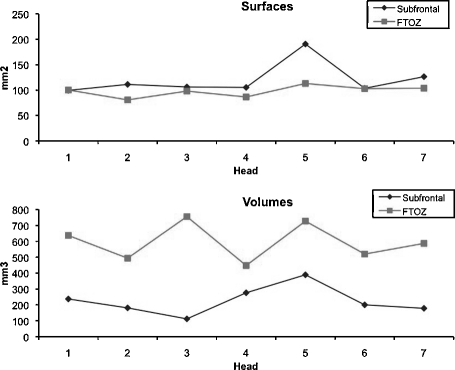
Mean ± standard deviation of the exposed surface area of diaphragma sellae was 115.3 ± 33 mm2 by a subfrontal approach and 94.7 ± 17.8 mm2 by an FTOZ approach (p = 0.1). Mean sellar volume accessible by a straight probe was 224.8 ± 94.3 mm3 by a subfrontal approach and 596.33 ± 193.5 mm3 by an FTOZ approach (p < 0.0001; Fig. 3).
Figure 3.
Graphic representation of the mean surface area of the exposed diaphragma sellae and mean sellar volumes measured in each cadaveric specimen via a bifrontal craniotomy subfrontal and anterolateral approach.
CLINICAL CASES
Eight consecutive patients underwent the transcranial removal of a pituitary adenoma by the senior author (H.V.L.) between 1998 and 2004. Our study protocol was approved by the Tampa General Hospital and University of South Florida Institutional Review Boards.
There were three men and five women. Their clinical characteristics are summarized in Table 1. Five patients had undergone previous surgery for attempted extirpation of the adenoma (four transsphenoidal and one transcranial), and three patients were referred to us without previous intervention because the tumor was considered inaccessible transsphenoidally. Pituitary function was normal in two patients and decreased in three. Three patients presented with functioning adenomas (one growth-hormone secreting, one corticotropin secreting, and one prolactinoma). The prolactinoma was a giant tumor that was resistant to a trial of bromocriptine (Fig. 4). All patients underwent a tailored anterolateral approach. Patient 3 required bilateral craniotomies and patient 4 declined a second surgery for further resection of her tumor.
Table 1.
Summary of Eight Patients Who Underwent an Anteroloateral (FTOZ) Transcranial Approach for Pituitary Adenomas
| Sex, Age (y) | Presentation | Preoperative Visual Function | Tumor Size (cm) | Previous surgery | Degree of resection | Visual Outcome | Other Cranial Nerves Deficits/Outcome | CSF Leak | Meningitis | |
|---|---|---|---|---|---|---|---|---|---|---|
| CSF, cerebrospinal fluid; TS, transsphenoidal; VFD, visual field defect. | ||||||||||
| 1 | F, 35 | Cushing | Normal | 2.8 | No | >90% | Normal | Yes | No | |
| 2 | F, 48 | Visual | Unilateral VFD | 3.5 | Transcranial | >90% | Unchanged | No | No | |
| 3 | M, 20 | Visual | Left eye light perception only; right eye normal | 9 | No | >90% | Improved to ability to read (continued and graduated from college) | Bilateral III, functional recovery (no diplopia) | Yes | No |
| 4 | F, 49 | Visual | Right eye blindness; left eye minimal light perception | 4 | TS | <90% | Left eye improved, able to ambulate and function independently, not able to read and drive; right eye persistent blindness | Right partial III, recovered eye movements. No diplopia (right eye blind) | No | No |
| 5 | F, 65 | Visual | Unilateral VFD | 2.6 | TS | >90% | Improved to normal | Right partial III Complete recovery (no diplopia, no ptosis) | No | No |
| 6 | F, 56 | Acromegaly | Normal | 2.5 | TS | >90% | Monocular blindness R side | No | No | |
| 7 | M, 57 | Visual | Monocular blindness | 4.3 | TS | Total | Unchanged | No | No | |
| 8 | M, 31 | Visual | Bilateral VFD | 2.8 | No | >90% | Improved to normal | No | No | |
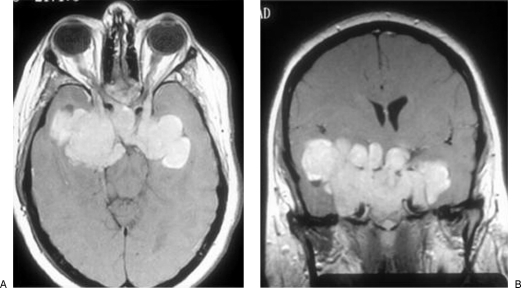
Figure 4.
Patient 3: preoperative gadolinium-enhanced axial (A) and coronal (B) T1-weighted images of a giant prolactinoma that was unresponsive to dopamine agonists.
Outcome Assessment
The extent of tumor removal was determined exclusively on the postoperative magnetic resonance imaging scans. It was classified as partial if <90% of the tumor was removed and subtotal if >90% was removed. Resection was only considered total with agreement of the surgeon's surgical report and the absence of visible tumor on postoperative magnetic resonance imaging scans.
Degree of Resection and Adjuvant Therapy
Six patients had a subtotal resection, one had complete resection, and one had partial resection (Table 1). All patients with subtotal or partial resection underwent postoperative radiation therapy.
Visual Outcome, Cranial Nerve Deficits, and Cerebrospinal Fluid Leak
VISION
Visual function remained unchanged in three patients (patient 1 with normal preoperative vision, patient 2 with preoperative unilateral visual field defect, and patient 7 with preoperative monocular blindness). Vision improved in patients 3, 4, 5, and 8. Patient 6 developed a postoperative monocular blindness on the side of the craniotomy.
CEREBROSPINAL FLUID LEAK
Although no patient developed meningitis, patients 1 and 3 suffered postoperative cerebrospinal fluid leakage. Patient 1 was successfully treated with a lumbar drain, and patient 3 underwent transsphenoidal repair of the leak.
CRANIAL NERVES OUTCOME
Postoperatively, patients 4 and 5 had transient oculomotor nerve palsies. Patient 3 had bilateral oculomotor nerve palsies after bilateral craniotomies; he also recovered and was able to successfully attend and graduate from college.
DISCUSSION
We describe the anterolateral approach as a safe and effective corridor for the transcranial resection of pituitary adenomas. Our cadaveric study further demonstrates that the volume of the intrasellar tumor that can be exposed is greater in the anterolateral approach rather than the midline subfrontal corridor.
These results are not meant to challenge the validity of transsphenoidal approaches as the default technique for the removal of pituitary adenomas but are rather presented to provide guidance when transsphenoidal approaches have either failed or appear contraindicated.
Pituitary adenomas that are approached transcranially are usually firm, large, and invasive. In this subset of tumors, the main goals of the resection are decompression of the optic apparatus and debulking of the mass in anticipation for postoperative irradiation. Long-lasting endocrine cure is not the main goal and is rarely, if ever, achieved.
The application of skull base techniques in the anterolateral corridor improves exposure by expanding both the superficial and the deep working windows—the former by removing the orbital rim, the later by removing the anterior clinoid process, sectioning the distal dural ring, and retracting the internal carotid artery laterally.1 Decompression of the optic nerve early in the case decreases the risk of optic nerve injury during tumor manipulation and removal.
Exposing the intrasellar compartment through the opticocarotid window relies on the ability to incise the dural membrane that covers the lateral sellar opening between the anterior and posterior clinoid processes. Cutting the lateral wall of the sella can only be safely accomplished after the opticocarotid window has been enlarged using the steps summarized above and described elsewhere.1 The key to safely open the lateral sellar wall is the identification of the posterior clinoid process. Because the anterior clinoid process has been removed, the carotid has been retracted laterally, and the normal anatomy is distorted by the tumor, the posterior clinoid process is the only stable and reliable landmark to the posterolateral insertion of the diaphragma sellae and the posterosuperior insertion of the lateral sellar wall. The incision should start at the posterior clinoid process and proceed anteriorly toward the optic strut or what is left of it. In cadaveric dissections, the pituitary gland is readily visible, whereas during surgery, the adenoma herniates under pressure through this incision.
In our cadaveric study, we were able to demonstrate that this approach provided greater exposure of the intrasellar content than the subfrontal corridor. Our results ignore the advantages of endoscopes and angled instruments as straight probes only were used to obtain these measurements. Although angled curettes are routinely used in pituitary surgery, we believe that direct visualization of the tip of the instruments, for the intrasellar resection of these often firm tumors, adds a significant degree of safety that should not be disregarded.
In 1997, Dolenc7 reported a similar transcranial approach to the sella to remove invasive pituitary adenomas. Our surgical strategy conceptually differs from Dolenc's, although we used same initial steps (e.g., resection of the orbital rim, resection of the anterior clinoid process, unroofing of the optic canal, and sectioning of the distal dural ring of the carotid). Once the osteotomies are performed, our approach remains strictly intradural, whereas in Dolenc's description, the tumor is removed extradurally through a sequential opening of the cavernous sinus triangles.
Despite the refinement of the approaches and the inclusion of skull base techniques, pituitary adenomas that require transcranial approaches remain formidable tumors that continue to challenge the skills of even an experienced skull base team. The high complication rate in our clinical series for both cerebrospinal fluid leak and postoperative visual deterioration are the testimony of the complexity of these tumors.
CONCLUSION
We present our clinical experience and an anatomic study using the anterolateral corridor as an effective approach to pituitary adenomas when classic or expanded transsphenoidal approaches fail. Despite the relatively high complication rate, we believe that complex cranial surgeons should maintain the skills and knowledge of these transcranial approaches to pituitary adenomas. Indeed, the rapid expansion of transsphenoidal techniques will continue to decrease the number of cases but will also continue to increase the complexity of those tumors that are referred for transcranial resection.
ACKNOWLEDGMENTS
During his research year in the skull base laboratory of the University of South Florida, Department of Neurosurgery, Siviero Agazzi, M.D., was supported by a research and educational grant by the SICPA foundation and Decker Fund, both in Lausanne Switzerland.
REFERENCES
- Youssef A S, Agazzi S, van Loveren H R van. Transcranial surgery for pituitary adenomas. Neurosurgery. 2005;57(1 Suppl):168–175. discussion 168–175. doi: 10.1227/01.neu.0000163602.05663.86. [DOI] [PubMed] [Google Scholar]
- Landolt A M. History of pituitary surgery from the technical aspect. Neurosurg Clin N Am. 2001;12:37–44. vii–viii. [PubMed] [Google Scholar]
- Pollock J R, Akinwunmi J, Scaravilli F, Powell M P. Transcranial surgery for pituitary tumors performed by Sir Victor Horsley. Neurosurgery. 2003;52:914–925. discussion 925–926. doi: 10.1227/01.neu.0000053148.34310.bb. [DOI] [PubMed] [Google Scholar]
- Symon L, Jakubowski J. Transcranial management of pituitary tumours with suprasellar extension. J Neurol Neurosurg Psychiatry. 1979;42:123–133. doi: 10.1136/jnnp.42.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symon L, Jakubowski J, Kendall B. Surgical treatment of giant pituitary adenomas. J Neurol Neurosurg Psychiatry. 1979;42:973–982. doi: 10.1136/jnnp.42.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen S, Myllymäki K. Outcome of patients after transcranial operation for pituitary adenoma. Ann Clin Res. 1986;18(Suppl 47):43–45. [PubMed] [Google Scholar]
- Dolenc V V. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery. 1997;41:542–550. discussion 551–552. doi: 10.1097/00006123-199709000-00007. [DOI] [PubMed] [Google Scholar]