ABSTRACT
Stereotactic radiosurgery has become a more frequently used treatment modality for vestibular schwannomas; a few reports of malignant transformation and/or radiation-associated tumors have surfaced. The majority of these reported cases were in patients with underlying neurofibromatosis. The authors report a case of a 74-year-old man with rapid progression of a cerebellar-pontine angle tumor 14 years after surgical resection of a vestibular schwannoma (VS) from the same site, and 6 years after stereotactic radiosurgery. A pathological study of the recent tumor showed a high-grade spindle cell neoplasm that bore no resemblance to the initial schwannoma. The patient had no diagnosis of neurofibromatosis. Secondary malignancy occurred in a non-neurofibromatosis patient 6 years after stereotactic radiosurgery. It is our belief that documentation of such cases will provide important evidence that helps evaluate the long-term effect of radiosurgery for VS. Such observations can influence clinical decisions regarding the choice of treatment modalities.
Keywords: Malignant transformation, secondary malignancy, vestibular schwannoma, radiosurgery
Vestibular schwannomas (VSs) usually are benign lesions that account for ∼10% of intracranial tumors. Malignant VS is a rare entity with only a few reported cases.1 Rare transformations of benign schwannomas to malignant nerve sheath tumors have been reported, typically in patients with neurofibromatosis 2 (NF2).2 With the more recent use of stereotactic radiosurgery for VS treatment, there have been some case reports on malignant transformation and/or radiation-associated malignancy, mostly in patients with NF2.3,4,5,6,7
CASE REPORT
Clinical Presentation
The patient was a 74-year-old man who underwent left suboccipital craniotomy and resection of a 2.5- to 3-cm diameter VS in 1993 in another hospital. The lesion was diagnosed as a VS, and this was confirmed by our pathological review. Since the original resection, the patient was followed with serial magnetic resonance imaging (MRI). Recurrence of the intracanalicular component was noted, and he was treated with one session of stereotactic radiosurgery in 2001 in the same hospital. He received 12.5 Gy to the 80% isodose surface. No change in size was detected of the intracanalicular lesion on subsequent MRIs until December of 2006. A head computed tomography (CT) was also obtained at that time. The patient received a pacemaker in December of 2006, and he received only CT scans thereafter (Fig. 1). In October of 2007, the patient developed worsening dizziness, ataxia, facial numbness, facial palsy, hoarseness, and difficulty swallowing over several weeks. A CT scan showed an enhancing lesion at the previous surgical site with significant mass effect on the brain stem (Fig. 2). This had significantly increased in size compared with the previous CT scan. The patient was referred to our institution for surgical management. On examination, he had a complete left facial paralysis, hearing loss (baseline since last resection), hoarseness, left deviation of uvula, decreased palate elevation on the left, and significant gait ataxia.
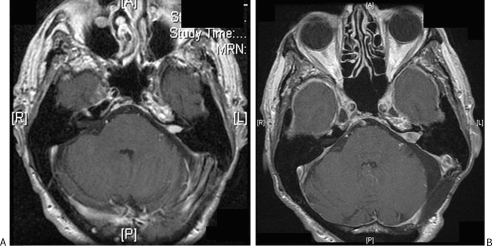
Figure 1.
Serial magnetic resonance imaging scans of the patient. (A) December 1999. (B) December 2006 prior to pacemaker insertion.
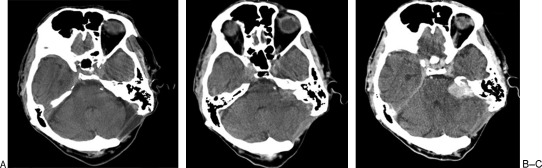
Figure 2.
Serial CT scans of the patient. (A) December 2006 prior to pacemaker insertion. (B) October 2007, noncontrast. (C) October 2007, with contrast.
Operation
The patient was taken to the operating room, and an operation was performed through a left retrosigmoid craniotomy and craniectomy. Once the tumor was identified, severe adhesions between the tumor and the blood vessels as well as the brain stem were noted. A total microsurgical resection of the tumor was performed. End-to-end repair of damaged anterior inferior cerebellar artery using 10–0 nylon sutures was done intraoperatively. The facial nerve stump was seen entering the tumor. It was dissected out, but even at stimulation of 5 mA intraoperatively, there was no response from the facial muscles, indicating the patient had a chronic facial paralysis without any recovery from his prior surgery.
Histological Examination
The slides from the initial surgery were obtained and demonstrated a schwannoma. The final diagnosis of the recent lesion was a high-grade, undifferentiated sarcoma. The new lesion appeared unrelated or so transformed that it bore no histological or immunohistological resemblance to schwannoma or nerve sheath tumors. The sarcoma was high grade with high mitotic activity, with variable but generally high nuclear-cytoplasmic ratios, primitive spindled cells, and focal necrosis. A de novo radiation-induced sarcoma was considered plausible. Other differential diagnoses were felt not adequately supported by histology, including malignant peripheral nerve sheath tumor, leiomyosarcoma, synovial sarcoma, meningeal sarcoma, hemangiopericytoma/solitary fibrous tumor, or fibrosarcoma (Fig. 3).
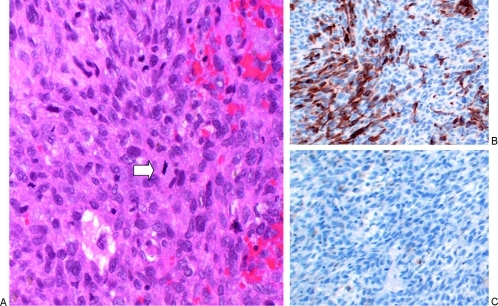
Figure 3.
Pathological findings of the postirradiation tumor. (A) A high-magnification (100×) image from a slide stained with hematoxylin and eosin shows a hypercellular neoplasm with spindle cells, pleomorphic, hyperchromatic nuclei, and mitotic figures (white arrow in A; 32 mitotic figures were found in 10 high-power fields). There is a small amount of extravasated blood. Areas of necrosis were also seen (not shown). (B) Desmin immunohistochemical stain shown at medium magnification (10×). A portion of the neoplastic cells are positive for desmin (a protein expressed in muscle). (C) S-100 immunohistochemical stain. Negative for S-100 (a protein expressed in neural crest–derived cells including Schwann cells).
Hospital Course
Postoperatively, the patient continued to have complete left facial nerve palsy. He also had left vocal cord dysfunction with dysphagia. A medialization of the vocal cords was performed by the otolaryngology voice specialist surgeon. Because of left lagophthalmos and ectropion, the ophthalmology service was consulted to perform a temporary tarsorrhaphy. A gold weight placement in the upper lid was planned. He was transferred to the inpatient rehabilitation service for further care. Palliative radiation treatment was also being considered. About a month after the operation, the patient experienced acute desaturation with copious secretions and supraventricular tachycardia with the heart rate into 170s. He was transferred to the intensive care unit. The patient remained alert and cognizant and decided with his family to implement comfort care and expired soon after.
DISCUSSION
Malignant transformation of a VS is a rare event and some of the previously described cases have been associated with prior radiation treatment for benign VS.4,5,6,7 Patients with NF2 mutations were found to account for most of the malignant transformations reported in the literature after surgical resection and radiation (8 of 12 patients; Table 1). The patient reported herein had no signs or symptoms of NF2. This patient's recurrent tumor fits the criteria defined by Cahan et al8 for a radiation-associated tumor. These criteria are: (1) a patient with a history of radiation, (2) a latency period on the order of months to years, (3) tumor recurrence within the radiation field, and (4) a recurrent tumor that is of different histology than the initial lesion.
Table 1.
Summary of Cases of Occurrence of New Tumors after Radiotherapy for Benign Conditions
| Ref | Patient (Age at Time of XRT [y] and Gender) | Primary Tumor | Initial Surgery | XRT* | Second Tumor | Time Elapsed from XRT (y) | Outcome |
|---|---|---|---|---|---|---|---|
| Adapted from Balasubramaniam et al.3 Ref, reference; XRT, radiotherapy; GBM, glioblastoma multiforme; GKS, gamma-knife surgery; NF2, neurofibromatosis 2; N/A, not available; VS, vestibular schwannoma; Translab, translabyrinthine; SFRT, stereotactic fractionated radiotherapy; mening, meningioma; dx, diagnosis; fx, fractions; VPS, ventriculoperitoneal shunt; ?, type of treatment and dose unknown; SOC, suboccipital craniotomy; MPNST, malignant peripheral nerve sheath tumor. | |||||||
| 3 | 64 F | VS, NF2− | Translab | SFRT 50 Gy/25 fx | GBM | 5 | Died 4 mo later |
| 19 | 63 F | Mening | No | GKS 40 Gy/20 Gy | GBM | 7 | N/A |
| 20 | 57 F | VS, NF2− | No | GKS 27.5 Gy/11 Gy | GBM | 7.5 | N/A |
| 21 | 14 M | AVM | VPS | GKS 40 Gy/20 Gy | GBM | 7 | Died 10 mo later |
| 22 | 66 F | Cavernoma | No | GKS 20 Gy/10 Gy | GBM | 13 | N/A |
| 23 | N/A | VS, NF2+ | N/A | Yes, ? | Malignant ependymoma | N/A | N/A |
| 23 | N/A | VS, NF2+ | N/A | Yes, ? | Malignant mening | N/A | N/A |
| 24 | 53 F | Mening | No | GKS×3, 60 Gy/30 Gy | Malignant osteosarcoma | 4 | N/A |
| 25 | 41 M | Pituitary | Yes | Proton 87 Gy/25 fx | Benign mening | 16 | N/A |
| 25 | 53 M | Pituitary | Yes | Proton 104 Gy/12 fx | VS | 19 | N/A |
| 6 | 26 F | VS, NF2− | SOC | GKS 34 Gy/17 Gy | MPNST | 6 | Died 10 mo later |
| 23 | N/A | VS, NF2+ | N/A | Yes, ? | MPNST | N/A | N/A |
| 23 | N/A | VS, NF2+ | N/A | Yes, ? | MPNST | N/A | N/A |
| 23 | N/A | VS, NF2+ | N/A | Yes, ? | MPNST | N/A | N/A |
| 26 | 30 F | VS, NF2+ | No | GKS ?/15 Gy | MPNST | 4 | Died 2 mo later |
| 4 | 44 M | VS, NF2− | No | GKS 34 Gy/14.36 Gy | Triton | 5 | Died 1 y later |
| 26 | 18 F | VS, NF2+ | No | Yes, ? ?/20 Gy | Triton | 6 | N/A |
| 27 | 20 F | VS, NF2+ | No | GKS 20 Gy/10–15 Gy | Meningiosarcoma | 6 | Died 2 y later |
| 28 | 23 M | VS, NF2+ | No | GKS ?/15 Gy | Rapid regrowth | 2 | Died |
| 5 | 56 F | VS, NF2− | Yes | GKS ?/15 Gy | Rapid regrowth | 0.5 | Died 1 y later |
For gamma-knife surgery treatment, central dose and peripheral dose are reported as: central dose Gy/peripheral dose Gy.
Radiation in the form of stereotactic radiosurgery or fractionated stereotactic radiotherapy is a commonly used modality for VS treatment.9 A series of retrospective studies, varying from 70 to 829 patients who were treated with radiotherapy (median margin dose 13 to 14.6 Gy or 54 Gy total with 1.8 Gy per fraction) for VSs, with 10- to 25-year follow-up, have demonstrated that radiation provides good tumor control (up to 97 to 98%), good functional preservation and low toxicity.10,11,12,13
It is well known from historical reports that radiation-associated tumors do occur.14 For benign tumors, the most concerning reports are of malignant transformation of the same tumor type or induction of a true secondary malignancy. Of the later, more aggressive gliomas and sarcomas are of greatest concern due to the lack of effective treatments. For VS, there have been three reported cases of malignant transformation of a benign VS treated with radiosurgery5,6,7 as well as one spontaneous transformation reported without radiation therapy.2
The University of Michigan reported their incidence of radiation-associated sarcomas throughout the body following ortho- and megavoltage radiation as 23 of 22,306 patients exposed (0.1%).15 Specifically for cranial sarcomas, University of California, San Francisco has reported 7 of 2868 (0.24%) patients exposed through cranial irradiation.16 These two previous reports may be somewhat limited in extrapolating to this current case because of the mixture of radiation energies and not being delivered in a stereotactic manner.
Fourteen cases of malignant transformation and/or radiation-associated VS have been reported in the literature (Table 1).3 Of all the patients whose outcomes were reported, all succumbed to the malignancy within 2 years. Four of the 14 cases reported pathologically confirmed radiation-associated tumors. Two of them did not have NF2, and both had glioblastoma multiforme as the final diagnosis. To put this in a proper prospective for patients, the incidence of death upfront from surgical resection has been reported to be as high as 0.5%.17 However, many of the mortalities probably occur with large or giant-sized tumors rather than tumors less than 3 cm, which are typically treated by radiosurgery. Thus, the surgical mortality for tumors of the latter size is unknown, but is probably very low.
The genetic abnormalities of NF2 patients, who are prone to develop VSs, have been located to a tumor suppressor gene called NF2 on chromosome 22, which encodes the protein named merlin. Biallelic inactivations of NF2 have also been found in nearly all sporadic schwannoma cases.18 It is possible that DNA damage resulting from radiation therapies contribute to the malignant changes in the tumorigenesis-prone background in VS.
CONCLUSIONS
Radiation-induced sarcomas can occur in patients treated for VSs even without an underlying genetic tumor predisposition such as neurofibromatosis.
REFERENCES
- Gonzalez L F, Lekovic G P, Eschbacher J, Coons S, Spetzler R F. A true malignant schwannoma of the eighth cranial nerve: case report. Neurosurgery. 2007;61:E421–E422. discussion E422. doi: 10.1227/01.NEU.0000255517.19709.FC. [DOI] [PubMed] [Google Scholar]
- McLean C A, Laidlaw J D, Brownbill D SB, Gonzales M F. Recurrence of acoustic neurilemoma as a malignant spindle-cell neoplasm. Case report. J Neurosurg. 1990;73:946–950. doi: 10.3171/jns.1990.73.6.0946. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A, Shannon P, Hodaie M, Laperriere N, Michaels H, Guha A. Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: case report and review of the literature. Neuro-oncol. 2007;9:447–453. doi: 10.1215/15228517-2007-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comey C H, McLaughlin M R, Jho H D, Martinez A J, Lunsford L D. Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg. 1998;89:653–658. doi: 10.3171/jns.1998.89.4.0653. [DOI] [PubMed] [Google Scholar]
- Hanabusa K, Morikawa A, Murata T, Taki W. Acoustic neuroma with malignant transformation. Case report. J Neurosurg. 2001;95:518–521. doi: 10.3171/jns.2001.95.3.0518. [DOI] [PubMed] [Google Scholar]
- Shin M, Ueki K, Kurita H, Kirino T. Malignant transformation of a vestibular schwannoma after gamma knife radiosurgery. Lancet. 2002;360:309–310. doi: 10.1016/S0140-6736(02)09521-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson J S, Reid H, Armstrong G R. Malignant transformation of a recurrent vestibular schwannoma. J Clin Pathol. 2004;57:109–110. doi: 10.1136/jcp.57.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahan W G, Woodard H Q, Higinbothan N L, et al. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Rutherford S A, King A T. Vestibular schwannoma management: what is the “best” option? Br J Neurosurg. 2005;19:309–316. doi: 10.1080/02688690500305399. [DOI] [PubMed] [Google Scholar]
- Chan A W, Black P M, Ojemann R G, et al. Stereotactic radiotherapy for vestibular schwannomas: favorable outcome with minimal toxicity. Neurosurgery. 2005;57:60–70. discussion 60–70. doi: 10.1227/01.neu.0000163091.12239.bb. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102:10–16. doi: 10.3171/jns.2005.102.1.0010. [DOI] [PubMed] [Google Scholar]
- Lunsford L D, Niranjan A, Flickinger J C, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195–199. [PubMed] [Google Scholar]
- Pollock B E, Driscoll C LW, Foote R L, et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77–85. discussion 77–85. doi: 10.1227/01.NEU.0000219217.14930.14. [DOI] [PubMed] [Google Scholar]
- Ron E, Modan B, Boice J D, Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- Amendola B E, Amendola M A, McClatchey K D, Miller C H., Jr Radiation-associated sarcoma: a review of 23 patients with postradiation sarcoma over a 50-year period. Am J Clin Oncol. 1989;12:411–415. [PubMed] [Google Scholar]
- Chang S M, Barker F G, II, Larson D A, Bollen A W, Prados M D. Sarcomas subsequent to cranial irradiation. Neurosurgery. 1995;36:685–690. doi: 10.1227/00006123-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Barker F G, II, Carter B S, Ojemann R G, Jyung R W, Poe D S, McKenna M J. Surgical excision of acoustic neuroma: patient outcome and provider caseload. Laryngoscope. 2003;113:1332–1343. doi: 10.1097/00005537-200308000-00013. [DOI] [PubMed] [Google Scholar]
- McClatchey A I. Neurofibromatosis. Annu Rev Pathol. 2007;2:191–216. doi: 10.1146/annurev.pathol.2.010506.091940. [DOI] [PubMed] [Google Scholar]
- Yu J S, Yong W H, Wilson D, Black K L. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576–1577. doi: 10.1016/S0140-6736(00)03134-2. [DOI] [PubMed] [Google Scholar]
- Shamisa A, Bance M, Nag S, et al. Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. Case report and review of the literature. J Neurosurg. 2001;94:816–821. doi: 10.3171/jns.2001.94.5.0816. [DOI] [PubMed] [Google Scholar]
- Kaido T, Hoshida T, Uranishi R, et al. Radiosurgery-induced brain tumor. Case report. J Neurosurg. 2001;95:710–713. doi: 10.3171/jns.2001.95.4.0710. [DOI] [PubMed] [Google Scholar]
- Salvati M, Frati A, Russo N, et al. Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol. 2003;60:60–67. discussion 67. doi: 10.1016/s0090-3019(03)00137-x. [DOI] [PubMed] [Google Scholar]
- Baser M E, Evans D GR, Jackler R K, Sujansky E, Rubenstein A. Neurofibromatosis 2, radiosurgery and malignant nervous system tumours. Br J Cancer. 2000;82:998. doi: 10.1054/bjoc.1999.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno N, Hayashi S, Shimura T, Maeda S, Teramoto A. Intracranial osteosarcoma after radiosurgery—case report. Neurol Med Chir (Tokyo) 2004;44:29–32. doi: 10.2176/nmc.44.29. [DOI] [PubMed] [Google Scholar]
- Loeffler J S, Niemierko A, Chapman P H. Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery. 2003;52:1436–1440. discussion 1440–1442. doi: 10.1227/01.neu.0000064809.59806.e8. [DOI] [PubMed] [Google Scholar]
- Bari M E, Forster D MC, Kemeny A A, Walton L, Hardy D, Anderson J R. Malignancy in a vestibular schwannoma. Report of a case with central neurofibromatosis, treated by both stereotactic radiosurgery and surgical excision, with a review of the literature. Br J Neurosurg. 2002;16:284–289. doi: 10.1080/02688690220148888. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Mirz F, Wetke R, Astrup J, Bojsen-Møller M, Nielsen E. Intracranial sarcoma in a patient with neurofibromatosis type 2 treated with gamma knife radiosurgery for vestibular schwannoma. Am J Otol. 2000;21:364–370. doi: 10.1016/s0196-0709(00)80046-0. [DOI] [PubMed] [Google Scholar]
- McEvoy A W, Kitchen N D. Rapid enlargement of a vestibular schwannoma following gamma knife treatment. Minim Invasive Neurosurg. 2003;46:254–256. doi: 10.1055/s-2003-42347. [DOI] [PubMed] [Google Scholar]