ABSTRACT
The literature regarding recurrences in patients with cranial chondrosarcoma is limited to small series performed at single institutions, raising the question if these data precisely reflect the true recurrence of this tumor for guiding the clinician in the management of these patients. An extensive systematic review of the English literature was performed. The patients were stratified according to treatment modality, treatment history, histological subtype, and histological grade, and the recurrence rates were analyzed. A total of 560 patients treated for cranial chondrosarcoma were included. Five-year recurrence rate among all patients was 22% with median follow-up of 60 months and median disease-free interval of 16 months. Tumor recurrence was more common in patients who only received surgery or had mesenchymal subtype tumors. Our systematic review closely reflects the actuarial recurrence rate and provides predictive factors in the recurrence of cranial chondrosarcoma.
Keywords: Cranial chondrosarcoma, recurrence, systematic review
Intracranial chondrosarcomas, rare tumors arising from the skull base, have high associated morbidity from the tumor itself as well as from treatment modalities. Although the pathogenesis of these tumors remains unclear, it has been proposed that intracranial chondrosarcomas develop from the chondrocytes within rests of endochondral cartilage present within the skull base.1 In contrast to the skull vault that develops primarily by intramembranous ossification, the bones of the skull base, including a large part of the petrous portion of the temporal bone, areas of the petro-occipital, spheno-occipital, and sphenopetrosal synchondrosis,2 mature predominantly by endochondral ossification.3 It is their anatomic location within the skull base that makes surgical resection of these tumors challenging.
Chondrosarcomas account for 6% of skull base neoplasms and 0.15% of all intracranial tumors.4 Although most chondrosarcomas arise de novo, they are common in patients with Ollier's disease, Maffucci syndrome, Paget's disease, and osteochondroma. Several histological subtypes of chondrosarcoma have been reported, including conventional, mesenchymal, clear cell, and dedifferentiated subtypes. Among intracranial chondrosarcomas, the conventional and mesenchymal subtypes are the predominant histological patterns described in the literature. The grading system for chondrosarcomas consists of three categories: grade I (well differentiated), grade II (moderately differentiated) and grade III (poorly differentiated).5
Surgical resection has traditionally been the mainstay of treatment for intracranial chondrosarcoma. This has been combined with adjuvant radiation and chemotherapy to improve recurrence rates and overall survival. Although there has been a large volume of literature published on intracranial chondrosarcomas relating histological patterns and treatment modalities to outcomes, most of the data come from small case series that lack the statistical power to draw significant conclusions about appropriate management. In this study, we have performed a comprehensive review of the English literature and meta-analysis on intracranial chondrosarcomas to investigate the factors associated with tumor recurrence. We examined the roles of degree of surgical resection, adjuvant therapy, and histological subtype on the rate of tumor recurrence in over 500 patients to identify key prognostic factors and guide clinical decision making in this rare and difficult disorder.
METHODOLOGY
Article Selection
Articles were identified via a PubMed search using the key phrases “cranial chondrosarcoma,” “clival chondrosarcoma,” “skull base chondrosarcoma,” and “intracranial chondrosarcoma” alone and in combination with “recurrence” as Boolean searches. We then searched all the references in these articles.
Inclusion criteria were: (1) All patients had to have follow-up data on recurrence available. (2) Articles had to have enough information for each patient to be completely disaggregated.
Exclusion criteria were: (1) Articles that combined patient outcomes of chondrosarcoma and chordoma were excluded, unless there was a clear distinction between the two separate groups of patients. (2) All chondrosarcomas of the head and neck region in origin were excluded. (3) All patients with Ollier's disease, Maffucci syndrome (enchondroma with multiple angiomas), Paget's disease, and osteochondroma were excluded from our analysis.
Data Extraction
Our search resulted in over 2000 patients treated for cranial chondrosarcoma. Of these, 630 patients were collected from 99 sources. Among the 630 patients, 560 of them had appropriate follow-up to document recurrence. All references for these articles were further scrutinized to ensure the nonduplication of patients.
In those cases where patients had more than one treatment for recurrence, only the initial treatment was considered. The age recorded for patients with recurrence was age of first presentation.
Data were analyzed as a whole and stratified into four groups. The first analysis divided the data according to treatment with patients undergoing surgery alone and the other group composed of those patients who received surgery in combination with postoperative adjuvant radiation treatment. A second stratification divided databases on treatment history into two groups: group one consisted of patients who received previous treatment and the other group consisted of those patients who were being treated for the first time. A third analysis divided patients into two groups based on histological subtypes: group one included those chondrosarcomas with conventional histology and group two represented the mesenchymal subtype. The final analysis stratified the data into three groups according to histological grading: grade I, grade II, and grade III, respectively.
Statistical Analysis
Pearson chi-square test was used for statistical evaluation of the data. A p value <0.05 was considered statistically significant.
RESULTS
A total of 560 nonduplicated chondrosarcoma patients met inclusion criteria for this systematic review.1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102 The recurrence rate among all patients was 22% (93 patients) with an average disease-free interval of 32.5 months (median, 16 months). Most of the chondrosarcomas in our study involved the clivus (32%), and the second most common location was the tempero-occipital junction (27%). The most common presenting symptom was diplopia (11%), closely followed by headache (9%). Patient data were analyzed for recurrence rate at 5 years from treatment based on treatment modality, history of previous treatment, and histological grade of the tumor. Sufficient data to evaluate all of these factors were not available for every patient, and therefore the analyses of each prognostic variable contain varying numbers of patients based on data availability.
The Effect of Treatment on Recurrence
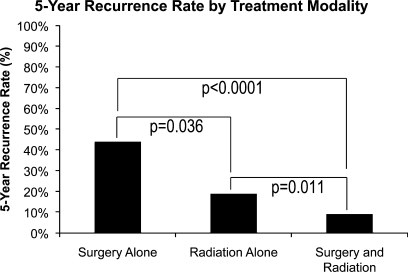
A total of 161 patients had surgery alone, and 325 patients had surgery in addition to postoperative adjuvant radiation therapy. Additionally, 46 patients underwent radiation therapy alone without surgical resection. The recurrence rate was higher in the group of patients that had surgery alone compared with surgery and radiation (44% versus 9%, p < 0.0001; Fig. 1). Unexpectedly, the rate of recurrence in patients undergoing radiation alone was also significantly lower than in patients undergoing surgery alone (19% versus 44%, p = 0.036) but significantly higher than in patients who had combined surgery and radiation (19% versus 9%, p = 0.011; Fig. 1).
Figure 1.
Five-year recurrence rate by treatment modality.
The Effect of Previous Treatment on Recurrence
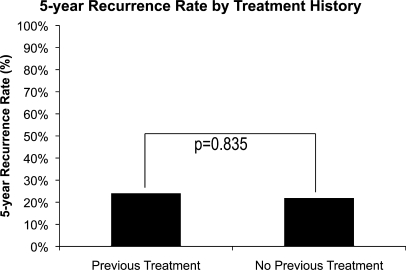
A total of 68 patients received previous treatment, and 492 patients were being treated for the first time. There was no difference in recurrence between these two groups of patients (24% versus 22%, p = 0.835; Fig. 2).
Figure 2.
Five-year recurrence rate by treatment history.
The Effect of Histological Subtype on Recurrence
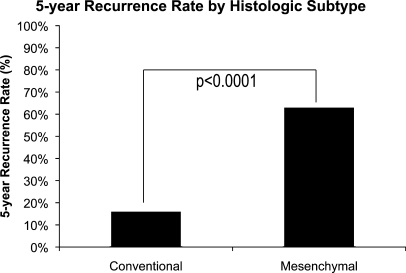
There were a total of 452 patients in our analysis who possessed chondrosarcomas of the conventional subtype, and 60 patients had the mesenchymal subtype. As expected, the recurrence rate was lower among patients with the conventional subtype (16% versus 63%, p < 0.0001; Fig. 3).
Figure 3.
Five-year recurrence rate by histological subtype.
The Effect of Histological Grade on Recurrence
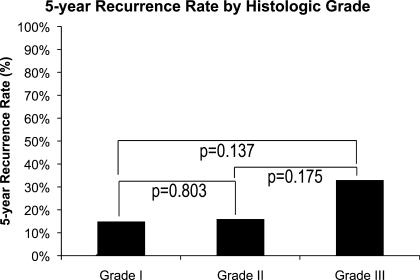
On pathological evaluation, a total of 364 patients had grade I disease, 80 patients had grade II disease, and eight patients had a grade III chondrosarcoma. Not surprising, the lowest recurrence rate was observed among the patients with grade I tumors (15%), and the highest recurrence rate was observed in the grade III group (33%), with grade II tumors in the middle (16%). There was no statistically significant difference in recurrence in patients with different grade tumors (15% versus 16%, p = 0.803; 15% versus 33%, p = 0.137; 16% versus 33%, p = 0.175; Fig. 4).
Figure 4.
Five-year recurrence rate by histological grade.
DISCUSSION
Intracranial chondrosarcomas, although rare among all intracranial tumors, pose a significant challenge to treating physicians given their location and local aggressiveness. These malignant extra-axial tumors occur primarily within the skull base in the petrous portion of the temporal bone and areas of the petro-occipital, spheno-occipital, and sphenopetrosal synchondroses.103 It has been proposed that these tumors occur preferentially in the skull base as they arise from chondrocytes within rests of endochondral cartilage responsible for the intramembranous growth of the bones of the skull base.93 Others have suggested that these tumors arise from pluripotent mesenchymal cells of the skull base or from mature fibroblasts.32 Although the cytological origin of these tumors remains controversial, their need for treatment does not. They often present with symptoms of brain stem or cranial nerve compression. Despite relatively slow growth, the consequences even small amounts of mass effect on tight skull base structures can be neurologically devastating. Even with appropriate treatment, the mortality from cranial chondrosarcoma ranges from 1 to 55% depending on histological subtype and morbidity from recurrent disease.
A large body of research has shown that many factors affect the prognosis in patients with cranial chondrosarcoma. Histological subtype, previous treatment (surgery or radiation therapy), and extent of tumor resection and of the use of adjuvant postoperative radiation therapy have all been shown to influence patient outcome; however, local recurrence is considered by many to be the most significant predictor of overall mortality in these patients.76 Unfortunately, given the rare nature of this disease, most of the data available on outcomes for cranial chondrosarcoma come from small case studies without sufficient statistical power to draw meaningful conclusions. For this reason, we have performed a systematic review of recurrence in a large population of patients from the literature who have undergone treatment for skull base chondrosarcomas to identify the key prognostic factors on the recurrence of this disease.
Examining the effect of treatment modality on recurrence, our study found that chondrosarcoma patients treated with surgery alone had a 5-year recurrence rate of 44%, and the addition of adjuvant radiation therapy reduced this rate dramatically to 9%. Although the benefit of postoperative radiation therapy has long been recognized, some authors have suggested that adjuvant radiation should be the standard of care, although others have merely suggested that it may be of benefit, especially when less than total resection can be achieved.104 Additionally, differences in the type of radiation have not been adequately addressed in the literature. To assess whether radiation was of benefit for all patients despite the degree of resection, we divided the cohort of surgical patients into those with gross total versus subtotal resection when these data were available. Although these data were only available for a subset of the patients reviewed in our analysis, the results demonstrated a clear, statistically significant benefit in recurrence-free survival for patients receiving postoperative radiation regardless of degree of resection. Furthermore, the overall rate of tumor recurrence was comparable for patients with gross total versus subtotal resection after postoperative radiation therapy was given, suggesting that a gross total resection does not improve recurrence-free survival if adjuvant radiation therapy is given. Because the greatest risk of treatment-related morbidity is associated with surgical resection of these hard-to-access tumors, the data would suggest that less aggressive resections leaving residual disease behind is preferable when combined with postoperative radiation to reduce operative morbidity without affecting outcome.
Despite the obvious benefit of radiation therapy in chondrosarcoma, surgical resection remains the mainstay for treatment. As we have demonstrated, the addition of radiation to surgical resection significantly improves recurrence-free survival and may overcome the need for complete tumor resection. Yet there are little data regarding the use of radiation as the primary treatment modality for chondrosarcoma. Of the 560 patients reviewed in this study, only 46 (8%) were treated with radiation alone. These patients were treated either for a recurrence with a history of resection confirming the diagnosis or as a new tumor with classic imaging characteristics of chondrosarcoma treated without tissue confirmation. The 5-year rate of recurrence following treatment with radiation alone was 19%, which was statistically lower than the recurrence rate of patients who received only surgical resection. Admittedly, these data are limited by the small number of cases, the lack of tissue confirmation of the diagnosis in all cases, and limited data on the size of tumors treated with radiation only compared with those treated with combined therapy. Although the data are insufficient to recommend nonsurgical management of this disease as a primary modality, it indicates that biopsy followed by radiation may be a reasonable alternative to attempted resection for small tumors in particularly challenging locations.
The histological classification of chondrosarcomas is known to include several subtypes. However, the four major subtypes are: conventional, mesenchymal, clear cell, and dedifferentiated. The review of literature revealed no clear cell or dedifferentiated subtypes occurring intracranially. Consequently, our analysis included only the mesenchymal and conventional subtypes. The conventional subtype has been reported to consist of hyaline or myxoid areas or a combination of both these histological features.105 The hyaline component consists of neoplastic chondrocytes residing within lacunar spaces surrounded by a hyaline matrix, and the myxoid component has been described as areas of chondrocytes surrounded by a frothy mucinous matrix.106,107 The mesenchymal subtype is known to display a more anaplastic appearance. In our study, those patients possessing chondrosarcomas of the conventional subtype had a 16% chance of recurrence, and those chondrosarcomas demonstrating the mesenchymal subtype carried a much higher recurrence rate of 63% (p < 0.0001). This was expected as the conventional subtype has been portrayed throughout the literature as the most benign. The mesenchymal subtype, on the other hand, demonstrates a much more aggressive behavior.108
Chondrosarcomas have been placed into categories based on varying cellularity and nuclear atypia. Three histological grades of cell differentiation exist: grade I (well differentiated), grade II (moderately differentiated), and grade III (poorly differentiated). This grading system is important because it reflects prognosis based on tumor biology distinct from its location or stage of presentation.105 Evans et al109 demonstrated this correlation between survival and histological grade perfectly in his study of chondrosarcomas from all body sites. They reported 5-year survival rates of chondrosarcomas grade I to be 90%, grade II to be 81%, and grade III to be 43%. Bearing this in mind, it was not surprising when the recurrence rate found in our study correlated directly with the histological grade of the chondrosarcomas. Our review revealed that a grade I chondrosarcoma had a 15% chance of recurrence, and grades II and III demonstrated a recurrence rate of 16% and 33%, respectively. The overall 5-year recurrence rate in our study came out to be 22% for a median follow-up time of 5 years. This falls well within the range of 12 to 60% recurrence, quoted in the literature for chondrosarcomas with a median follow-up time of 1.9 to 30 years.110,111
In conclusion, we report our results from a sizeable disaggregated meta-analysis of the English language literature regarding recurrence among cranial chondrosarcoma patients. It is our hope, that by using such a large data set, we are able to minimize the effects of individual surgeons and individual institution bias on the outcome of these patients, thereby creating a more impartial guide for physicians and patients in the future management of these tumors.
REFERENCES
- Korten A G, ter Berg H J, Spincemaille G H, van der Laan R T, Van de Wel A M. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry. 1998;65:88–92. doi: 10.1136/jnnp.65.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters G W, Brookes G B. Chondrosarcoma of the temporal bone. Clin Otolaryngol Allied Sci. 1995;20:53–58. doi: 10.1111/j.1365-2273.1995.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Lau D P, Wharton S B, Antoun N M, Bottrill I D, Moffat D A. Chondrosarcoma of the petrous apex. Dilemmas in diagnosis and treatment. J Laryngol Otol. 1997;111:368–371. doi: 10.1017/s002221510013734x. [DOI] [PubMed] [Google Scholar]
- Cianfriglia F, Pompili A, Occhipinti E. Intracranial malignant cartilaginous tumours. Report of two cases and review of literature. Acta Neurochir (Wien) 1978;45:163–175. doi: 10.1007/BF01774391. [DOI] [PubMed] [Google Scholar]
- Baehring J M, Piepmeier J, Duncan C, Ogle E, Kim J, Liebsch N. Chondrosarcoma of the skull base. J Neurooncol. 2006;76:49. doi: 10.1007/s11060-005-5981-3. [DOI] [PubMed] [Google Scholar]
- Seth H N, Singh M. Intracranial mesenchymal chondrosarcoma. Acta Neuropathol. 1973;24:86–89. doi: 10.1007/BF00691422. [DOI] [PubMed] [Google Scholar]
- Wojno K J, Hruban R H, Garin-Chesa P, Huvos A G. Chondroid chordomas and low-grade chondrosarcomas of the craniospinal axis. An immunohistochemical analysis of 17 cases. Am J Surg Pathol. 1992;16:1144–1152. doi: 10.1097/00000478-199212000-00002. [DOI] [PubMed] [Google Scholar]
- Masuzawa T, Nakahara N, Saito K, Sato F. Parasellar chondrosarcoma—case report. Neurol Med Chir (Tokyo) 1986;26:44–48. doi: 10.2176/nmc.26.44. [DOI] [PubMed] [Google Scholar]
- Hassounah M, Al-Mefty O, Akhtar M, Jinkins J R, Fox J L. Primary cranial and intracranial chondrosarcoma. A survey. Acta Neurochir (Wien) 1985;78:123–132. doi: 10.1007/BF01808691. [DOI] [PubMed] [Google Scholar]
- Bahr A L, Gayler B W. Cranial chondrosarcomas. Report of four cases and review of the literature. Radiology. 1977;124:151–156. doi: 10.1148/124.1.151. [DOI] [PubMed] [Google Scholar]
- Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery. 2006;59(1 Suppl 1):ONS50–ONS57. discussion ONS50–ONS57. doi: 10.1227/01.NEU.0000219914.17221.55. [DOI] [PubMed] [Google Scholar]
- Lin E M, Ray M E, Telian S A. Cochlear implantation with ipsilateral petroclival chondrosarcoma. Otol Neurotol. 2006;27:337–341. doi: 10.1097/00129492-200604000-00008. [DOI] [PubMed] [Google Scholar]
- Wanebo J E, Bristol R E, Porter R R, Coons S W, Spetzler R F. Management of cranial base chondrosarcomas. Neurosurgery. 2006;58:249–255. discussion 249–255. doi: 10.1227/01.NEU.0000194834.74873.FB. [DOI] [PubMed] [Google Scholar]
- Inenaga C, Morii K, Tamura T, Tanaka R, Takahashi H. Mesenchymal chondrosarcoma of the sellar region. Acta Neurochir (Wien) 2003;145:593–597. discussion 597. doi: 10.1007/s00701-003-0059-5. [DOI] [PubMed] [Google Scholar]
- Neff B, Sataloff R T, Storey L, Hawkshaw M, Spiegel J R. Chondrosarcoma of the skull base. Laryngoscope. 2002;112:134–139. doi: 10.1097/00005537-200201000-00023. [DOI] [PubMed] [Google Scholar]
- Crockard H, Cheeseman A, Steel T, et al. A multidisciplinary team approach to skull base chondrosarcomas. J Neurosurg. 2001;95:184–189. doi: 10.3171/jns.2001.95.2.0184. [DOI] [PubMed] [Google Scholar]
- Amirjamshidi A, Abbassioun K. Radiation-induced tumors of the central nervous system occurring in childhood and adolescence. Four unusual lesions in three patients and a review of the literature. Childs Nerv Syst. 2000;16:390–397. doi: 10.1007/s003819900125. [DOI] [PubMed] [Google Scholar]
- Blevins N H, Jackler R K, Kaplan M J, Gutin P H. Combined transpetrosal-subtemporal craniotomy for clival tumors with extension into the posterior fossa. Laryngoscope. 1995;105(9 Pt 1):975–982. doi: 10.1288/00005537-199509000-00018. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Sawamura Y, Ikeda J, Ishii N, Abe H. Successful chemoradiation therapy for high-grade skull base chondrosarcoma in a child. Childs Nerv Syst. 1995;11:250–253. doi: 10.1007/BF00277662. [DOI] [PubMed] [Google Scholar]
- Reid C B, Fagan P A, Turner J. Low-grade myxoid chondrosarcoma of the temporal bone: differential diagnosis and report of two cases. Am J Otol. 1994;15:419–422. [PubMed] [Google Scholar]
- Stapleton S R, Wilkins P R, Archer D J, Uttley D. Chondrosarcoma of the skull base: a series of eight cases. Neurosurgery. 1993;32:348–355. discussion 355–356. doi: 10.1227/00006123-199303000-00003. [DOI] [PubMed] [Google Scholar]
- Eavey R D, Janfaza P, Chapman P H, et al. Skull base dumbbell tumor: surgical experience with two adolescents. Ann Otol Rhinol Laryngol. 1992;101:939–945. doi: 10.1177/000348949210101110. [DOI] [PubMed] [Google Scholar]
- Colak A, Berker M, Saglam S, Onol B. Chondrosarcoma of the temporal bone in an infant: case report and review of the literature. Neurosurgery. 1992;31:956–957. [PubMed] [Google Scholar]
- Morimoto T, Sasaki T, Takakura K, Ishida T. Chondrosarcoma of the skull base: report of six cases. Skull Base Surg. 1992;2:177–185. doi: 10.1055/s-2008-1057131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton J C, Kuhwoede R, Fisch U. Mesenchymal tumors of the skull base with particular reference to surgical management and outcome. Skull Base Surg. 1992;2:112–117. doi: 10.1055/s-2008-1057120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford L D, Flickinger J C. The role of radiosurgery in the management of chordoma and chondrosarcoma of the cranial base. Neurosurgery. 1991;29:38–45. discussion 45–46. doi: 10.1097/00006123-199107000-00007. [DOI] [PubMed] [Google Scholar]
- Miyamori T, Mizukoshi H, Yamano K, et al. Intracranial chondrosarcoma—case report. Neurol Med Chir (Tokyo) 1990;30:263–267. doi: 10.2176/nmc.30.263. [DOI] [PubMed] [Google Scholar]
- Charabi S, Engel P, Bonding P. Myxoid tumours in the temporal bone. J Laryngol Otol. 1989;103:1206–1209. doi: 10.1017/s0022215100111351. [DOI] [PubMed] [Google Scholar]
- Sen C N, Sekhar L N, Schramm V L, Janecka I P. Chordoma and chondrosarcoma of the cranial base: an 8-year experience. Neurosurgery. 1989;25:931–940. discussion 940–941. doi: 10.1097/00006123-198912000-00013. [DOI] [PubMed] [Google Scholar]
- Oguro K, Nakahara N, Yamaguchi Y, Shimabukuro H, Masuzawa T. Chondrosarcoma of the posterior fossa—case report. Neurol Med Chir (Tokyo) 1989;29:1030–1038. doi: 10.2176/nmc.29.1030. [DOI] [PubMed] [Google Scholar]
- Seidman M D, Nichols R D, Raju U B, Mehta B, Levy H G. Extracranial skull base chondrosarcoma. Ear Nose Throat J. 1989;68:626–632, 635. [PubMed] [Google Scholar]
- Coltrera M D, Googe P B, Harrist T J, Hyams V J, Schiller A L, Goodman M L. Chondrosarcoma of the temporal bone. Diagnosis and treatment of 13 cases and review of the literature. Cancer. 1986;58:2689–2696. doi: 10.1002/1097-0142(19861215)58:12<2689::aid-cncr2820581224>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kveton J F, Brackmann D E, Glasscock M E, III, House W F, Hitselberger W E. Chondrosarcoma of the skull base. Otolaryngol Head Neck Surg. 1986;94:23–32. doi: 10.1177/019459988609400104. [DOI] [PubMed] [Google Scholar]
- Adegbite A B, McQueen J D, Paine K W, Rozdilsky B. Primary intracranial chondrosarcoma: a report of two cases. Neurosurgery. 1985;17:490–494. doi: 10.1227/00006123-198509000-00017. [DOI] [PubMed] [Google Scholar]
- Kubota T, Yamamoto S, Hirano A, Murata H. Chondrosarcoma in the optic canal—a case report with ultrastructural study. Neurol Med Chir (Tokyo) 1985;25:36–41. doi: 10.2176/nmc.25.36. [DOI] [PubMed] [Google Scholar]
- Cianfriglia F, Pompili A, Occhipinti E. Intracranial malignant cartilaginous tumours. Report of two cases and review of literature. Acta Neurochir (Wien) 1978;45:163–175. doi: 10.1007/BF01774391. [DOI] [PubMed] [Google Scholar]
- Chandler J P, Yashar P, Laskin W B, Russell E J. Intracranial chondrosarcoma: a case report and review of the literature. J Neurooncol. 2004;68:33–39. doi: 10.1023/b:neon.0000024728.72998.7d. [DOI] [PubMed] [Google Scholar]
- Marshman L A, Gunasekera L, Rose P E, Olney J S. Primary intracerebral mesenchymal chondrosarcoma with rhabdomyosarcomatous differentiation: case report and literature review. Br J Neurosurg. 2001;15:419–424. doi: 10.1080/02688690120082431. [DOI] [PubMed] [Google Scholar]
- Rapidis A D, Archondakis G, Anteriotis D, Skouteris C A. Chondrosarcomas of the skull base: review of the literature and report of two cases. J Craniomaxillofac Surg. 1997;25:322–327. doi: 10.1016/s1010-5182(97)80034-0. [DOI] [PubMed] [Google Scholar]
- Volpe N J, Lessell S. Remitting sixth nerve palsy in skull base tumors. Arch Ophthalmol. 1993;111:1391–1395. doi: 10.1001/archopht.1993.01090100099035. [DOI] [PubMed] [Google Scholar]
- Aksoy S, Abali H, Kiliçkap S, Güler N. Successful treatment of a chemoresistant tumor with temozolomide in an adult patient: report of a recurrent intracranial mesenchymal chondrosarcoma. J Neurooncol. 2005;71:333–334. doi: 10.1007/s11060-004-1725-z. [DOI] [PubMed] [Google Scholar]
- González-Lois C, Cuevas C, Abdullah O, Ricoy J R. Intracranial extraskeletal myxoid chondrosarcoma: case report and review of the literature. Acta Neurochir (Wien) 2002;144:735–740. doi: 10.1007/s00701-002-0949-y. [DOI] [PubMed] [Google Scholar]
- Oruckaptan H H, Berker M, Soylemezoglu F, Ozcan O E. Parafalcine chondrosarcoma: an unusual localization for a classical variant. Case report and review of the literature. Surg Neurol. 2001;55:174–179. doi: 10.1016/s0090-3019(01)00329-9. [DOI] [PubMed] [Google Scholar]
- Crosswell H, Buchino J J, Sweetman R, Reisner A. Intracranial mesenchymal chondrosarcoma in an infant. Med Pediatr Oncol. 2000;34:370–374. doi: 10.1002/(sici)1096-911x(200005)34:5<370::aid-mpo14>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Salcman M, Scholtz H, Kristt D, Numaguchi Y. Extraskeletal myxoid chondrosarcoma of the falx. Neurosurgery. 1992;31:344–348. doi: 10.1227/00006123-199208000-00021. [DOI] [PubMed] [Google Scholar]
- Pagès A, Pagès M, Ramos J, Bénézech J. Radiation-induced intracranial fibrochondrosarcoma. J Neurol. 1986;233:309–310. doi: 10.1007/BF00314165. [DOI] [PubMed] [Google Scholar]
- Cybulski G R, Russell E J, D'Angelo C M, Bailey O T. Falcine chondrosarcoma: case report and literature review. Neurosurgery. 1985;16:412–415. doi: 10.1227/00006123-198503000-00024. [DOI] [PubMed] [Google Scholar]
- Rodda R A, Franklin C I. Intracranial meningeal chondrosarcoma—probable mesenchymal type. Aust N Z J Surg. 1984;54:387–390. [PubMed] [Google Scholar]
- Bernstein M, Perrin R G, Platts M E, Simpson W J. Radiation-induced cerebellar chondrosarcoma. Case report. J Neurosurg. 1984;61:174–177. doi: 10.3171/jns.1984.61.1.0174. [DOI] [PubMed] [Google Scholar]
- Kubota T, Hayashi M, Yamamoto S. Primary intracranial mesenchymal chondrosarcoma: case report with review of the literature. Neurosurgery. 1982;10:105–110. [PubMed] [Google Scholar]
- Wu W Q, Lapi A. Primary non-skeletal intracranial cartilaginous neoplasms: report of a chondroma and a mesenchymal chondrosarcoma. J Neurol Neurosurg Psychiatry. 1970;33:469–475. doi: 10.1136/jnnp.33.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott S, Bordley J E. A radiosensitive chondrosarcoma of the sphenoid sinus and base of the skull. Report of a case. Laryngoscope. 1972;82:57–60. doi: 10.1002/lary.5540820109. [DOI] [PubMed] [Google Scholar]
- Arlen M, Tollefsen H R, Huvos A G, Marcove R C. Chondrosarcoma of the head and neck. Am J Surg. 1970;120:456–460. doi: 10.1016/s0002-9610(70)80006-x. [DOI] [PubMed] [Google Scholar]
- Minagi H, Newton T H. Cartilaginous tumors of the base of skull. Am J Roentgenol Radium Ther Nucl Med. 1969;105:308–313. doi: 10.2214/ajr.105.2.308. [DOI] [PubMed] [Google Scholar]
- George B, Ferrario C A, Blanquet A, Kolb F. Cavernous sinus exenteration for invasive cranial base tumors. Neurosurgery. 2003;52:772–780. discussion 780–782. doi: 10.1227/01.neu.0000053364.33375.c2. [DOI] [PubMed] [Google Scholar]
- Lalwani A K, Kaplan M J, Gutin P H. The transsphenoethmoid approach to the sphenoid sinus and clivus. Neurosurgery. 1992;31:1008–1014. discussion 1014. doi: 10.1227/00006123-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Leedham P W, Swash M. Chondrosarcoma with subarachnoid dissemination. J Pathol. 1972;107:59–61. doi: 10.1002/path.1711070111. [DOI] [PubMed] [Google Scholar]
- Debus J, Schulz-Ertner D, Schad L, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys. 2000;47:591–596. doi: 10.1016/s0360-3016(00)00464-8. [DOI] [PubMed] [Google Scholar]
- Hug E B, Loredo L N, Slater J D, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–439. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- Austin J P, Urie M M, Cardenosa G, Munzenrider J E. Probable causes of recurrence in patients with chordoma and chondrosarcoma of the base of skull and cervical spine. Int J Radiat Oncol Biol Phys. 1993;25:439–444. doi: 10.1016/0360-3016(93)90065-4. [DOI] [PubMed] [Google Scholar]
- Suit H D, Goitein M, Munzenrider J, et al. Definitive radiation therapy for chordoma and chondrosarcoma of base of skull and cervical spine. J Neurosurg. 1982;56:377–385. doi: 10.3171/jns.1982.56.3.0377. [DOI] [PubMed] [Google Scholar]
- Lustig L R, Sciubba J, Holliday M J. Chondrosarcomas of the skull base and temporal bone. J Laryngol Otol. 2007;121:725–735. doi: 10.1017/S0022215107006081. [DOI] [PubMed] [Google Scholar]
- Brackmann D E, Teufert K B. Chondrosarcoma of the skull base: long-term follow-up. Otol Neurotol. 2006;27:981–991. doi: 10.1097/01.mao.0000233812.48800.b4. [DOI] [PubMed] [Google Scholar]
- Raghu M, Moumoulidis I, De R, Moffat D. Chondrosarcomas of the temporal bone: presentation and management. J Laryngol Otol. 2004;118:551–555. doi: 10.1258/0022215041615272. [DOI] [PubMed] [Google Scholar]
- Satyarthee G D, Mahapatra A K. Unusual presentation of petro-clival chondrosarcoma: short report. J Clin Neurosci. 2004;11:539–542. doi: 10.1016/j.jocn.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Im S H, Kim D G, Park I A, Chi J G. Primary intracranial myxoid chondrosarcoma: report of a case and review of the literature. J Korean Med Sci. 2003;18:301–307. doi: 10.3346/jkms.2003.18.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan C A, Kaltsas G, Evanson J, et al. Pituitary chondrosarcoma: an unusual cause of a sellar mass presenting as a pituitary adenoma. J Clin Endocrinol Metab. 2001;86:386–391. doi: 10.1210/jcem.86.1.7111. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Satoh T, Masuda T, Arai H, Ehara S, Muro-Oka G. Intracranial low-grade chondrosarcoma with hyperostosis of the skull: a case report. J Neurooncol. 2000;49:249–254. doi: 10.1023/a:1006498209279. [DOI] [PubMed] [Google Scholar]
- La Rocca R V, Morgan K W, Paris K, Baeker T R. Recurrent chondrosarcoma of the cranial base: a durable response to ifosfamide-doxorubicin chemotherapy. J Neurooncol. 1999;41:281–283. doi: 10.1023/a:1006154904014. [DOI] [PubMed] [Google Scholar]
- Megerian C A, Chiocca E A, McKenna M J, Harsh G F, IV, Ojemann R G. The subtemporal-transpetrous approach for excision of petroclival tumors. Am J Otol. 1996;17:773–779. [PubMed] [Google Scholar]
- Kletzker G R, Smith P G, McIntire L D, Leonetti J P. Presentation and management of uncommon lesions of the middle ear. Am J Otol. 1995;16:634–642. [PubMed] [Google Scholar]
- Sepehrnia A, Samii M, Tatagiba M. Management of intracavernous tumours: an 11-year experience. Acta Neurochir Suppl (Wien) 1991;53:122–126. doi: 10.1007/978-3-7091-9183-5_21. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Schramm V L, Jr, Jones N F. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67:488–499. doi: 10.3171/jns.1987.67.4.0488. [DOI] [PubMed] [Google Scholar]
- Sekhar L N, Møller A R. Operative management of tumors involving the cavernous sinus. J Neurosurg. 1986;64:879–889. doi: 10.3171/jns.1986.64.6.0879. [DOI] [PubMed] [Google Scholar]
- Schulz-Ertner D, Nikoghosyan A, Hof H, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. 2007;67:171–177. doi: 10.1016/j.ijrobp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Rosenberg A E, Nielsen G P, Keel S B, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23:1370–1378. doi: 10.1097/00000478-199911000-00007. [DOI] [PubMed] [Google Scholar]
- Gerszten P C, Pollack I F, Hamilton R L. Primary parafalcine chondrosarcoma in a child. Acta Neuropathol. 1998;95:111–114. doi: 10.1007/s004010050773. [DOI] [PubMed] [Google Scholar]
- Harvey S A, Wiet R J, Kazan R. Chondrosarcoma of the jugular foramen. Am J Otol. 1994;15:257–263. [PubMed] [Google Scholar]
- Stenstam B H, Pellettieri L, Sorteberg W, Rezaei A, Sköld K. BNCT for recurrent intracranial meningeal tumours—case reports. Acta Neurol Scand. 2007;115:243–247. doi: 10.1111/j.1600-0404.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- Bingaman K D, Alleyne C H, Jr, Olson J J. Intracranial extraskeletal mesenchymal chondrosarcoma: case report. Neurosurgery. 2000;46:207–211. discussion 211–212. [PubMed] [Google Scholar]
- el-Gindi S, Abd-el-Hafeez M, Salama M. Extracranial skeletal metastases from an intracranial meningeal chondrosarcoma. Case report. J Neurosurg. 1974;40:651–653. doi: 10.3171/jns.1974.40.5.0651. [DOI] [PubMed] [Google Scholar]
- Waga S, Matsushima M, Ando K, Morii S. Intracranial chondrosarcoma with extracranial metastases. Case report. J Neurosurg. 1972;36:790–794. doi: 10.3171/jns.1972.36.6.0790. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H A, Eggert H R. Mesenchymal chondrosarcoma of the craniocervical junction. Clin Neurol Neurosurg. 1990;92:343–347. doi: 10.1016/0303-8467(90)90062-a. [DOI] [PubMed] [Google Scholar]
- La Spina M, Dollo C, Giangaspero F, Bertolini P, Russo G. Intracranial mesenchymal chondrosarcoma with osteoid formation: report of a pediatric case. Childs Nerv Syst. 2003;19:680–682. doi: 10.1007/s00381-003-0727-z. [DOI] [PubMed] [Google Scholar]
- Ruark D S, Schlehaider U K, Shah J P. Chondrosarcomas of the head and neck. World J Surg. 1992;16:1010–1015. discussion 1015–1016. doi: 10.1007/BF02067021. [DOI] [PubMed] [Google Scholar]
- Feiz-Erfan I, Han P P, Spetzler R F, et al. Exposure of midline cranial base without a facial incision through a combined craniofacial-transfacial procedure. Neurosurgery. 2005;56(1 Suppl):28–35. discussion 28–35. doi: 10.1227/01.neu.0000144209.03703.c6. [DOI] [PubMed] [Google Scholar]
- Finn D G, Goepfert H, Batsakis J G. Chondrosarcoma of the head and neck. Laryngoscope. 1984;94(12 Pt 1):1539–1544. [PubMed] [Google Scholar]
- Jereczek-Fossa B A, Krengli M, Orecchia R. Particle beam radiotherapy for head and neck tumors: radiobiological basis and clinical experience. Head Neck. 2006;28:750–760. doi: 10.1002/hed.20448. [DOI] [PubMed] [Google Scholar]
- Castelnuovo P, Pagella F, Semino L, De Bernardi F, Delù G. Endoscopic treatment of the isolated sphenoid sinus lesions. Eur Arch Otorhinolaryngol. 2005;262:142–147. doi: 10.1007/s00405-004-0764-6. [DOI] [PubMed] [Google Scholar]
- Gadwal S R, Fanburg-Smith J C, Gannon F H, Thompson L D. Primary chondrosarcoma of the head and neck in pediatric patients: a clinicopathologic study of 14 cases with a review of the literature. Cancer. 2000;88:2181–2188. [PubMed] [Google Scholar]
- Berkmen Y M, Blatt E S. Cranial and intracranial cartilaginous tumours. Clin Radiol. 1968;19:327–333. doi: 10.1016/s0009-9260(68)80019-4. [DOI] [PubMed] [Google Scholar]
- Herskowitz A, el-Gammal T. Supratentorial cartilaginous tumors. (A report of 2 cases) Dis Nerv Syst. 1973;34:384–388. [PubMed] [Google Scholar]
- Lau D P, Wharton S B, Antoun N M, Bottrill I D, Moffat D A. Chondrosarcoma of the petrous apex. Dilemmas in diagnosis and treatment. J Laryngol Otol. 1997;111:368–371. doi: 10.1017/s002221510013734x. [DOI] [PubMed] [Google Scholar]
- Gay I, Elidan J, Kopolovic J. Chondrosarcoma at the skull base. Ann Otol Rhinol Laryngol. 1981;90(1 Pt 1):53–55. doi: 10.1177/000348948109000113. [DOI] [PubMed] [Google Scholar]
- Miller R C, Foote R L, Coffey R J, et al. The role of stereotactic radiosurgery in the treatment of malignant skull base tumors. Int J Radiat Oncol Biol Phys. 1997;39:977–981. doi: 10.1016/s0360-3016(97)00377-5. [DOI] [PubMed] [Google Scholar]
- Boorstein J M, Spizarny D L. Case report 476: chondrosarcoma of base of skull (CBS) Skeletal Radiol. 1988;17:208–211. doi: 10.1007/BF00351012. [DOI] [PubMed] [Google Scholar]
- Nokes S R, Dauito R, Murtagh F R, Love L C, Arrington J A. Intracranial mesenchymal chondrosarcoma. AJNR Am J Neuroradiol. 1987;8:1137–1138. [PMC free article] [PubMed] [Google Scholar]
- Labram E K, Pobereskin L H, Siraj M U. Intracranial chondrosarcoma. Acta Neurochir (Wien) 1997;139:156–157. doi: 10.1007/BF02747198. [DOI] [PubMed] [Google Scholar]
- Gentry L R, Thompson B, Godersky J C. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol. 1988;9:1129–1138. [PMC free article] [PubMed] [Google Scholar]
- Al-Mefty O, Fox J L, Rifai A, Smith R R. A combined infratemporal and posterior fossa approach for the removal of giant glomus tumors and chondrosarcomas. Surg Neurol. 1987;28:423–431. doi: 10.1016/0090-3019(87)90224-2. [DOI] [PubMed] [Google Scholar]
- Shuangshoti S, Kasantikul V. View from beneath—pathology in focus. Primary intracranial mesenchymal chondrosarcoma. J Laryngol Otol. 1989;103:545–549. doi: 10.1017/s0022215100156853. [DOI] [PubMed] [Google Scholar]
- Kothary N, Law M, Cha S, Zagzag D. Conventional and perfusion MR imaging of parafalcine chondrosarcoma. AJNR Am J Neuroradiol. 2003;24:245–248. [PMC free article] [PubMed] [Google Scholar]
- Watters G W, Brookes G B. Chondrosarcoma of the temporal bone. Clin Otolaryngol Allied Sci. 1995;20:53–58. doi: 10.1111/j.1365-2273.1995.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Isaacson B, Kutz J W, Roland P S. Lesions of the petrous apex: diagnosis and management. Otolaryngol Clin North Am. 2007;40:479–519. doi: 10.1016/j.otc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Neff B, Sataloff R T, Storey L, Hawkshaw M, Spiegel J R. Chondrosarcoma of the skull base. Laryngoscope. 2002;112:134–139. doi: 10.1097/00005537-200201000-00023. [DOI] [PubMed] [Google Scholar]
- Coltrera M D, Googe P B, Harrist T J, Hyams V J, Schiller A L, Goodman M L. Chondrosarcoma of the temporal bone. Diagnosis and treatment of 13 cases and review of the literature. Cancer. 1986;58:2689–2696. doi: 10.1002/1097-0142(19861215)58:12<2689::aid-cncr2820581224>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rosenberg A E, Nielsen G P, Keel S B, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999;23:1370–1378. doi: 10.1097/00000478-199911000-00007. [DOI] [PubMed] [Google Scholar]
- Tzortzidis F, Elahi F, Wright D C, Temkin N, Natarajan S K, Sekhar L N. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chondrosarcomas. Neurosurgery. 2006;58:1090–1098. discussion 1090–1098. doi: 10.1227/01.NEU.0000215892.65663.54. [DOI] [PubMed] [Google Scholar]
- Evans H L, Ayala A G, Romsdahl M M. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–831. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gay E, Sekhar L N, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery. 1995;36:887–896. discussion 896–897. doi: 10.1227/00006123-199505000-00001. [DOI] [PubMed] [Google Scholar]
- Watkins L, Khudados E S, Kaleoglu M, Revesz T, Sacares P, Crockard H A. Skull base chordomas: a review of 38 patients, 1958–88. Br J Neurosurg. 1993;7:241–248. doi: 10.3109/02688699309023805. [DOI] [PubMed] [Google Scholar]