ABSTRACT
We report a novel technique for closure using titanium mesh cranioplasty in addition to hydroxyapatite cement and abdominal fat graft for acoustic neuroma. We reviewed 15 patients who underwent translabyrinthine craniectomy for resection of acoustic neuroma. Hearing loss was documented prior to surgical procedure. Over 2 years, patients underwent titanium mesh and hydroxyapatite cranioplasty with abdominal fat graft. Participants included seven men and eight women, age range 38 to 65. Main outcome measures included cosmetic outcome and incidence of cerebrospinal fluid (CSF) leak. The lesion was right-sided in seven patients and left-sided in eight. Cosmetic outcome was excellent in all. There were no cases of CSF leak. Closure used one-third the hydroxyapatite required for traditional closure. Our technique yields cosmetic results equivalent to hydroxyapatite cement alone and a comparable incidence of CSF leakage without leaving a drain in place postoperatively. The technique is easy to adopt, is more cost-effective than hydroxyapatite cement cranioplasty alone, offers greater ease of access for reoperation, and does not preclude later implantation of bone-anchored hearing aid.
Keywords: Translabyrinthine approach, acoustic tumors, hydroxyapatite, titanium mesh
The translabyrinthine approach to the cerebellopontine angle was first proposed by Panse in 1904 and subsequently refined by Zange in 1915.1 The approach led uniformly to loss of hearing and facial function and was frequently complicated by cerebrospinal fluid (CSF) fistula formation and the concomitant high mortality that accompanied the condition in the prepenicillin era. With the advent of improved diagnostic techniques, earlier detection, and microscopic techniques of bone extirpation, House and Hitselberger standardized the translabyrinthine approach and described how it can safely be applied for the resection of small to medium-sized cerebellopontine angle tumors, which had already caused hearing loss.2,3 However, abdominal fat grafting (AFG) remained the standard closure, and delayed development of CSF fistula still occurred in 6 to 15% of cases.4,5
In 2002, Arriaga and Chen reported a method of closure using hydroxyapatite cement (HAC) reconstruction over the top of AFG, significantly reducing the occurrence of CSF leakage.6 Subsequent experience at several centers confirmed that HAC reconstruction reduced rates of CSF fistula formation, was well tolerated by patients, and was relatively easy to adopt. Moreover, it has recently been reported that, with newer formulations of hydroxyapatite, it may not be necessary to leave a drain postoperatively. With any formulation, however, the amount of cement required for HAC alone remains substantial; this incurs significant expense, can be time-consuming, and has the potential to make reoperation cumbersome.
We evaluate a novel approach to closure of the translabyrinthine craniectomy using molded titanium mesh over AFG with HAC overlay. Titanium mesh cranioplasty (TMC) has been well tolerated in other contexts, is relatively inexpensive, substantially reduces the amount of HAC required for closure, and can provide for rapid access should reoperation become necessary. In this retrospective study, we report on the first 16 patients to undergo TMC for translabyrinthine resection of tumor at our institution. All were patients at University Hospitals Case Medical Center and were operated on by members of our neuro-otology and skull base team.
METHODS
Study Design
We retrospectively reviewed all patients who underwent translabyrinthine craniectomy between November 2006 and August 2008 for resection of acoustic neuroma. Hearing loss was documented in all cases prior to the surgical procedure.
Surgical Technique
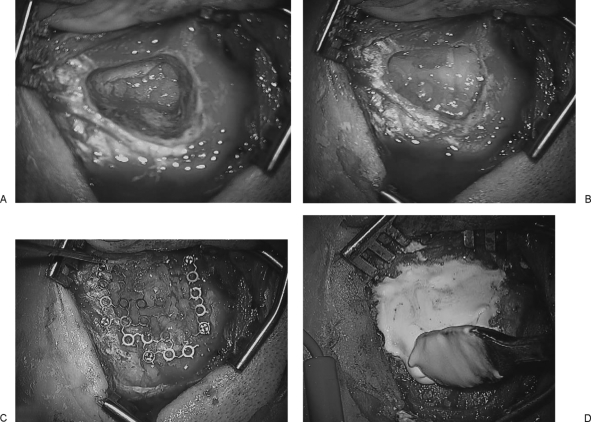
The translabyrinthine approach at our institution is performed collaboratively by members of our neurosurgical and neuro-otology services. We recommend this procedure as being most appropriate for patients with smaller tumors (less than 3 cm maximal diameter) and certainly for those mostly confined to the internal auditory canal (IAC) in whom severe or complete hearing loss has been documented (Gardner-Robertson grade III or IV) or in whom an attempt at hearing preservation is inadvisable.7 The translabyrinthine procedure itself has been well described,8,9 and the novel advance described in the current report is related to the procedure and implemented upon closure. Following the mastoidectomy and resection of the tumor, temporalis fascia is used to seal the defect in the dura over the IAC and posterior fossa, and the bony defect is filled with fat obtained from a small abdominal incision (Fig. 1A, B). The fat is layered laterally to the level of the facial recess and aditus, at which point bone wax is used to seal off the pathways to the middle ear. Further layers of fat are placed to the level of the cortical bone. This fat is then covered with a piece of titanium mesh secured by miniscrews and deflected medially to compress the fat graft (Fig. 1C). Finally, the mesh is covered with HAC and contoured into the bone of the mastoid cortex (Fig. 1D). Once the HAC fully hardens, the periosteum is closed over the HAC in a watertight fashion, followed by the usual layered closure of soft tissue. A mastoid dressing is not necessary as the HAC has hardened and compression of the wound is not required.
Figure 1.
Temporalis fascia is placed to cover the dural defect in the internal auditory canal and posterior fossa and sealed with fibrin glue. Bone wax is placed over the posterior epitympanum to prevent cerebrospinal fluid–middle ear communication (A). Abdominal fat is placed in the mastoid and labyrinthine defect (B). Titanium mesh is secured in the mastoid cortex with self-tapping screws, and the mesh is then bowed medially to compress the fat (C). Hydroxyapatite cement is spread over the mesh and contoured to the edges of the cortical bone (D).
RESULTS
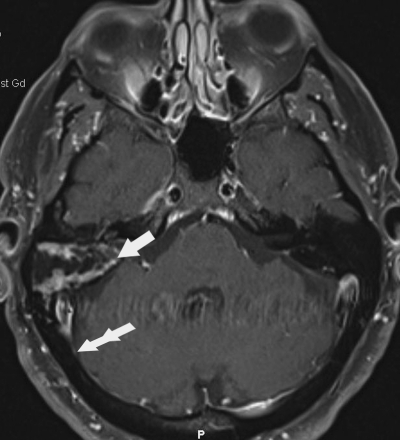
Fifteen cases were included in this review (Table 1): seven men and eight women, with an age range of 38 to 65 years (mean age 53.5 years). Of these 15 patients, two later underwent implantation of a bone-anchored hearing aid ∼1 cm posterior to the HAC repair into normal cortical bone without adverse sequelae. There were no reported complications following the procedures in any patient. Gross total resection of tumor was achieved in every case. All patients harbored acoustic tumors with a median size of 1.2 cm in maximal diameter (range 0.6 to 1.5 cm). At 1 year, postoperative computed tomographic scanning was performed to document the bony assimilation of the HAC and lack of defect over the area of the mastoidectomy. The scan demonstrated evidence of HAC incorporation without indentation of the graft site and no evidence of cosmetic deformity (Fig. 2).
Table 1.
Clinical Characteristics of Patients
| Patient Number | Age (mean 53 years) | Gender | Tumor Size (mean 1.2 cm) | Laterality | Date of BAHA Placement |
|---|---|---|---|---|---|
| BAHA, bone-anchored hearing aid. | |||||
| 1 | 49 | M | 0.8 | L | |
| 2 | 53 | F | 0.6 | R | |
| 3 | 39 | F | 1.2 | R | |
| 4 | 64 | M | 1.4 | L | |
| 5 | 56 | M | 1.5 | R | |
| 6 | 59 | F | 1.1 | L | |
| 7 | 45 | F | 1.3 | R | 7/11/2008 |
| 8 | 38 | F | 1.0 | R | |
| 9 | 51 | M | 0.9 | L | |
| 10 | 58 | F | 1.1 | L | 3/14/2008 |
| 11 | 65 | F | 1.5 | L | |
| 12 | 65 | M | 1.8 | R | |
| 13 | 47 | M | 0.8 | L | |
| 14 | 57 | M | 1.7 | L | |
| 15 | 57 | F | 0.9 | R | |
Figure 2.
Axial T1-contrasted image demonstrating postoperative changes following translabyrinthine craniectomy 6 months after resection of acoustic tumor and titanium mesh cranioplasty with hydroxyapatite cement (HAC). Fat is noticeable within the mastoid resection cavity (arrow). The contour of the skull is normal where the HAC has incorporated into the normal bone (double arrow).
DISCUSSION
Traditional methods of closure following translabyrinthine approaches for acoustic tumors utilize autologous fat graft to replace the mastoidectomy defect.7 There are several drawbacks to this approach, including the need for a postoperative pressure dressing and cosmetic indentation of the surgical site as the fat is reabsorbed over time. A physiological closure may be achieved via the use of materials designed to resemble the consistency and shape of the removed bone. Such materials include various types of polymethylmethacrylate or acrylic.10 Recently, reports of the use of HAC to replace the bony defect have been associated with a significant improvement in the CSF leakage rate in addition to cosmetic benefits.6,11 Animal models using the same materials in the repair of cranial bone defects have failed to provide any evidence of inflammatory reaction or negative histological reaction, with evidence of integration of new bone into the HAC around the periphery of the lesion.12 HAC has been reported to cause no foreign body reaction or inflammatory response.13 In a series of 54 patients treated with HAC, Arriaga and Chen report a reduction of the CSF leak rate from 12.5 to 3.7% when compared with a similar-sized cohort in which only autologous fat was used.6 In an update to the study, expanded to include a total of 108 patients followed over 4 years, the authors report one additional CSF leak when including an additional 90 patients who had no wound complications.11 An additional study using HAC in both translabyrinthine and retrosigmoid approaches for the resection of 33 acoustic neuromas by Kruger and colleagues noted one reported case of CSF leakage.14
Despite these findings, several difficulties with the approach described by Arriaga and Chen have been described.6 Other authors have described significant infectious complications associated with large-volume use of HAC in the mastoid cavity and in the paranasal sinuses.13,15,16 For example, in a series by Ridenour and colleagues, all patients in whom this technique was used required revision to remove the cement due to extensive skull base osteitis and delayed failure of integration.17 In a discussion of a large surgical experience in the use of bone cement materials involving more than 120 patients, Zins and colleagues issue a cautionary note regarding the use of such materials because of a significant complication rate primarily due to infection.18 In their series, 50% of patients presented with a major complication as late as 6 years after the initial operation when bone cement was used in the repair of large skull defects, and they conclude that its use should be restricted in a large majority of patients.18 Another issue is the cost of the cement itself, which can be significant. Because of this factor, some authors have advocated the use of a vascularized bone flap to achieve the same end point without added cost.19
Such cost concerns must be considered, but one of the advantages of the technique described here is that only a thin layer of HAC is used over the titanium mesh at the level of the mastoid cortex as opposed to filling the mastoid defect with HAC, thus reducing costs. We have not observed a significant incidence of infections using our technique, as it limits the amount of HAC applied, despite the addition of a second foreign body in the form of titanium mesh. This likely is a reflection of the fact that, in our technique, the HAC does not come into contact with the mastoid air cells.
The HAC reconstruction introduced by Arriaga and Chen in 2002 substantially reduced rates of CSF leak after translabyrinthine craniectomy.6 In our current study, we have similarly found that a low rate of CSF leakage is associated with the use of this material with our modified method of closure. It is likely that reduction in the rate of CSF leak associated with these methods is related to direct pressure upon the porus acusticus in the immediate postoperative period until scarring around the dural defect created prevents CSF egress. Baird and colleagues have described a limited method of HAC use wherein cement is placed only around the drilled posterior wall of the porus acusticus, creating a significant reduction of their CSF leak rate from 12 to 2.3%.20 We propose that the addition of titanium mesh to the closure limits the amount of HAC that must be used in replacing the bony defect caused by the mastoidectomy. In addition, we have found that the introduction of medial bowing to the mesh once it is secured to the cortex provides uniform and permanent pressure to the fat graft, allowing for a more effective seal of air-cell tracts than standard fat application techniques and potentially contributing to the lack of not only CSF leakage but also the incidence of subgaleal CSF effusions. Alternatively, use of a periosteal flap may accomplish a similar goal, though we have found it time-efficient to use mesh rather than harvest and close the periosteum. Unlike the method described by Baird and colleagues, our method maintains cosmesis postoperatively by reestablishing preoperative cranial contours. Additionally, in cases necessitating urgent reentry into the surgical cavity, significant time must be spent removing HAC from the bony defect in techniques where the entire mastoid defect has been filled with HAC. This is in stark contrast to the method described in the present study wherein the HAC may be removed along with the titanium mesh in an expeditious fashion, allowing access to fat and the dural defect.
CONCLUSION
The addition of titanium mesh to the HAC cranioplasty is a promising alternative to HAC alone. The use of this simple modification does not increase infection rates and is simple to perform. It provides excellent cosmetic outcomes and low rates of CSF leakage, is more cost-effective than HAC reconstruction, and allows for rapid access if reoperation is required.
REFERENCES
- Penzholz H. Development and present state of cerebellopontine angle surgery from the neuro- and otosurgical point of view. Arch Otolaryngol. 1984;240:164–174. doi: 10.1007/BF00453475. [DOI] [PubMed] [Google Scholar]
- House W F, Hitselberger W E. Transtemporal bone microsurgical removal of acoustic neuromas. Total versus subtotal removal of acoustic tumors. Arch Otolaryngol. 1964;80:751–752. doi: 10.1001/archotol.1964.00750040767020. [DOI] [PubMed] [Google Scholar]
- Winn R, editor. Youman's Neurological Surgery. 5th ed. Philadelphia: Elsevier Inc.; 2009.
- Falcioni M, Mulder J J, Taibah A, De Donato G, Sanna M. No cerebrospinal fluid leaks in translabyrinthine vestibular schwannoma removal: reappraisal of 200 consecutive patients. Am J Otol. 1999;20:660–666. [PubMed] [Google Scholar]
- Fishman A J, Marrinan M S, Golfinos J G, Cohen N L, Roland J T., Jr Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope. 2004;114:501–505. doi: 10.1097/00005537-200403000-00022. [DOI] [PubMed] [Google Scholar]
- Arriaga M A, Chen D A. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2002;126:512–517. doi: 10.1067/mhn.2002.124436. [DOI] [PubMed] [Google Scholar]
- Day J D, Chen D A, Arriaga M. Translabyrinthine approach for acoustic neuroma. Neurosurgery. 2004;54:391–395. doi: 10.1227/01.neu.0000103668.26590.5a. [DOI] [PubMed] [Google Scholar]
- Bambakidis NC, Megerian CA, Spetzler RF, editor. Surgery of the Cerebellopontine Angle. Shelton, CT: BC Decker Inc.; 2009.
- Gonzalez L F, Lekovic G P, Kakarla U K, Reis C VC, Weisskopf P, Daspit C P. In: Bambakidis NC, Megerian CA, Spetzler RF, editor. Surgery of the Cerebellopontine Angle. Shelton, CT: BC Decker Inc.; 2009. Surgical approaches to the cerebellopontine angle.
- Couldwell W T, Fukushima T. Cosmetic mastoidectomy for the combined supra/infratentorial transtemporal approach. Technical note. J Neurosurg. 1993;79:460–461. doi: 10.3171/jns.1993.79.3.0460. [DOI] [PubMed] [Google Scholar]
- Arriaga M A, Chen D A, Burke E L. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery-update. Otol Neurotol. 2007;28:538–540. doi: 10.1097/mao.0b013e3180423ad9. [DOI] [PubMed] [Google Scholar]
- Ascherman J A, Foo R, Nanda D, Parisien M. Reconstruction of cranial bone defects using a quick-setting hydroxyapatite cement and absorbable plates. J Craniofac Surg. 2008;19:1131–1135. doi: 10.1097/SCS.0b013e31817bd83e. [DOI] [PubMed] [Google Scholar]
- Costantino P D, Hiltzik D H, Sen C, et al. Sphenoethmoid cerebrospinal fluid leak repair with hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 2001;127:588–593. doi: 10.1001/archotol.127.5.588. [DOI] [PubMed] [Google Scholar]
- Kruger E, Arriaga M, Chen D, Hillman T. S241-acoustic neuroma surgery: Hydroxyapatite cement cranioplasty. Otolaryngol Head Neck Surg. 2008;139:(2 Suppl 1):P156. doi: 10.1067/mhn.2002.124436. [DOI] [PubMed] [Google Scholar]
- Snyderman C H, Scioscia K, Carrau R L, Weissman J L. Hydroxyapatite: an alternative method of frontal sinus obliteration. Otolaryngol Clin North Am. 2001;34:179–191. doi: 10.1016/s0030-6665(05)70305-4. [DOI] [PubMed] [Google Scholar]
- Verret D J, Ducic Y, Oxford L, Smith J. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol Head Neck Surg. 2005;133:897–899. doi: 10.1016/j.otohns.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ridenour J S, Poe D S, Roberson D W. Complications with hydroxyapatite cement in mastoid cavity obliteration. Otolaryngol Head Neck Surg. 2008;139:641–645. doi: 10.1016/j.otohns.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Zins J E, Moreira-Gonzalez A, Papay F A. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg. 2007;120:1332–1342. doi: 10.1097/01.prs.0000279557.29134.cd. [DOI] [PubMed] [Google Scholar]
- Yuen H W, Chen J M. Reconstructive options for skull defects following translabyrinthine surgery for vestibular schwannomas. Curr Opin Otolaryngol Head Neck Surg. 2008;16:318–324. doi: 10.1097/MOO.0b013e32830139b8. [DOI] [PubMed] [Google Scholar]
- Baird C J, Hdeib A, Suk I, et al. Reduction of cerebrospinal fluid rhinorrhea after vestibular schwannoma surgery by reconstruction of the drilled porus acusticus with hydroxyapatite bone cement. J Neurosurg. 2007;107:347–351. doi: 10.3171/JNS-07/08/0347. [DOI] [PubMed] [Google Scholar]