Abstract
Purpose
To compare standard of care nuclear SPECT imaging with cardiac magnetic resonance imaging (MRI) for emergency room (ER) patients with chest pain and intermediate probability for coronary artery disease.
Materials and Methods
Thirty-one patients with chest pain, negative electrocardiogram (ECG), and negative cardiac enzymes who underwent cardiac single photon emission tomography (SPECT) within 24 h of ER admission were enrolled. Patients underwent a comprehensive cardiac MRI exam including gated cine imaging, adenosine stress and rest perfusion imaging and delayed enhancement imaging. Patients were followed for 14 ± 4.7 months.
Results
Of 27 patients, 8 (30%) showed subendocardial hypoperfusion on MRI that was not detected on SPECT. These patients had a higher rate of diabetes (P = 0.01) and hypertension (P = 0.01) and a lower global myocardial perfusion reserve (P = 0.01) compared with patients with a normal cardiac MRI (n = 10). Patients with subendocardial hypoperfusion had more risk factors for cardiovascular disease (mean 4.4) compared with patients with a normal MRI (mean 2.5; P = 0.005). During the follow-up period, patients with subendocardial hypoperfusion on stress MRI were more likely to return to the ER with chest pain compared with patients who had a normal cardiac MRI (P = 0.02). Four patients did not finish the MR exam due to claustrophobia.
Conclusion
In patients with chest pain, diabetes and hypertension, cardiac stress perfusion MRI identified diffuse subendocardial hypoperfusion defects in the ER setting not seen on cardiac SPECT, which is suspected to reflect microvascular disease.
Keywords: adenosine stress perfusion cardiac MRI, emergency room, chest pain, microvascular disease
The evaluation and triage of patients with chest pain is a common challenge for emergency room (ER) physicians. Fast and accurate assessment of myocardial ischemia in a patient presenting to the ER with chest pain is an essential component for further diagnostic and therapeutic decision making. Analysis of electrocardiograms (ECG) and cardiac enzymes are the first line tests to “rule out” acute myocardial infarction (1). In patients with a negative ECG, negative cardiac enzymes and an intermediate probability for coronary artery disease (CAD), nuclear stress perfusion tests (single photon emission computed tomography, SPECT) are well established means to evaluate for stress induced myocardial ischemia (2,3).
New technical developments over the past decade allow a comprehensive cardiac MRI examination, which includes myocardial perfusion, function, and viability assessment (4,5). Stress perfusion with MRI is an emerging noninvasive method for the evaluation of myocardial ischemia (6–9). Myocardial scar imaging with MRI aids in identifying small subendocardial myocardial infarctions that are not seen by cardiac SPECT (10). Furthermore, cardiac SPECT exposes the patient to 17–20 mSv of ionizing radiation (11) that is not present with MRI.
Some patients presenting to the ER with chest pain likely of cardiac origin may not have flow limiting stenosis of the coronary arteries, but instead have small vessel or other cardiac disease that could potentially be identified by MRI (12–14). Therefore, the aim of this study was to compare standard of care nuclear SPECT imaging with cardiac MRI for the evaluation of emergency room patients with chest pain and intermediate probability for coronary artery disease.
Materials and Methods
Study Population
During a 12-month period, we prospectively and consecutively enrolled ER patients with chest pain, scheduled for a clinical cardiac SPECT who had negative cardiac enzymes and no signs of acute ischemia on ECG. The exclusion criteria were an internal pacemaker, defibrillator, positive cardiac enzymes, or contraindications for adenosine infusion. This study was approved by the institutional review board, and written informed consent was obtained from all patients.
Patients with a history of prior myocardial infarction and cardiac surgery were included in the study. All beverages containing caffeine were stopped at least 12 h before MRI examination.
Study Protocol
The MRI examination was performed within 24 h of presentation to the ER and within three hours of the nuclear SPECT stress test. During the MRI exam, blood pressure and ECG were monitored. Cardiovascular risk factors such as hypertension, diabetes mellitus, hypercholesterolemia, smoking, and family history of CAD were assessed. All patients were followed to assess for cardiac events for an average time period of 14 ± 4.7 months after noninvasive cardiac testing.
MR Imaging
Cardiac MRI was performed at 1.5 Tesla (T) (Siemens Avanto, Erlangen, Germany). A 6-element body matrix coil and 6 elements of a 24-element spine matrix coil were used for signal reception. For functional analysis, retrospectively ECG-gated steady state free precession (SSFP) cine MRI was performed in the short and long axis planes. The temporal resolution was 40 ms, with a slice thickness of 8 mm and 2-mm gap between slices on short axis images.
For the stress perfusion MRI, adenosine (Astellas Pharma US, Inc, IL) was infused intravenously at a rate of 140 (μg/kg per min over 6 min. At four minutes into the adenosine infusion, stress perfusion MRI was obtained with a Saturation Recovery (SR) SSFP sequence. Scan parameters per slice for the SR-SSFP perfusion images were repetition time/echo time (TR/TE) 2.4 ms / 1.0 ms, SR time 180 ms, flip angle 50°, FOV 36 × 27 cm, matrix 192 × 115, acquisition duration 150 ms, slice thickness 8 mm, and an acceleration factor of 2 (GRAPPA). Gadopentetate dimeglumine (Magnevist®, Bayer, Schering, Berlin, Germany) was injected at 5 cc/s followed immediately by a 20 cc of normal saline flush at 5 cc/s for the rest and stress perfusion MR images (0.075 mmol/kg each for rest and stress MR imaging, 0.15 mmol/kg total dose). Three evenly spaced short axis slices and one horizontal long axis slice were acquired with a temporal resolution of two ECG R-to-R intervals to cover the entire left ventricle for each patient. After 10 min, the perfusion examination was repeated to obtain rest perfusion images.
Following a delay of 5 to 10 min after rest perfusion imaging, gradient echo delayed enhancement (DE) MRI was obtained using an inversion recovery technique with nulling of the normal myocardium. Scan parameters per slice for the DE MRI were TR/TE 5.4 ms / 3.0 ms, flip angle 20°, field of view (FOV) 36 × 27 cm, matrix 256 × 160, slice thickness 8 mm with 2-mm spacing between each slice. Short axis images were acquired as well as one horizontal long axis image to cover the entire heart.
In addition, coronary sinus flow measurements were obtained at rest and during adenosine stress using breath hold two-dimensional (2D) phase contrast MR imaging as described by Koskenvuo et al in detail (15). The entire protocol was completed within 60 min.
SPECT Myocardial Perfusion Test
All patients underwent routine SPECT myocardial perfusion imaging using Tc99 sestamibi for rest and stress imaging. Of the 27 included patients, 13 underwent symptom-limited treadmill exercise testing (Bruce Protocol), 13 underwent a dobutamine stress protocol, and for 1 patient, adenosine stress protocol was used. Because the SPECT exam was part of the clinical routine, the type of stressor could not be influenced by the study team members. The SPECT exam is accepted as the clinical gold standard at our institution. Dobutamine was infused in incremental doses, starting at 5 μg/kg/ min for 3 min with increases to 10, 20, 30, and 40 (μg/kg/min until the stress end point was reached (e.g., target heart rate, chest pain with ECG changes, or hypotension). One patient received adenosine stress testing, with an identical stress regimen compared with the MRI stress protocol. Myocardial SPECT perfusion studies were performed using technetium 99m-sestamibi at rest and in the postexercise state according to widely accepted guidelines (16). The high-count rest scans were acquired as gated-SPECT studies (8 frames per cardiac cycle), and the left ventricular ejection fraction as well as end-diastolic volume were calculated.
Coronary Angiography
Patients with a positive SPECT and / or MRI stress test for reversible myocardial ischemia underwent conventional coronary angiography (n = 4) or coronary multi-detector computed tomography (n = 1) using a 256 detector scanner (Toshiba Aquilion, Japan). All angiography examinations were completed within 30 days (mean 15.5 ± 16.9 days) of the initial ER presentation.
MRI Analysis
Two experienced cardiac MRI physicians who were blinded to patient history (JVC and DD) evaluated all MRI studies separate from each other. If there was disagreement between the two readers, the cases were reviewed together and interpreted in consensus.
The analysis of the MRI perfusion examination was performed visually, as previously reported (17). We compared stress with rest perfusion to reduce the potential rate of artifacts. If a deficit was equally present at stress and rest, if it did not follow the subendocardial border, if ghosting artifacts could be seen or if it “blinked” bright and dark it was not regarded as an evident hypoperfusion, but as a potential artifact. Patients were classified according to following criteria as previously described similarly by Pilz et al (13): (1) Patients with a reversible regional perfusion deficit in a coronary artery territory, lasting for more than six heart beats under adenosine stress, and without evidence of DE were classified as having significant obstructive CAD. (2) Patients with DE due to ischemic scar, history of coronary stent placement or coronary artery bypass graft without stress induced reversible perfusion deficit were categorized as “significant large vessel disease without reversible ischemia”. (3) Patients with diffuse stress induced subendocardial hypoperfusion (<1/2 of the myocardial wall thickness) in at least two different coronary artery territories or circumferentially lasting for up to six heartbeats after the time of maximal signal peak intensity in the left ventricle were classified as having “small vessel disease” (13). (4) Patients without ischemic or nonischemic cardiac MR findings were categorized as “normal”.
For the analysis, groups 1 and 2 were combined to a “large vessel disease” group. Additionally, other noncoronary findings that could explain the patients' chest pain were recorded.
Coronary sinus flow volumes in mL/min were calculated at rest and adenosine stress using dedicated flow software (Medis®, Netherlands). The coronary sinus was traced on the magnitude images. To compensate for the through-plane motion, a second region of interest was determined for each phase image on the myocardial tissue close to the vessel.
Coronary sinus blood flow (mL/min) was calculated by summing the flow per cardiac phase over the cardiac cycle and multiplying by the heart rate during the measurement. Coronary flow reserve was calculated by dividing the ratio of hyperemic to baseline coronary sinus flow.
SPECT Myocardial Perfusion Test
Cardiac SPECT studies were interpreted by an experienced nuclear medicine physician as part of routine clinical care for the patient. For this interpretation, the physician had access to the patients' medical records but not to the MRI results. Presence or absence of reversible or nonreversible stress induced perfusion deficits was recorded.
Statistical Analysis
Data are reported as mean ± standard deviation. The data were compared using Fisher's exact test or a two-tailed Wilcoxon signed rank test for matched pairs. In all cases, a P value < 0.05 was considered statistically significant. Interobserver agreement was measured using kappa statistics. Analyses were performed with commercially available statistic software (JMP®, SAS Institute, Cary, NC). The authors had full access to the data and take responsibility for its integrity.
Results
Thirty-one patients were enrolled who were referred for SPECT stress test within 24 h after presentation with chest pain. Four patients (13%) were claustrophobic and did not complete the MRI exam. They were excluded from further analysis. The mean age of the remaining 27 patients (15 male) was 56.3 ± 13.2 years (Table 1). Five of 27 (19%) had a prior coronary revascularization procedure (one stent, four coronary artery bypass grafts).
Table 1. Patient Characteristics.
| Age (mean ± standard deviation) | 56.3 ± 13.2 years |
|---|---|
| Male | 15/27 (56%) |
| Diabetes | 9/27 (33%) |
| Hypertension | 21/27 (78%) |
| Elevated Cholesterol | 21/27 (78%) |
| Smoking | 18/27 (67%) |
| Family history of CAD | 23/27 (85%) |
| Prior revascularization (stent, CABG) | 5/27 (19%) |
| Negative cardiac enzymes | 27/27 (100%) |
| Cardiac SPECT within 24 h of chest pain | 27/27 (100%) |
| MRI within 3 h of SPECT | 27/27 (100%) |
CAD = coronary artery disease, CABG = coronary artery bypass graft, SPECT = single photon emission computed tomography.
Image quality was sufficient for analysis in all patients, with reader consensus in 24/27 cases (kappa = 0.70). Of 27 patients, 8 (30%) showed diffuse subendocardial hypoperfusion with adenosine stress. Five of 27 patients (19%) had reversible large vessel ischemia on MRI (Fig. 1), confirmed by a ≥ 70% stenosis on angiography. One patient had both small and significant large vessel reversible ischemia.
Figure 1.
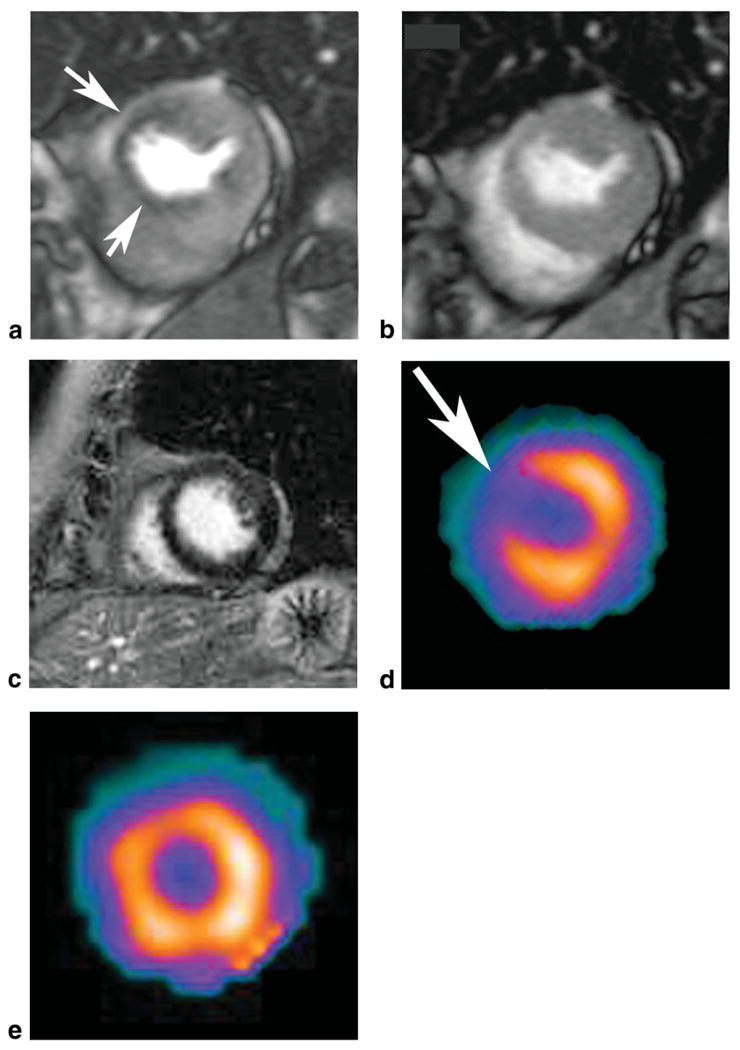
Short axis first pass adenosine stress perfusion MRI image shows a perfusion defect in the anterior-septal left ventricular wall (a, arrows), which is reversible at rest (b). Short axis delayed enhancement MR image shows no evidence of myocardial infarction (c) in this 63-year-old female patient with a high grade stenosis in the left anterior descending coronary artery on catheter directed coronary angiography. The MRI findings match the SPECT findings at stress (d) and rest (e).
All eight patients with subendocardial hypoperfusion on MRI (Fig. 2) were normal on cardiac SPECT. Of the five patients showing large vessel reversible ischemia on MRI, two patients also had corresponding transient ischemia on SPECT (Table 2). Of the three cases not detected by SPECT, two had a prior coronary artery bypass graft (CABG), one did not reach the target heart rate, and one had significant triple vessel disease (Table 2). Of 27 patients, 4 (15%) had an ischemic scar on MRI (Fig. 3). One patient had a small subendocardial scar that was not detected on SPECT.
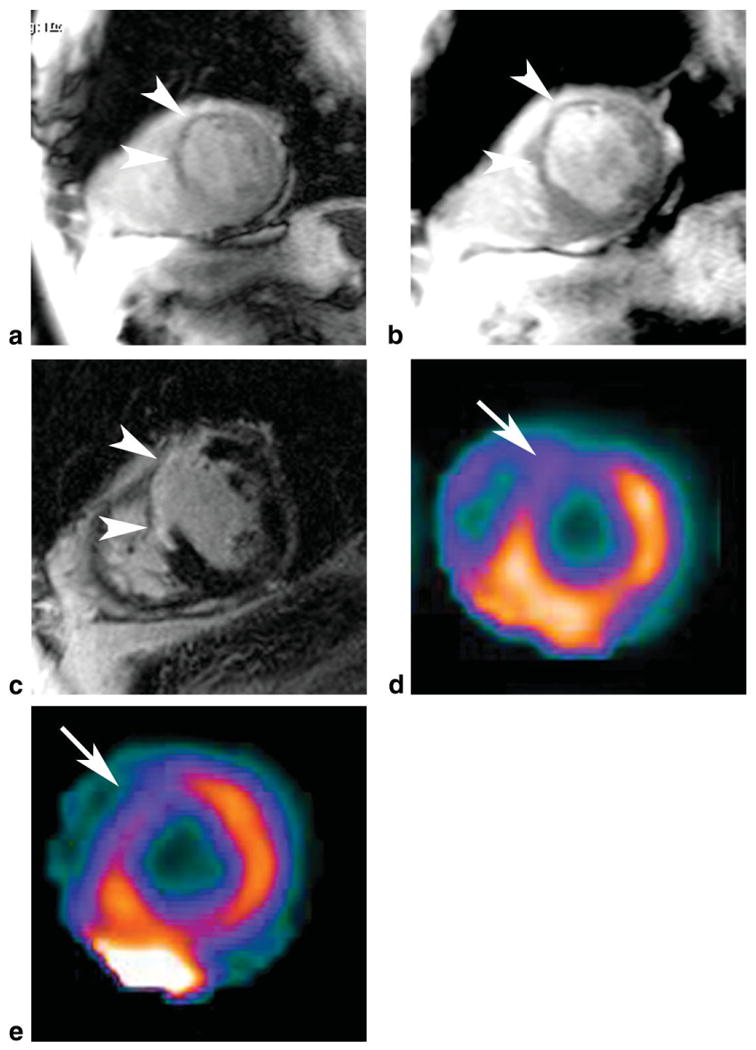
Figure 2.

Short axis MR images (a–c) in a 53-year-old female with five traditional cardiac risk factors, chest pain, and negative cardiac enzymes show diffuse transient subendocardial hypoperfusion on the adenosine stress first pass perfusion image (a), which is reversible at rest (b). On delayed enhancement MRI there was no evidence of scar (c). The cardiac SPECT exam was normal at stress (d) and rest (e).
Table 2. SPECT and MRI findings of study patients (n=27).
| Sex | Age | Nonischemic cardiac findings | Subendocardial hypoperfusion | Reversible ischemia | Angiogram ≥70% | Ischemic scar | Recurrent CP | Prior CAD | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPECT | MRI | SPECT | MRI | SPECT | MRI | SPECT | MRI | |||||
| f | 78.0 | − | − | − | + | − | − | N/A | − | − | + | |
| f | 41.2 | − | − | − | + | − | − | N/A | − | − | − | |
| f | 71.3 | − | moderate pericardial effusion | − | + | − | − | N/A | − | − | + | |
| m | 67.5 | − | − | − | + | − | − | N/A | − | − | − | |
| f | 57.9 | − | − | − | + | − | − | N/A | − | − | + | |
| f | 53.4 | − | − | − | + | − | − | N/A | − | − | − | |
| m | 39.1 | − | − | − | + | − | − | N/A | − | − | + | |
| f | 59.1 | − | − | − | + | − | + | +a | − | − | − | + |
| m | 54.5 | − | − | − | − | + | + | + | − | + | + | + |
| f | 63.0 | − | − | − | − | + | + | + | − | − | + | |
| m | 65.1 | − | moderate mitral regurgitation | − | − | − | + | + | + | + | + | + |
| m | 53.1 | − | − | − | − | − | + | + | + | + | + | + |
| m | 75.6 | − | − | − | − | − | − | N/A | + | + | − | + |
| m | 44.6 | − | mitral valve repair, non–ischemic CM | − | − | − | − | N/A | − | − | + | |
| m | 83.5 | − | scar RV attachment site, PH | − | − | − | − | N/A | − | − | + | |
| m | 52.2 | − | DCM ETOH | − | − | − | − | N/A | − | − | + | |
| m | 76.6 | − | mild LVH, moderate Aortic regurgitation, LV diastolic dysfunction | − | − | − | − | N/A | − | − | − | |
| m | 47.0 | − | − | − | − | − | − | N/A | − | − | − | |
| f | 47.4 | − | − | − | − | − | − | N/A | − | − | − | |
| m | 46.3 | − | − | − | − | − | − | N/A | − | − | − | |
| m | 43.4 | − | − | − | − | − | − | N/A | − | − | − | |
| f | 45.2 | − | − | − | − | − | − | N/A | − | − | − | |
| f | 50.1 | − | − | − | − | − | − | N/A | − | − | − | |
| m | 59.0 | − | − | − | − | − | − | N/A | − | − | − | |
| f | 29.7 | − | − | − | − | − | − | N/A | − | − | − | |
| m | 59.3 | − | − | − | − | − | − | N/A | − | − | − | |
| f | 57.6 | − | − | − | − | − | − | N/A | − | − | − | |
| 15m/12f | 56.3 | 0 | 6 | 0 | 8 | 2 | 5 | 6 | 3 | 4 | 11 | 5 |
cardiac CT angiography.
CP = chest pain; CAD = coronary artery disease; CM = cardiomyopathy; DCM = dilated cardiomyopathy; ETOH = alcohol abuse; LVH = left ventricular hypertrophy; RV = right ventricle; PH = pulmonary hypertension.
Figure 3.
Short axis MRI images of a 75-year-old male patient with an old myocardial infarction and chest pain in the emergency room show thinning of the anterior septal wall with decreased first pass perfusion at adenosine stress (a), which persists at rest (b). On delayed enhancement imaging the scar (c) matches the fixed first pass perfusion defect. The MRI findings match the SPECT findings at stress (d) and rest (e).
Of 27 patients, 14 (52%) did not have small or large vessel disease on MRI and were also normal on SPECT. Of these 14 patients, 4 (29%) had nonischemic myocardial disease on MRI (Fig. 4), which was not detected on SPECT and may have contributed to the patients' chest pain: delayed enhancement at the right ventricular attachment site in a patient with pulmonary artery hypertension, moderate aortic regurgitation, mitral valve replacement with concomitant mitral valve stenosis, and alcohol related nonischemic cardiomyopathy (Table 2). One patient with subendocardial hypoperfusion also had a moderate pericardial effusion not identified at SPECT imaging.
Figure 4.
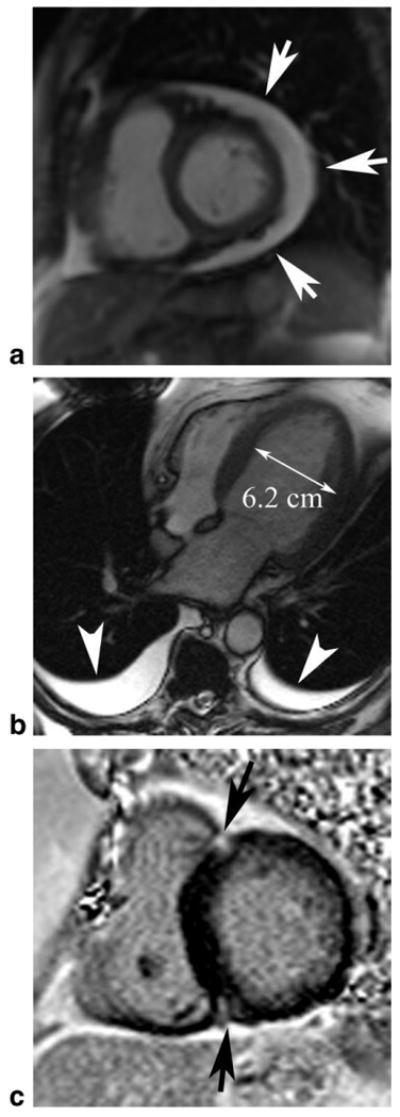
Nonischemic cardiac findings identified on cardiac MRI in patients presenting with chest pain. a: Moderate pericardial effusion; (b) dilated nonischemic cardiomyopathy with absent LV scar (not shown), bilateral pleural effusions (arrowheads); (c) delayed enhancement at the right ventricular attachment sites (arrows) in a patient with severe pulmonary hypertension (after MRI confirmed with right heart catheterization, pulmonary artery pressure: mean, 57 mmHg; systolic, 85 mmHg).
Risk Factor Analysis
Patients with subendocardial hypoperfusion and the patient group with large vessel disease on MRI had a higher number of risk factors for cardiovascular disease (mean 4.4 and 4.0, respectively) compared with patients with a normal cardiac MRI (mean 2.5; P = 0.005 and P = 0.03, respectively). The group with large vessel disease (mean age, 58.9 ± 8.2 years) was significantly older compared with the group with normal MRI (mean age, 48.5 ± 8.9 years; P = 0.01).
Patients with subendocardial hypoperfusion had a significantly higher rate of diabetes (P = 0.01) and hypertension (P = 0.01) compared with patients with a normal cardiac MRI (Table 3). The majority (75%) of patients with subendocardial perfusion defects were women.
Table 3. Comparison of Small Vessel Disease, Large Vessel Disease, and Normal Patient Groups.
| Small vessel disease group | Large vessel disease group | Normal MRI° | |
|---|---|---|---|
| n | 8 | 6 | 10 |
| Sex (male/female in%) | 25/75 | 67/33 | 50/50 |
| Age | 58.4 | 61.7† | 48.5 |
| Diabetes | 6** | 1 | 1 |
| Hypertension | 8** | 6* | 4 |
| Elevated cholesterol | 8 | 5 | 6 |
| Family history of CAD | 6 | 6 | 8 |
| Smoking | 6 | 5 | 5 |
| Recurrent chest pain | 4* | 4** | 0 |
| Major CV event | 0 | 1 | 0 |
| Mean no. of risk factors | 4.4†† | 4.0† | 2.5 |
*<0.05, **<0.01, two-tailed Fisher exact test compared to the normal MRI group.
†<0.05, ††<0.01, two-tailed Wilcoxon test compared to the normal MRI group.
absence of MRI findings (ischemic or non-ischemic) to explain the patient's chest pain.
Patients with subendocardial hypoperfusion had a significant lower coronary flow reserve (1.9 ± 0.44) assessed by coronary sinus flow measurements compared with patients with a normal perfusion MRI (3.0 ± 0.88; P = 0.01). The age of patients with normal MRI was not significantly different from that of patients with subendocardial hypoperfusion (48.5 ± 8.9 years versus 58.4 ± 13.8 years; P = 0.17).
Event Ascertainment
All patients were followed for an average period of 14 ± 4.7 months. During this time, there were no deaths, myocardial infarctions or strokes. One patient with a positive MRI stress test and negative SPECT for transient ischemia was found to have significant triple vessel disease on catheter directed angiography and underwent coronary artery bypass surgery 1 month after the initial admission. Three patients with chest pain, history of coronary artery bypass and reversible ischemia on MRI did not receive additional revascularization therapy, because the significant coronary artery disease was mainly affecting only coronary side branches on angiography.
During the follow-up period, 11 patients presented to the hospital with recurrent chest pain, but negative cardiac enzymes. All of these patients had abnormal findings on the initial cardiac MRI, including nonischemic findings, ischemic scar, subendocardial left ventricular (LV) hypoperfusion and transient ischemia (Table 2). None of the patients with a normal cardiac MRI had recurrent chest pain (n = 10; Table 2). Thus, patients with any abnormality on cardiac MRI (n = 17) were more likely to have recurrent chest pain than those with normal cardiac MRI (n = 10) (P = 0.001). Patients with subendocardial hypoperfusion on stress MRI were significantly more likely to return to the ER with angina-like chest pain compared with patients with a normal cardiac MRI (4 of 8 patients, compared to 0 of 10 patients, respectively; P = 0.02). For recurrent presentations to the hospital with chest pain during the follow-up period, there was no significant difference between patients with (4 of 5 patients) or without (7 of 22 patients) reversible ischemia and/or myocardial scar on the initial SPECT exam (P = 0.13) (Table 2).
Discussion
The results of this study suggest that cardiac MRI with stress evaluation may help define the etiology of chest pain in emergency room patients with a negative ECG, negative cardiac enzymes, and intermediate risk for ischemic heart disease. Patients with subendocardial hypoperfusion on MRI returned to the hospital more often with recurrent chest pain and had diabetes and hypertension more frequently compared with patients with a normal cardiac MRI. This same group of patients had a lower perfusion reserve measured by coronary sinus flow measurements compared with ER patients with a normal cardiac MRI and a normal cardiac SPECT examination.
Patients with angina pectoris but normal coronary arteries without coronary spasm have previously been described (18). There are 10% to 30% of patients diagnosed with ischemia who have normal angiograms, thought to be due to microvascular disease (19,20). In our study, 8 of 27 (30%) chest pain patients with negative cardiac enzymes in the ER showed diffuse subendocardial hypoperfusion on MRI. It seems likely that this could be caused by microvascular disease. In comparison, a multi center study in 159 women showed that coronary microvascular dysfunction was present in approximately half of women with chest pain in the absence of obstructive CAD (21).
Coronary microangiopathy, causing increased resistance in prearteriolar coronary vessels, consequently lowering myocardial perfusion and thus leading to impaired coronary flow reserve, has been suggested to be the underlying cause for the adenosine-induced diffuse subendocardial hypoperfusion (22,23). Pilz et al also reported adenosine-induced subendocardial hypoperfusion in the left ventricular myocardium, using first pass perfusion MRI (13). As in our study, patients with adenosine-induced diffuse subendocardial hypoperfusion had an increased frequency of hypertension or diabetes. Pilz et al showed that the subendocardial perfusion deficit as seen by cardiac MRI was highly correlated to lower coronary artery flow on catheter directed coronary angiography. In addition, in our study ER patients with diffuse stress induced myocardial hypoperfusion and chest pain had a lower perfusion reserve compared with symptomatic ER patients with normal first pass perfusion MRI.
In 222 participants of the MESA (Multi Ethnic Study of Atherosclerosis) study, coronary vasoreactivity was reduced in asymptomatic individuals with a greater coronary risk factor burden (24). In our study, patients with chest pain and adenosine-induced microvascular hypoperfusion also had significantly more traditional cardiovascular risk factors compared with the group without small or large vessel disease. The data suggest that the traditional risk factors not only affect the conductive coronary arteries but also myocardial microvascular vasoreactivity.
MRI findings of subendocardial hypoperfusion need to be carefully distinguished from hypoperfusion due to hemodynamically significant coronary artery stenosis. Both findings are seen only with adenosine stress perfusion. In general, perfusion defects due to coronary artery stenosis are more persistent and more focal than diffuse subendocardial perfusion defects. Both occur after the peak contrast bolus has reached the LV cavity at the time the myocardium starts to enhance. Dark rim artifacts typically start to occur earlier just before the peak bolus reaches the LV cavity. Dark rim artifacts may occur particularly with older perfusion sequences with lower spatial resolution, likely due to susceptibility differences between the blood pool and myocardium (25,26). Diffuse subendocardial hypoperfusion is located in the endocardium and is not confined to the blood pool/myocardial border as typically seen with dark rim artifacts. Dark rim artifacts are frequent (52% in our study) and are typically recognized on both resting and stress perfusion MRI studies and are usually more focal than subendocardial perfusion defects.
Three patients with CABG and reversible ischemia on MRI did not receive any additional revascularization therapy in our study, as the coronary artery narrowing was affecting only coronary side branches on conventional angiography. In all three patients adenosine-induced regional reversible perfusion defects involved less than one-third of the myocardial thickness, but lasted longer than six heart beats. These perfusion deficits were not thought to be clinically significant for coronary revascularization; nevertheless, during the follow-up period, two of these patients presented to the hospital with recurring chest pain and negative cardiac enzymes. Dobutamine stress examinations may have higher specificity in this setting (27,28).
Compared with cardiac SPECT, PET, and CT, MRI does not expose patients to radiation, which is a strong motivation to further work on implementing cardiac MRI in the emergency room (29–31). Cardiac stress perfusion MRI has higher spatial resolution (2 mm in our study) compared with SPECT (10 mm) and PET (5–6 mm) (32), which is likely the cause for the detection of subendocardial perfusion defects on cardiac MRI in our ER patient cohort with normal SPECT exams.
Limitations
A limitation of our study is that only a portion of our patients received conventional coronary angiography, because it was not routine clinical practice to perform catheter directed angiography after a negative SPECT examination. Patients in this category were instead followed for cardiovascular events. Nevertheless, reviewer agreement was high. Four patients (13%) were claustrophobic and did not complete the MRI exam, while all patients could tolerate the nuclear cardiac SPECT exam. Adenosine was used for all MRI cases but often different stress agents were used for the SPECT stress exam, which may have influenced the rate of discordant results. We acknowledge that this is a pilot study and future larger trials have to show if adenosine induced diffuse subendocardial hypoperfusion on first pass perfusion MRI is an independent predictor of future cardiovascular events.
In conclusion, chest pain patients presenting to the emergency room may have ischemic or nonischemic etiologies causing their pain. Cardiac stress perfusion MRI can identify subendocardial hypoperfusion that may represent microvascular disease in patients with chest pain and negative cardiac enzymes; these perfusion abnormalities are not otherwise detected on SPECT imaging. In our patient cohort, adenosine stress induced left ventricular diffuse subendocardial hypoperfusion found on MRI was associated with recurrent chest pain, diabetes, hypertension and decreased global myocardial perfusion reserve. It remains to be determined if patients with chest pain and adenosine-induced diffuse subendocardial hypoperfusion on MRI benefit from more aggressive cardiovascular risk reduction treatment.
Acknowledgments
J.V.C. was supported by the Radiological Society of North America Research and Education Foundation and Siemens Medical Solutions, USA, Inc.
Contract grant sponsor: Siemens Medical Solutions, Inc.; Contract grant number: numbers; Contract grant number: JHU-2006-MR-27-01.
References
- 1.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 2.Dakik HA, Hwang WS, Jafar A, Kimball K, Verani MS, Mahmarian JJ. Prognostic value of quantitative stress myocardial perfusion imaging in unstable angina patients with negative cardiac enzymes and no new ischemic ECG changes. J Nucl Cardiol. 2005;12:32–36. doi: 10.1016/j.nuclcard.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bulow H, Schwaiger M. Nuclear cardiology in acute coronary syndromes. Q J Nucl Med Mol Imaging. 2005;49:59–71. [PubMed] [Google Scholar]
- 4.Foo TK, Ho VB, Saranathan M, et al. Feasibility of integrating high-spatial-resolution 3D breath-hold coronary MR angiography with myocardial perfusion and viability examinations. Radiology. 2005;235:1025–1030. doi: 10.1148/radiol.2353040090. [DOI] [PubMed] [Google Scholar]
- 5.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237:316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 6.Pilz G, Jeske A, Klos M, et al. Prognostic value of normal adenosine-stress cardiac magnetic resonance imaging. Am J Cardiol. 2008;101:1408–1412. doi: 10.1016/j.amjcard.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343–1353. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Klem I, Heitner JF, Shah DJ, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 9.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma H, Suzawa N, Ichikawa Y, et al. Diagnostic accuracy of stress first-pass contrast-enhanced myocardial perfusion MRI compared with stress myocardial perfusion scintigraphy. AJR Am J Roentgenol. 2005;185:95–102. doi: 10.2214/ajr.185.1.01850095. [DOI] [PubMed] [Google Scholar]
- 12.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 13.Pilz G, Klos M, Ali E, Hoefling B, Scheck R, Bernhardt P. Angiographic correlations of patients with small vessel disease diagnosed by adenosine-stress cardiac magnetic resonance imaging. J Cardiovasc Magn Reson. 2008;10:8. doi: 10.1186/1532-429X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhardt P, Levenson B, Albrecht A, Engels T, Strohm O. Detection of cardiac small vessel disease by adenosine-stress magnetic resonance. Int J Cardiol. 2007;121:261–266. doi: 10.1016/j.ijcard.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Koskenvuo JW, Hartiala JJ, Knuuti J, et al. Assessing coronary sinus blood flow in patients with coronary artery disease: a comparison of phase-contrast MR imaging with positron emission tomography. AJR Am J Roentgenol. 2001;177:1161–1166. doi: 10.2214/ajr.177.5.1771161. [DOI] [PubMed] [Google Scholar]
- 16.Georgoulias P, Orfanakis A, Demakopoulos N, et al. Abnormal heart rate recovery immediately after treadmill testing: correlation with clinical, exercise testing, and myocardial perfusion parameters. J Nucl Cardiol. 2003;10:498–505. doi: 10.1016/s1071-3581(03)00530-0. [DOI] [PubMed] [Google Scholar]
- 17.Plein S, Ryf S, Schwitter J, Radjenovic A, Boesiger P, Kozerke S. Dynamic contrast-enhanced myocardial perfusion MRI accelerated with k-t sense. Magn Reson Med. 2007;58:777–785. doi: 10.1002/mrm.21381. [DOI] [PubMed] [Google Scholar]
- 18.Cannon RO, III, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 19.Proudfit WL, Shirey EK, Sones FM., Jr Selective cine coronary arteriography. Correlation with clinical findings in 1,000 patients. Circulation. 1966;33:901–910. doi: 10.1161/01.cir.33.6.901. [DOI] [PubMed] [Google Scholar]
- 20.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 21.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 22.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 23.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. doi: 10.1016/s0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Jerosch-Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Meyer C, Strach K, Thomas D, et al. High-resolution myocardial stress perfusion at 3 T in patients with suspected coronary artery disease. Eur Radiol. 2008;18:226–233. doi: 10.1007/s00330-007-0746-3. [DOI] [PubMed] [Google Scholar]
- 26.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54:1295–1299. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 28.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 29.Cody DD, Mahesh M. AAPM/RSNA physics tutorial for residents: technologic advances in multidetector CT with a focus on cardiac imaging. Radiographics. 2007;27:1829–1837. doi: 10.1148/rg.276075120. [DOI] [PubMed] [Google Scholar]
- 30.Javadi M, Mahesh M, McBride G, et al. Lowering radiation dose for integrated assessment of coronary morphology and physiology: first experience with step-and-shoot CT angiography in a rubidium 82 PET-CT protocol. J Nucl Cardiol. 2008;15:783–790. doi: 10.1007/BF03007359. [DOI] [PubMed] [Google Scholar]
- 31.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 32.Jadvar H, Strauss HW, Segall GM. SPECT and PET in the evaluation of coronary artery disease. Radiographics. 1999;19:915–926. doi: 10.1148/radiographics.19.4.g99jl08915. [DOI] [PubMed] [Google Scholar]


